Abstract
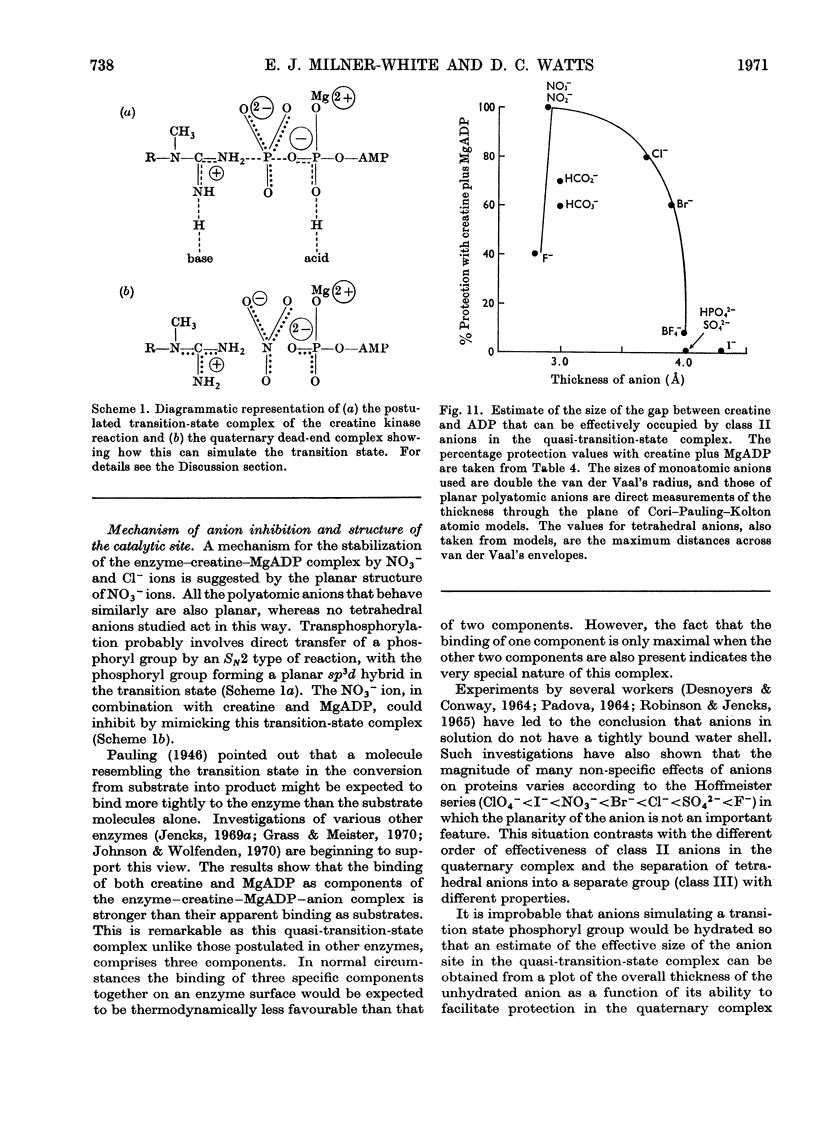
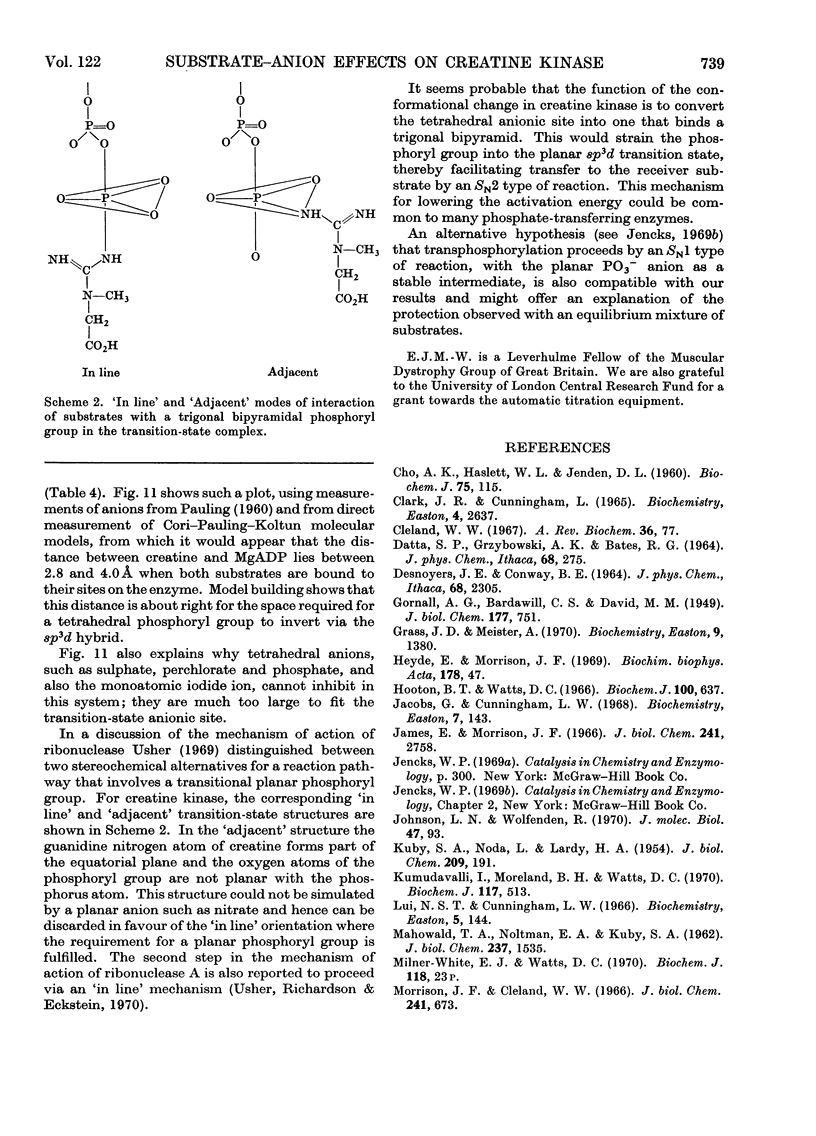
1. The substrate combination creatine–MgADP does not significantly protect creatine kinase against inhibition by iodoacetamide in the absence of small anions. 2. Small anions can be divided into three groups according to the way in which they affect creatine kinase: I, acetate reversibly increases enzyme activity in the forward reaction but does not affect the rate of inhibition by iodoacetamide in the presence of creatine plus MgADP; II, planar anions and some halides (HCO3−, HCO2−, NO3−, NO2−, Cl−, Br−, F−) in the presence of creatine plus MgADP protect the enzyme from inhibition by iodoacetamide; III, tetrahedral anions (SO42−, HPO42−, ClO4−, BF4−) and iodide do not affect the rate of inhibition by iodoacetamide in the presence of creatine plus MgADP but may decrease the protection by class II anions under these conditions. Anions of class II and class III also reversibly inhibit enzyme activity. 3. It is concluded that class II anions form a stable and inactive quaternary enzyme–creatine–MgADP–anion complex and this is responsible for the effect attributed by previous workers to the ternary complex lacking anion. Formation of this complex, particularly in the forward reaction, can lead to markedly non-linear enzyme progress curves. Some previous observations are re-appraised in the light of these findings. 4. From the behaviour of chloride and nitrate ions, and the marked lowering of the Ki values for creatine and MgADP they produce, it is inferred that planar or monoatomic anions act in the quaternary complex by simulating the transferable phosphoryl group in the transition state (or another intermediate state) of the reaction. 5. It is suggested that, in the course of the reaction, the tetrahedral phosphate-binding site for the transferable phosphoryl group of the substrate (that also binds class II and class III anions) changes into a trigonal bipyramid site (also occupied by class II anions). This strains the phosphoryl group to adopt the transitional sp3d hybridized state and must contribute significantly to the low activation energy of the reaction. 6. Catalysis is deduced to proceed by an `in line' transfer reaction and from the effects of class II anions it is possible to estimate the approximate dimensions of the anionic site in the transition-state complex. 