Abstract
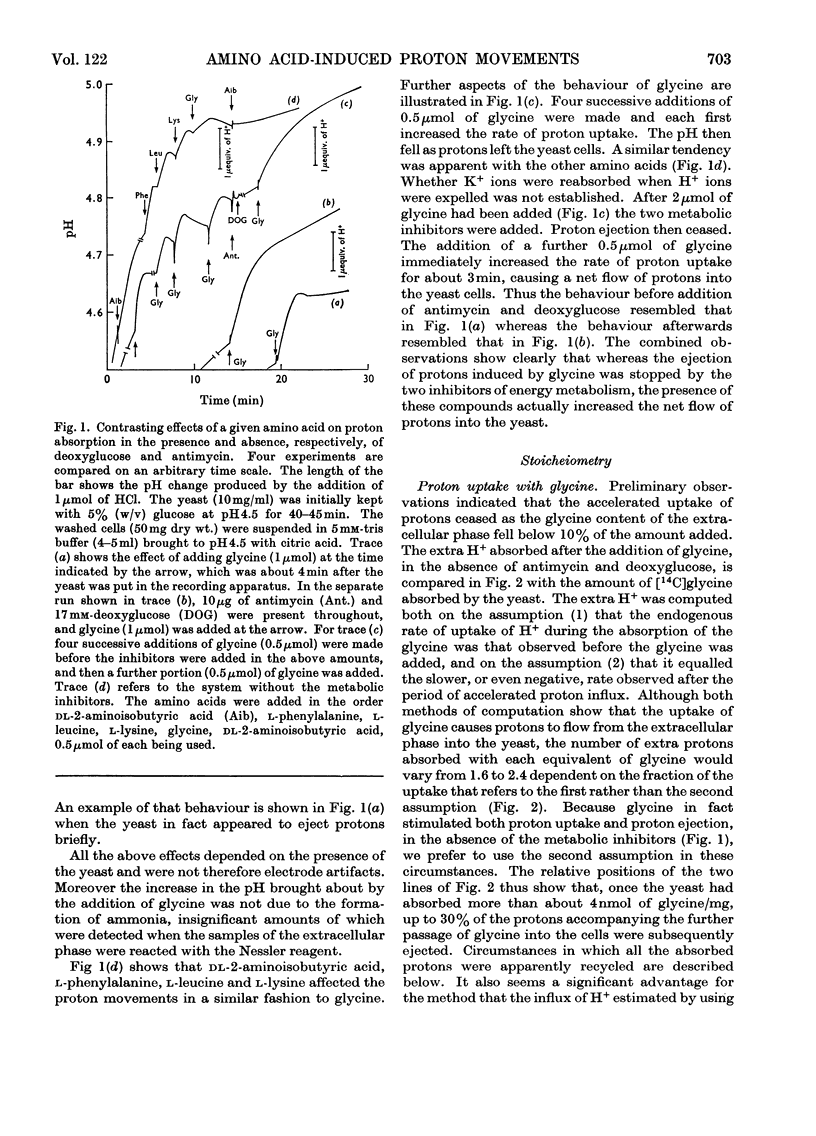
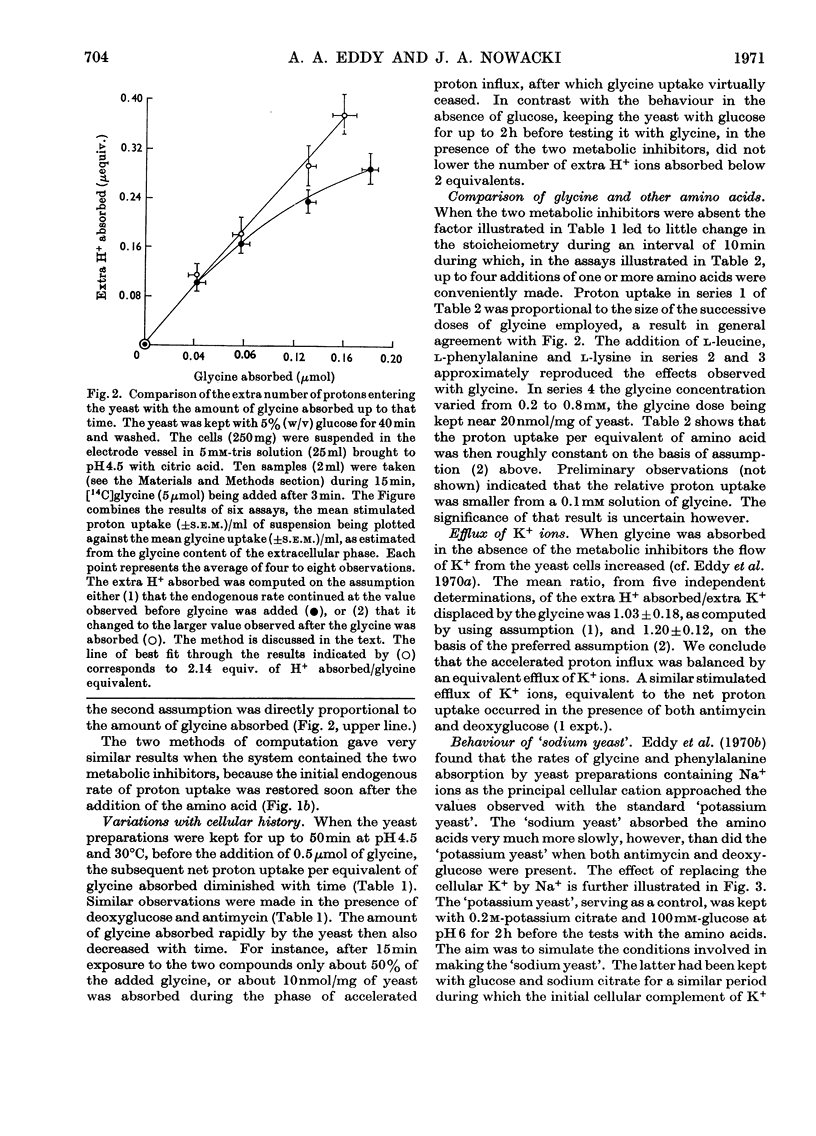
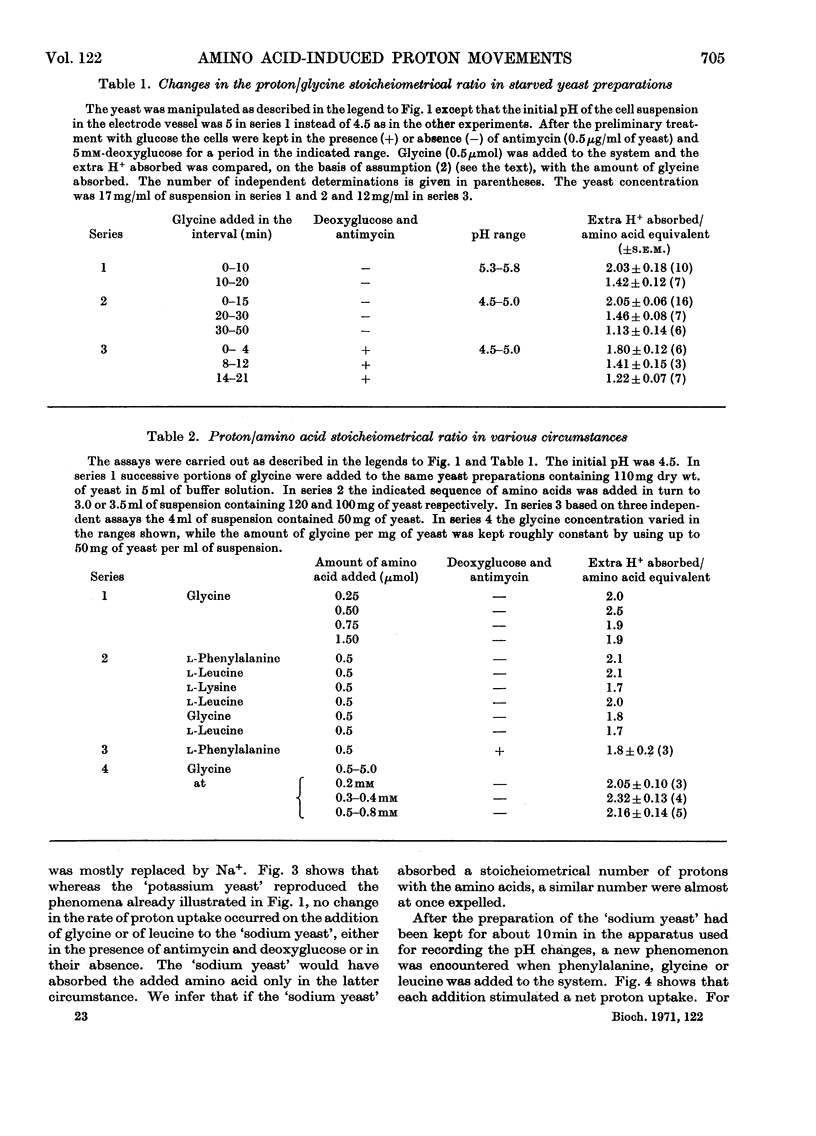
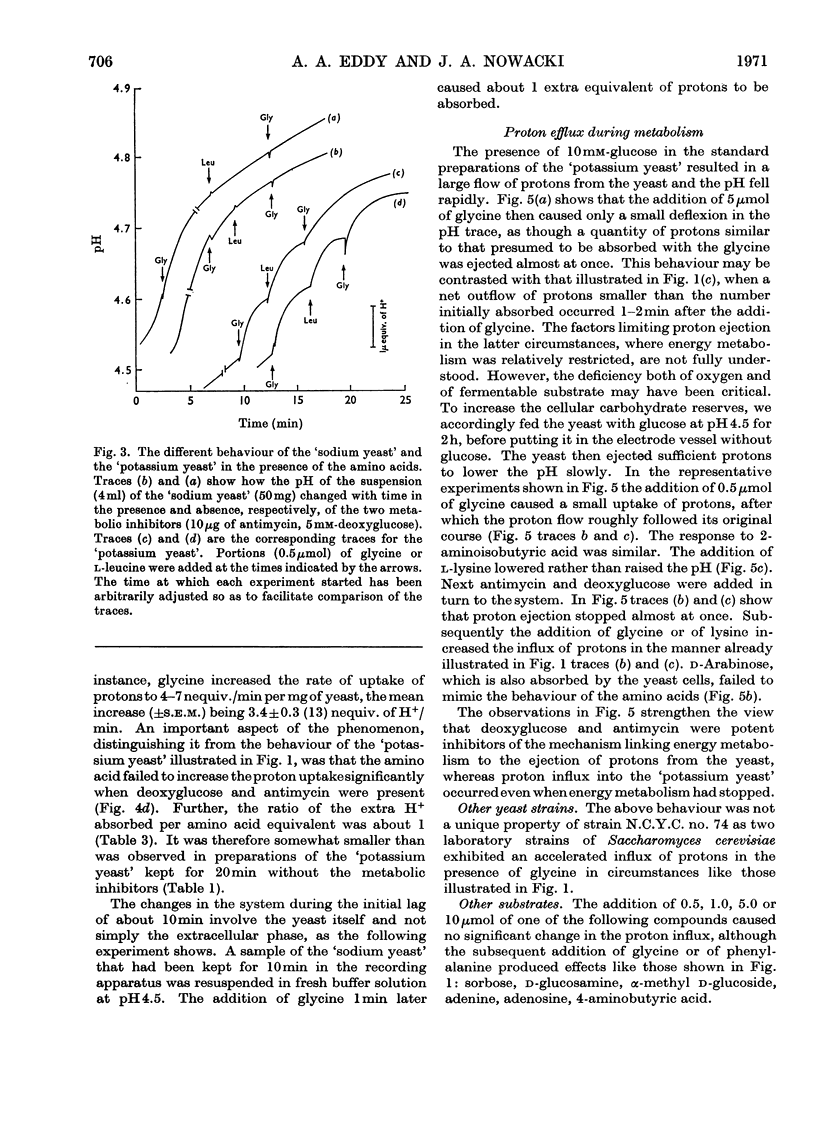
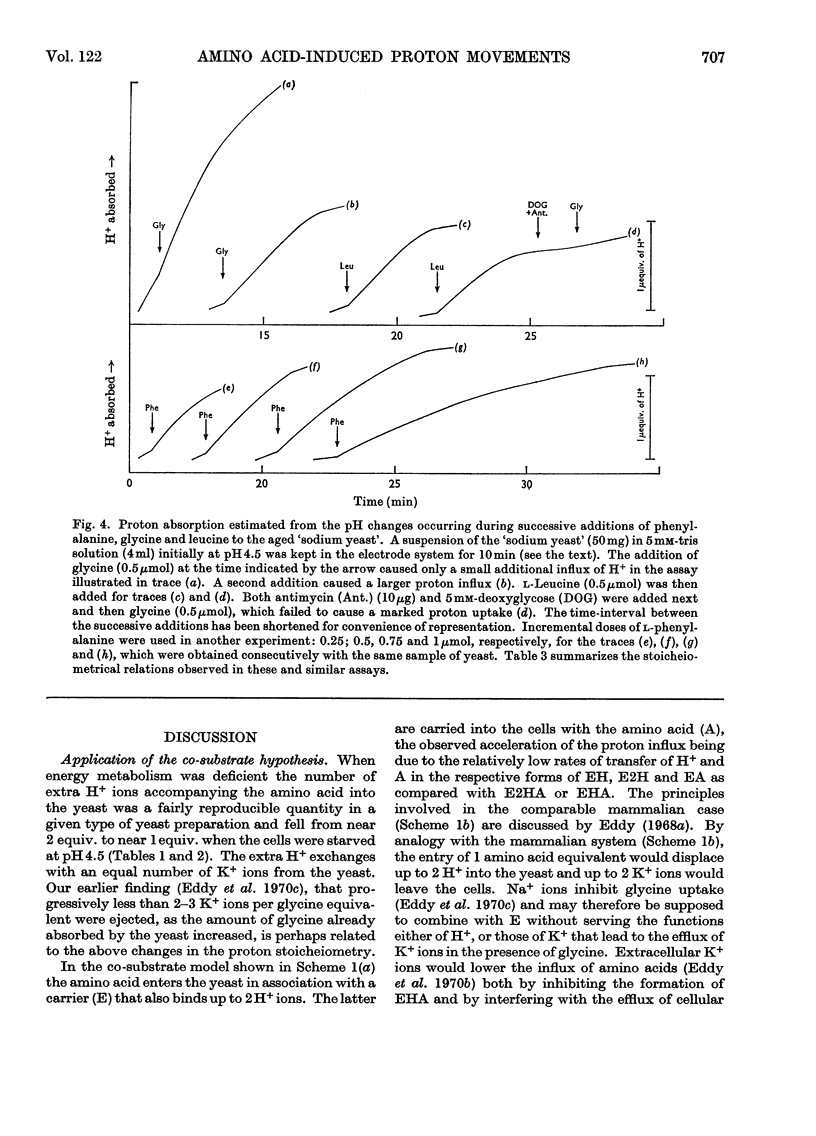
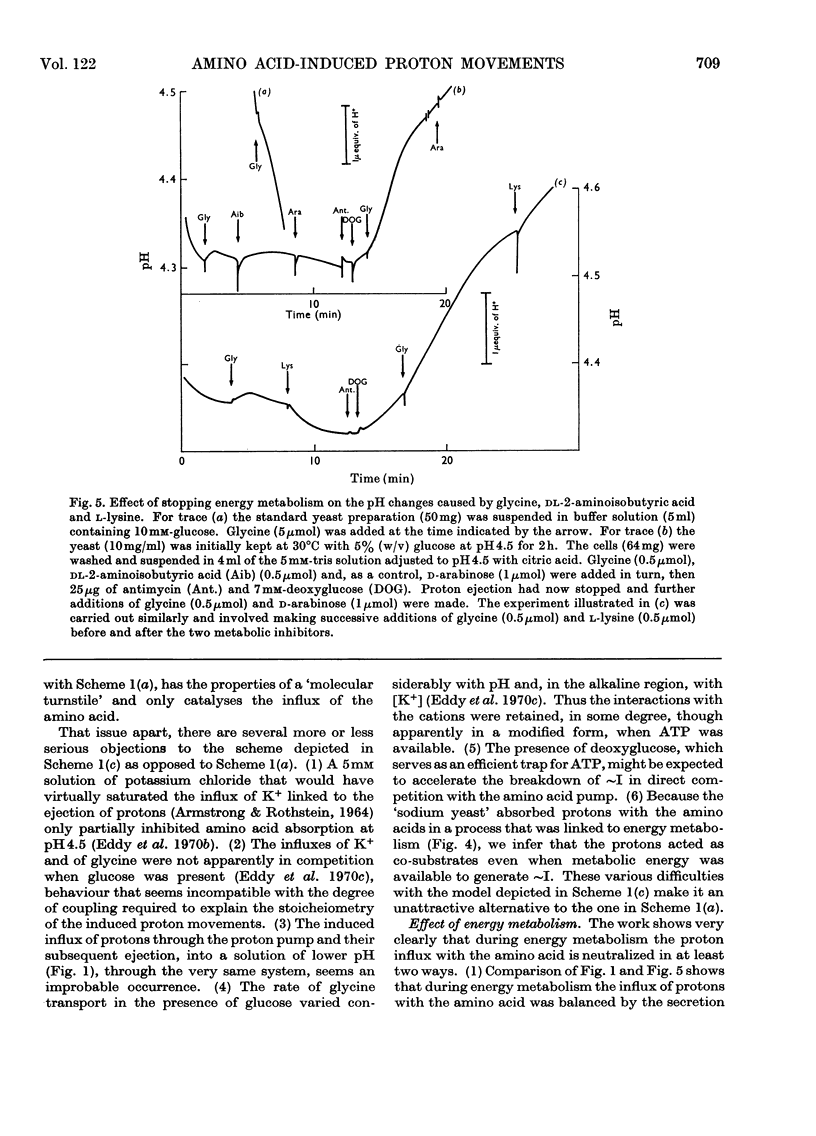
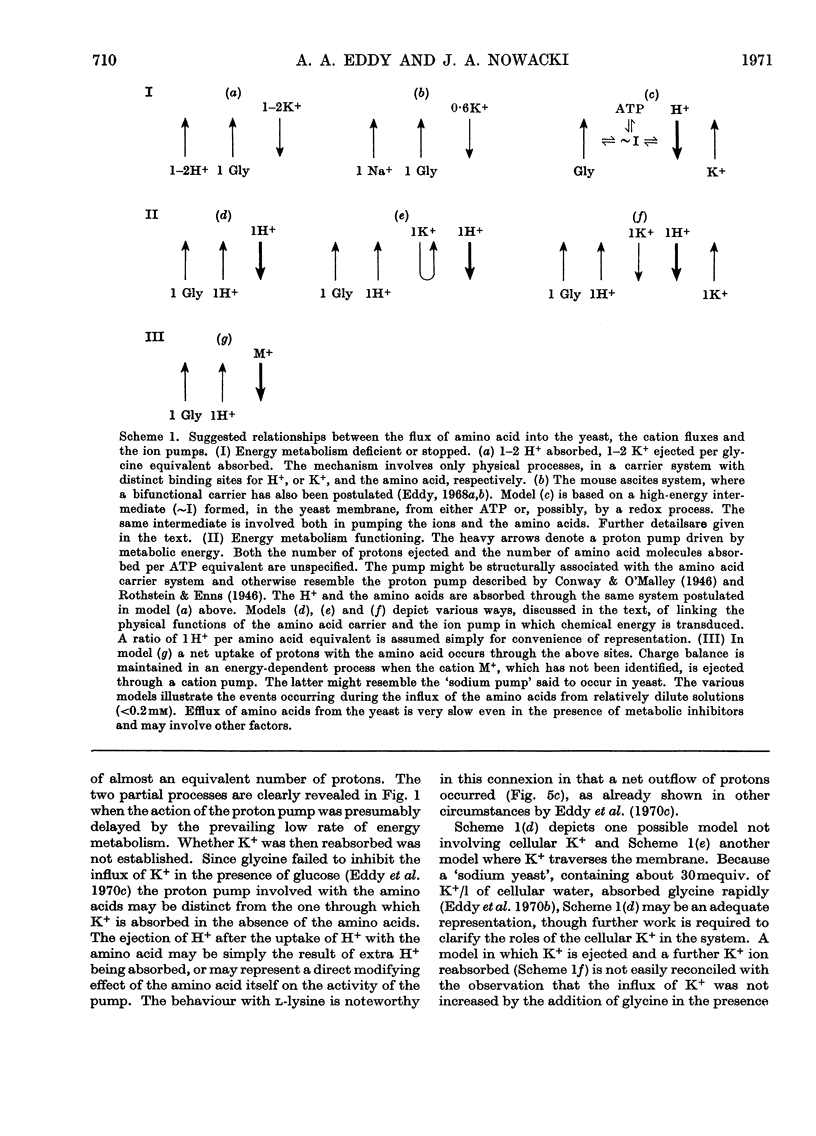
1. Proton uptake into the yeast Saccharomyces carlsbergensis, was studied at pH4.5–5.5 in the presence of both antimycin and 2-deoxyglucose to inhibit energy metabolism. Previous work had shown that the cells then absorbed about 20nmol of glycine or l-phenylalanine against a considerable amino acid concentration gradient. The addition of the amino acid immediately stimulated the rate of uptake of protons two- to three-fold. About 