Abstract
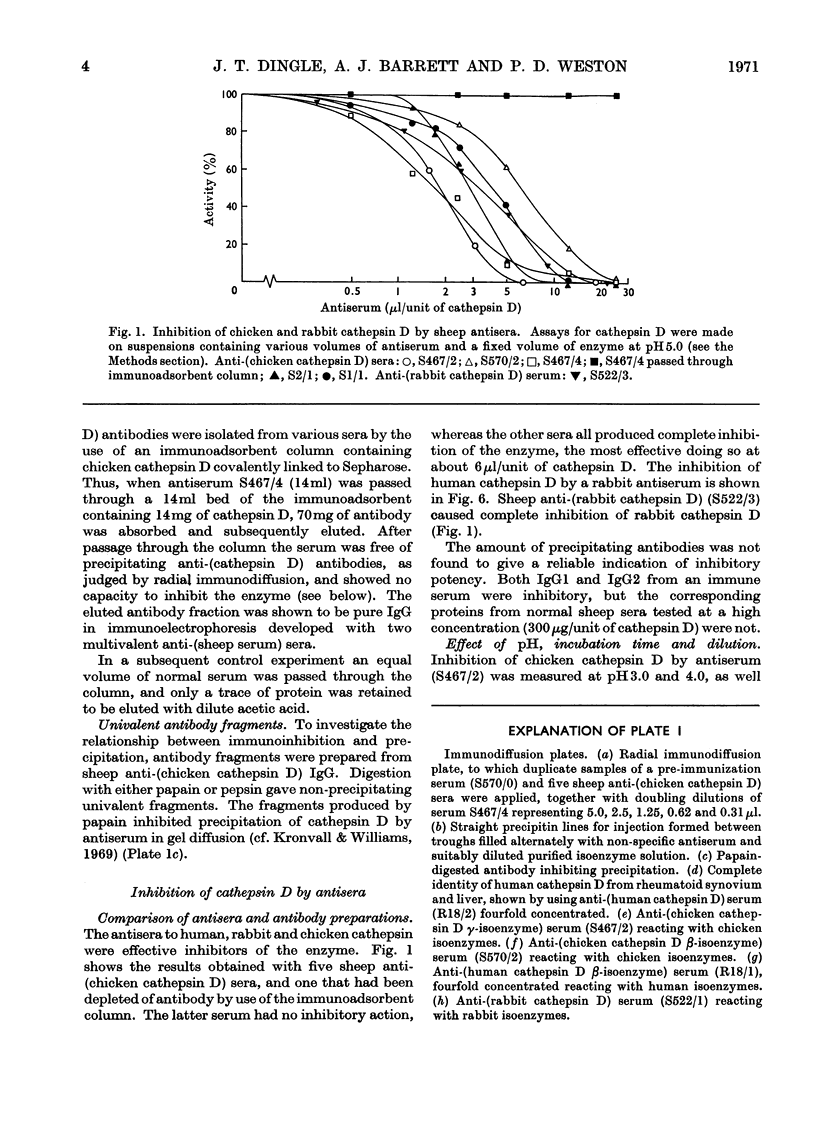
