Abstract
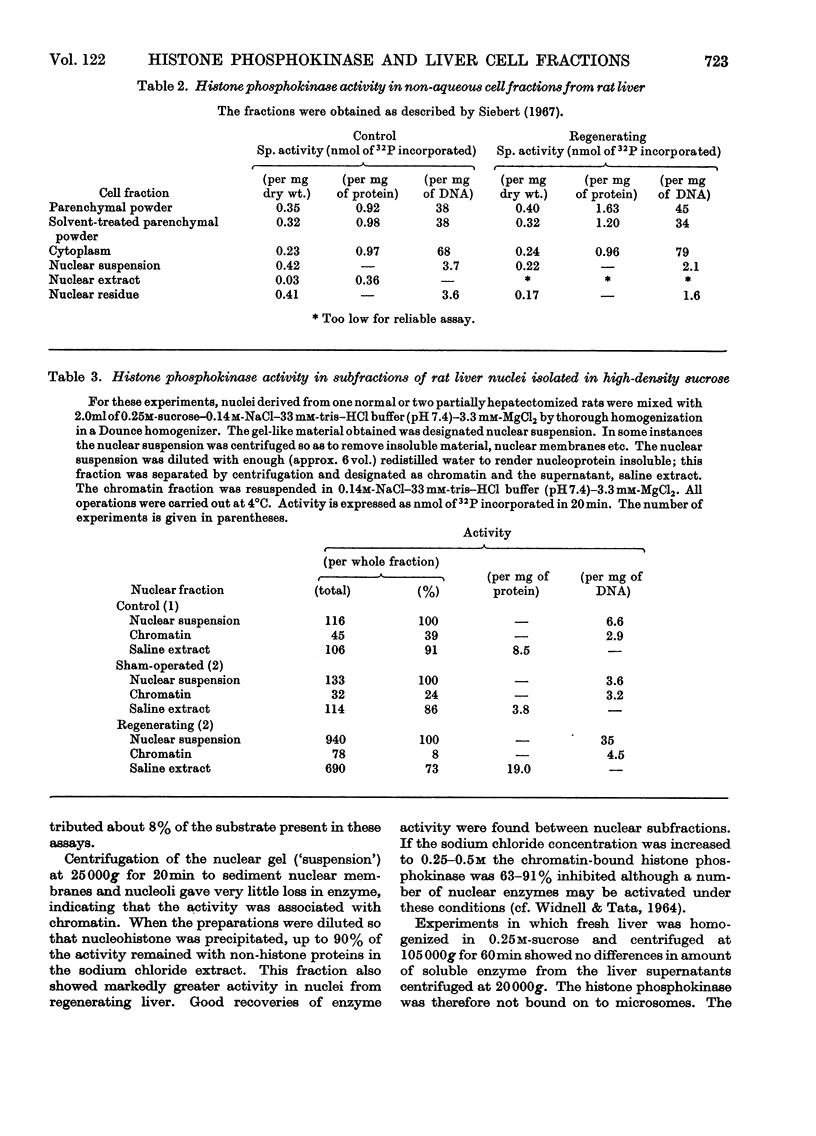
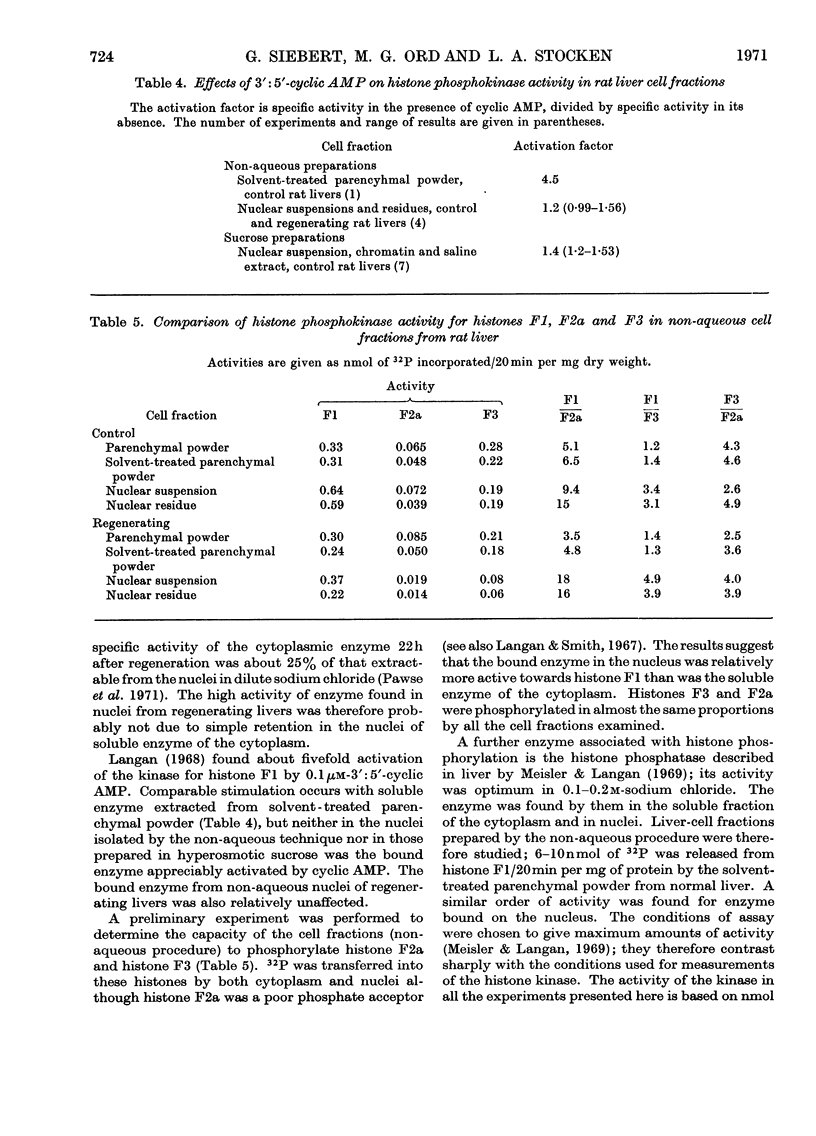
1. Liver cell fractions were prepared by non-aqueous procedures and nuclei were also obtained in a hyperosmotic sucrose medium. Histone phosphokinase activity, assayed with histone F1 as substrate, was present in the soluble fraction of the cytoplasm and also bound on to the chromatin fraction of the nucleus. 2. The activity of the enzyme increased sixfold in nuclei from regenerating livers 22h after partial hepatectomy. 3. The enzyme bound in the nucleus was only marginally activated by 1μm-3′:5′-cyclic AMP which stimulated the cytoplasmic soluble enzyme fourfold. 4. Nuclei prepared by the non-aqueous technique were also able to phosphorylate histones F2a and F3 and showed histone phosphatase activity with histone F1 phosphate as substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allfrey V. G. Changes in chromosomal proteins at times of gene activation. Fed Proc. 1970 Jul-Aug;29(4):1447–1460. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy S. C., Roberts S., Morelos B. S. Histone-acetylating enzyme of brain. Biochem J. 1970 Oct;119(4):665–672. doi: 10.1042/bj1190665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Gallwitz D. Extraction and partial purification of two histone-specific transacetylases from rat liver nuclei. Biochem Biophys Res Commun. 1970 Jul 13;40(1):236–242. doi: 10.1016/0006-291x(70)91072-7. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Pawse A. R., Ord M. G., Stocken L. A. Histone kinase and cell division. Biochem J. 1971 May;122(5):713–719. doi: 10.1042/bj1220713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevely W. S., Stocken L. A. Variations in the phosphate content of histone F1 in normal and irradiated tissues. Biochem J. 1968 Nov;110(2):187–191. doi: 10.1042/bj1100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDNELL C. C., TATA J. R. EVIDENCE FOR TWO DNA-DEPENDENT RNA POLYMERASE ACTIVITIES IN ISOLATED RAT-LIVER NUCLEI. Biochim Biophys Acta. 1964 Jul 22;87:531–533. doi: 10.1016/0926-6550(64)90133-1. [DOI] [PubMed] [Google Scholar]