2 extra equivalents of H+ accompanied a given amount of the amino acids into the yeast preparations exposed to the metabolic inhibitors for 2–4min and about 1.2 equivalents after 20min exposure. 2. Analogous observations were made during serial additions of glycine, l-phenylalanine, l-leucine and l-lysine to preparations lacking the metabolic inhibitors and deficient in substrates needed for energy metabolism. In fresh cellular preparations the influx of glycine was then closely coupled to a stimulated flow of 2.1 equiv. of H+ into the yeast. A similar number of K+ ions left the cells. About 30% of the extra protons was subsequently ejected from the yeast. Deoxyglucose and antimycin together inhibited the ejection of protons. When the yeast had been fed with glucose energy metabolism was stimulated and almost as many protons as were absorbed with the amino acid were apparently ejected again. 3. Yeast preparations containing Na+, instead of K+, as the principal cation absorbed about 1 extra equivalent of H+ after the addition of phenylalanine, glycine or leucine. This response was not observed in the presence of both deoxyglucose and antimycin. 4. The observations show that H+ and, in certain circumstances, K+ are co-substrates in the transport of the amino acids into the yeast. An analogy is drawn with the roles of Na+ and K+ as co-substrates in certain mammalian systems. The results lead to various models relating the physical flow of the co-substrate ions on the amino acid carrier to the transduction of chemical energy in an associated ion pump forming part of the mechanism for transporting amino acids into the yeast.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONWAY E. J., RYAN H., CARTON E. Active transport of sodium ions from the yeast cell. Biochem J. 1954 Sep;58(1):158–167. doi: 10.1042/bj0580158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway E. J., O'malley E. The nature of the cation exchanges during yeast fermentation, with formation of 0.02n-H ion. Biochem J. 1946;40(1):59–67. [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A. A net gain of sodium ions and a net loss of potassium ions accompanying the