Abstract
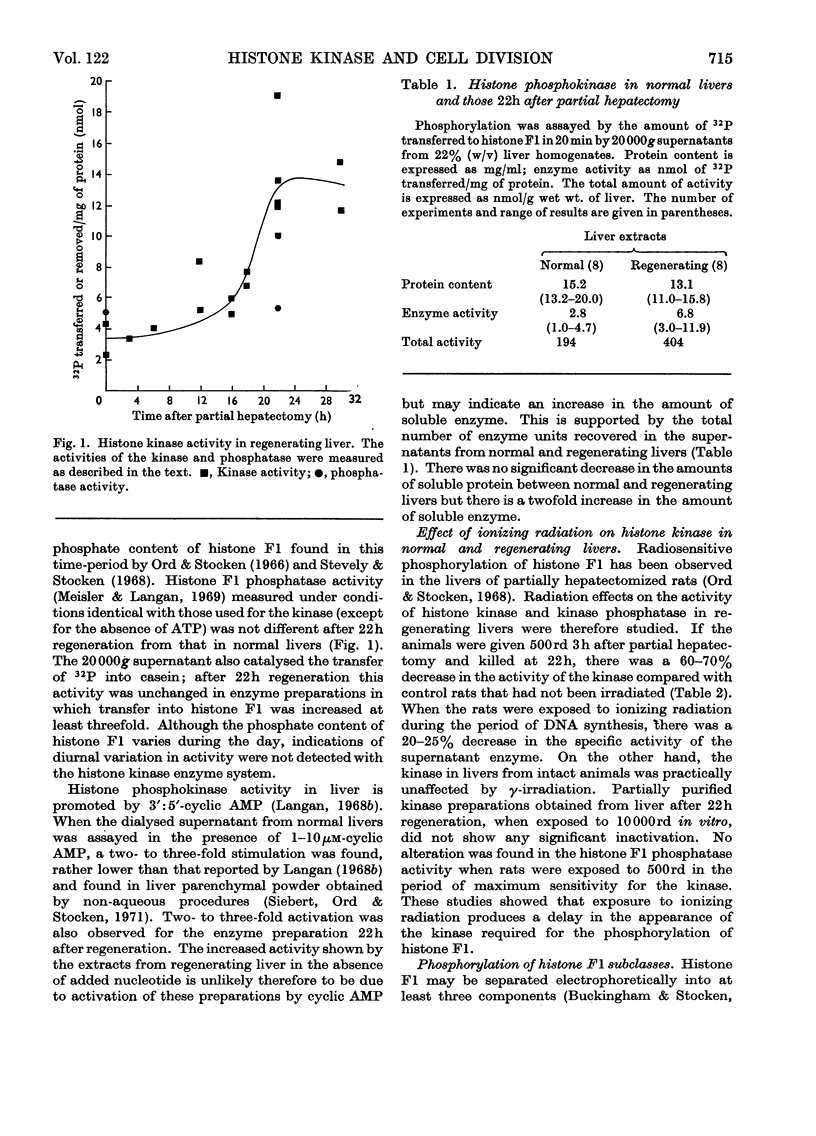
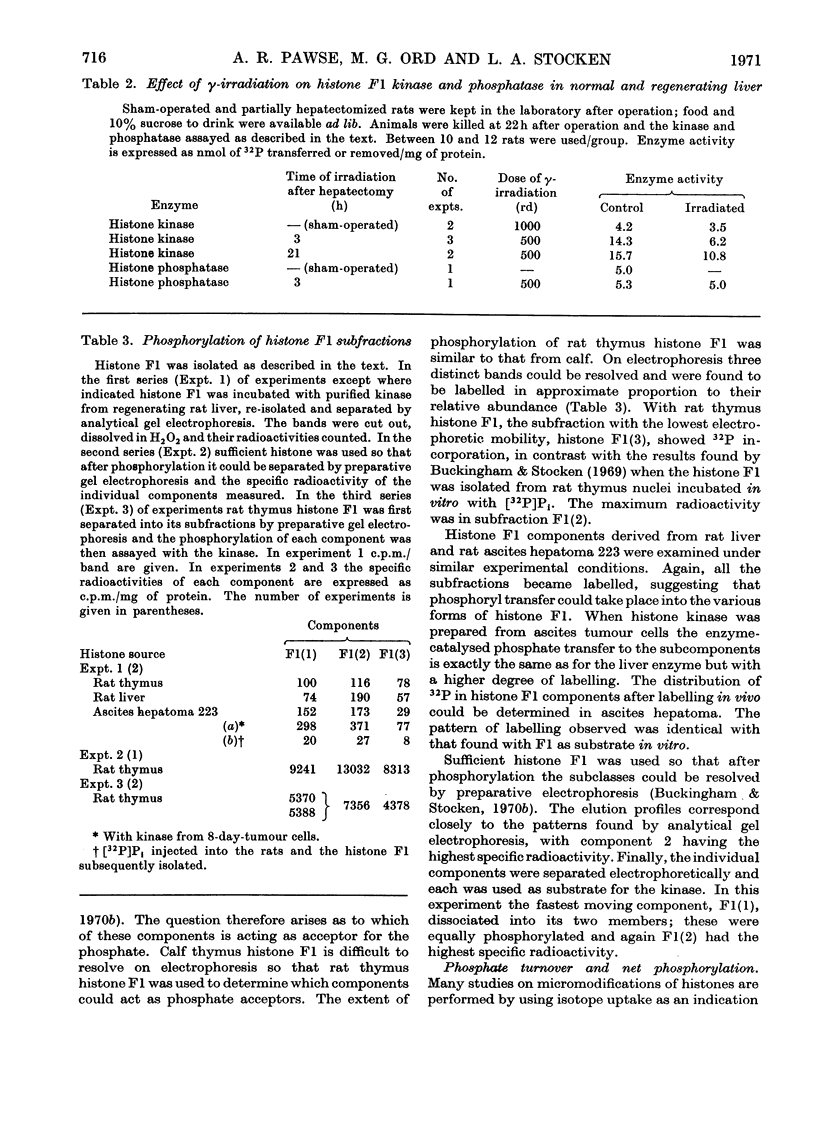
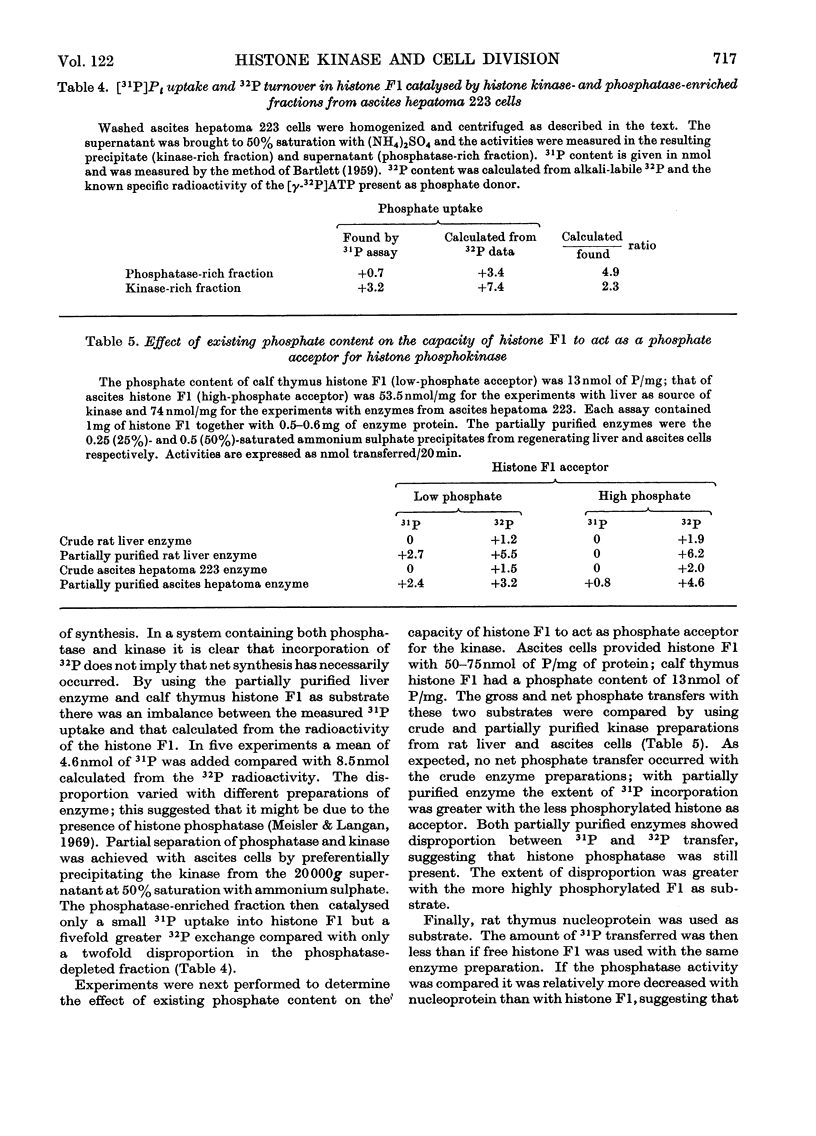
1. The activity of the soluble phosphokinase for histone F1 increases in regenerating rat liver during the first period of DNA synthesis after partial hepatectomy. The increase probably represents new enzyme synthesis. 2. A dose of 500rd of γ-irradiation given early in G1 decreases the amount of histone F1 phosphokinase found 22h after partial hepatectomy by 60–70%. 3. The enzyme preparations also contained a histone F1 phosphatase; the presence together of the kinase and phosphatase caused a disproportion between net 31P uptake and 32P incorporation into histone F1. 4. All four subclasses of histone F1 could accept phosphate from ATP. 5. Crude enzyme preparations transferred more 31P into histone F1 with an initially low phosphate content than into one with a high phosphate content; conversely, more 32P was transferred into the latter.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., LITTAU V. C., MIRSKY A. E. METHODS FOR THE PURIFICATION OF THYMUS NUCLEI AND THEIR APPLICATION TO STUDIES OF NUCLEAR PROTEIN SYNTHESIS. J Cell Biol. 1964 May;21:213–231. doi: 10.1083/jcb.21.