7. The specific protecting effect of an equilibrium mixture of substrates against inhibition by iodoacetamide provides further evidence for the conformational change suggested above as a step in the catalytic process.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHO A. K., HASLETT W. L., JENDEN D. J. A titrimetric method for the determination of creating phosphokinase. Biochem J. 1960 Apr;75:115–119. doi: 10.1042/bj0750115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. R., Cunningham L. The acetylation of creatine phosphokinase with p-nitrophenyl acetate. Biochemistry. 1965 Dec;4(12):2637–2643. doi: 10.1021/bi00888a013. [DOI] [PubMed] [Google Scholar]
- Heyde E., Morrison J. F. The interaction of Mg2+ and ATP4- with ATP: creatine phosphotransferase. Biochim Biophys Acta. 1969 Mar 18;178(1):47–60. doi: 10.1016/0005-2744(69)90130-2. [DOI] [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Adenosine 5 -triphosphate--creatine phosphotransferase from dystrophic mouse skeletal muscle. A genetic lesion associated with the catalytic-site thiol group. Biochem J. 1966 Sep;100(3):637–646. doi: 10.1042/bj1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs G., Cunningham L. W. Creatine kinase. The relationship of trypsin susceptibility to substrate binding. Biochemistry. 1968 Jan;7(1):143–151. doi: 10.1021/bi00841a019. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Kumudavalli I., Moreland B. H., Watts D. C. Properties and reaction with iodoacetamide of adenosine 5'-triphosphate-creatine phosphotransferase from human skeletal muscle. Further evidence about the role of the essential thiol group in relation to the mechanism of action. Biochem J. 1970 Apr;117(3):513–523. doi: 10.1042/bj1170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui N. S., Cunningham L. Cooperative effects of substrates and substrate analogs on the conformation of creatine phosphokinase. Biochemistry. 1966 Jan;5(1):144–149. doi: 10.1021/bi00865a019. [DOI] [PubMed] [Google Scholar]
- MAHOWALD T. A., NOLTMANN E. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. III. Inhibition reactions. J Biol Chem. 1962 May;237:1535–1548. [PubMed] [Google Scholar]