uptake of glycine by mouse ascites-tumour cells in the presence of sodium cyanide. Biochem J. 1968 Jun;108(2):195–206. doi: 10.1042/bj1080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Backen K., Watson G. The concentration of amino acids by yeast cells depleted of adenosine triphosphate. Biochem J. 1970 Dec;120(4):853–858. doi: 10.1042/bj1200853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Indge K. J., Backen K., Nowacki J. A. Interctions between potassium ions and glycine transport in the yeast Saccharomyces carlsbergensis. Biochem J. 1970 Dec;120(4):845–852. doi: 10.1042/bj1200845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A. The effects of varying the cellular and extracellular concentrations of sodium and potassium ions on the uptake of glycine by mouse ascites-tumour cells in the presence and absence of sodium cyanide. Biochem J. 1968 Jul;108(3):489–498. doi: 10.1042/bj1080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lant A. F., Priestland R. N., Whittam R. The coupling of downhill ion movements associated with reversal of the sodium pump in human red cells. J Physiol. 1970 Apr;207(2):291–301. doi: 10.1113/jphysiol.1970.sp009062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Slater E. C. An evaluation of the Mitchell hypothesis of chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Eur J Biochem. 1967 May;1(3):317–326. doi: 10.1007/978-3-662-25813-2_43. [DOI] [PubMed] [Google Scholar]