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BURNETT G., KENNEDY E. P. The enzymatic phosphorylation of proteins. J Biol Chem. 1954 Dec;211(2):969–980. [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham R. H., Stocken L. A. Histone F1 from rat thymus. Subfractionation and incorporation of [32P] phosphate in vitro. Biochem J. 1970 Apr;117(3):509–512. doi: 10.1042/bj1170509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham R. H., Stocken L. A. Histone F1. Purification and phosphorus content. Biochem J. 1970 Mar;117(1):157–160. doi: 10.1042/bj1170157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- CREASEY W. A., STOCKEN L. A. The effect of ionizing radiation on nuclear phosphorylation in the radio-sensitive tissues of the rat. Biochem J. 1959 Jul;72:519–523. doi: 10.1042/bj0720519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronow M., Todd P. The synthesis of deoxyribonucleic acid and nuclear proteins by rat hepatoma cells immediately after gamma irradiation. Radiat Res. 1969 Sep;39(3):705–715. [PubMed] [Google Scholar]
- Jergil B., Sung M., Dixon G. H. Species- and tissue-specific patterns of phosphorylation of very lysine-rich histones. J Biol Chem. 1970 Nov 10;245(21):5867–5870. [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinsmith L. J., Allfrey V. G., Mirsky A. E. Phosphoprotein metabolism in isolated lymphocyte nuclei. Proc Natl Acad Sci U S A. 1966 May;55(5):1182–1189. doi: 10.1073/pnas.55.