- MORRISON J. F., O'SULLIVAN W. J. KINETIC STUDIES OF THE REVERSE REACTION CATALYSED BY ADENOSINE TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE. THE INHIBITION BY MAGNESIUM IONS AND ADENOSINE DIPHOSPHATE. Biochem J. 1965 Jan;94:221–235. doi: 10.1042/bj0940221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-White E. J., Watts D. C. The significance of anions in the protection by substrates of adenosine triphosphate-creatine phosphotransferase against inhibition by iodoacetamide. Biochem J. 1970 Jun;118(2):23P–24P. doi: 10.1042/bj1180023p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison J. F., Cleland W. W. Isotope exchange studies of the mechanism of the reaction catalyzed by adenosine triphosphate: creatine phosphotransferase. J Biol Chem. 1966 Feb 10;241(3):673–683. [PubMed] [Google Scholar]
- Morrison J. F., James E. The mechanism of the reaction catalysed by adenosine triphosphate-creatine phosphotransferase. Biochem J. 1965 Oct;97(1):37–52. doi: 10.1042/bj0970037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIHEI T., NODA L., MORALES M. F. Kinetic properties and equilibrium constant of the adenosine triphosphate-creatine transphosphorylase-catalyzed reaction. J Biol Chem. 1961 Dec;236:3203–3209. [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. II. Homogeneity and physicochemical properties. J Biol Chem. 1954 Jul;209(1):203–210. [PubMed] [Google Scholar]
- O'Sullivan W. J., Cohn M. Nucleotide specificity and conformation of the active site of creatine kinase. Magnetic resonance and sulfhydryl reactivity studies. J Biol Chem. 1966 Jul 10;241(13):3116–3125. [PubMed] [Google Scholar]
- ROBINSON D. R., JENCKS W. P. THE EFFECT OF COMPOUNDS OF THE UREA-GUANIDINIUM CLASS ON THE ACTIVITY COEFFICIENT OF ACETYLTETRAGLYCINE ETHYL ESTER AND RELATED COMPOUNDS. J Am Chem Soc. 1965 Jun 5;87:2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- Usher D. A., Richardson D. I., Jr, Eckstein F. Absolute stereochemistry of the second step of ribonuclease action. Nature. 1970 Nov 14;228(5272):663–665. doi: 10.1038/228663a0. [DOI] [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R. A study of the 'reactive' sulphydryl groups of adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1962 Dec;85:507–516. doi: 10.1042/bj0850507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS D. C. STUDIES ON THE MECHANISM OF ACTION OF ADENOSINE 5'-TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE. INHIBITION BY MANGANESE IONS AND BY P-NITROPHENYL ACETATE. Biochem J. 1963 Nov;89:220–229. doi: 10.1042/bj0890220. [DOI] [PMC free article] [PubMed] [Google Scholar]