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Further studies on phosphorylation and the thiol-disulphide ratio of histones in growth and development. Biochem J. 1969 Mar;112(1):81–89. doi: 10.1042/bj1120081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Metabolic properties of histones from rat liver and thymus gland. Biochem J. 1966 Mar;98(3):888–897. doi: 10.1042/bj0980888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Variations in the phosphate content and thiołdisulphide ratio of histones during the cell cycle. Studies with regenerating rat liver and sea urchins. Biochem J. 1968 Apr;107(3):403–410. doi: 10.1042/bj1070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Shepherd G. R., Noland B. J., Roberts C. N. Phosphorus in histones. Biochim Biophys Acta. 1970 Jan 21;199(1):265–276. doi: 10.1016/0005-2787(70)90715-x. [DOI] [PubMed] [Google Scholar]
- Siebert G., Ord M. G., Stocken L. A. Histone phosphokinase activity in nuclear and cytoplasmic cell fractions from normal and regenerating rat livers. Biochem J. 1971 May;122(5):721–725. doi: 10.1042/bj1220721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S., Stocken L. A. Variations in the phosphate content of histone F1 in normal and irradiated tissues. Biochem J. 1968 Nov;110(2):187–191. doi: 10.1042/bj1100187. [DOI] [PMC free article] [PubMed] [Google Scholar]