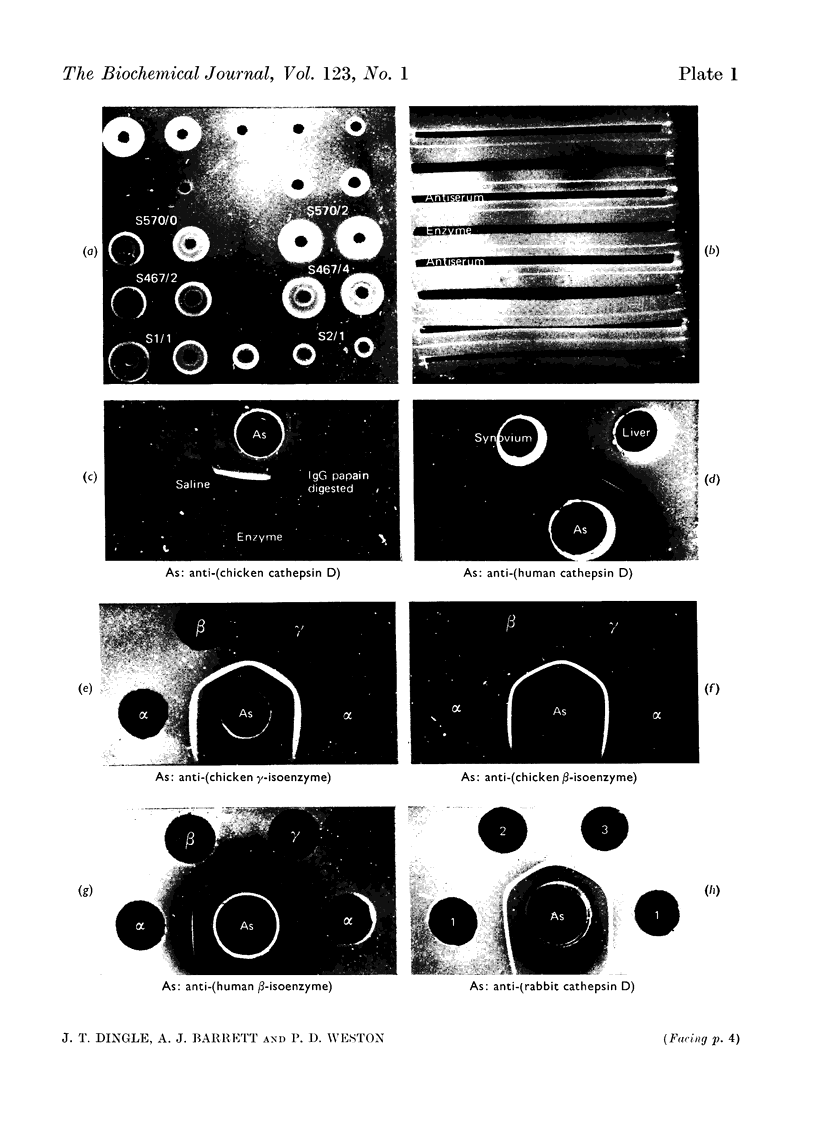
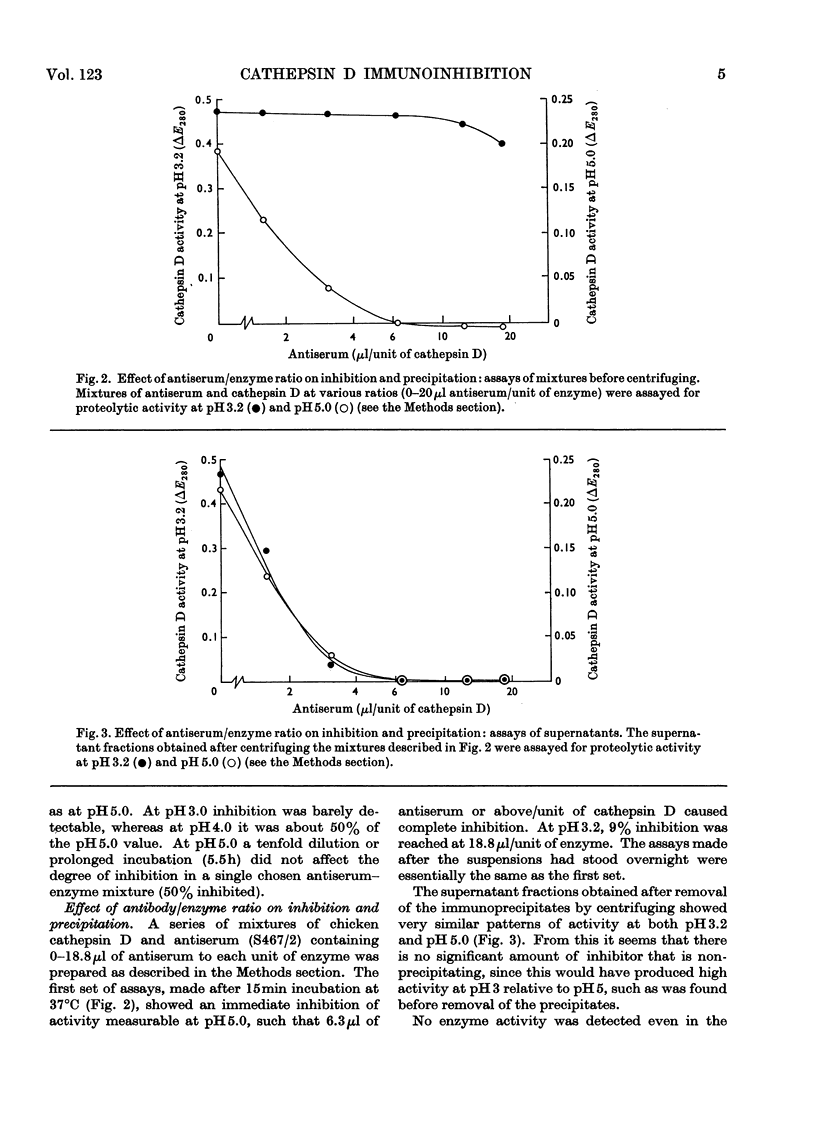
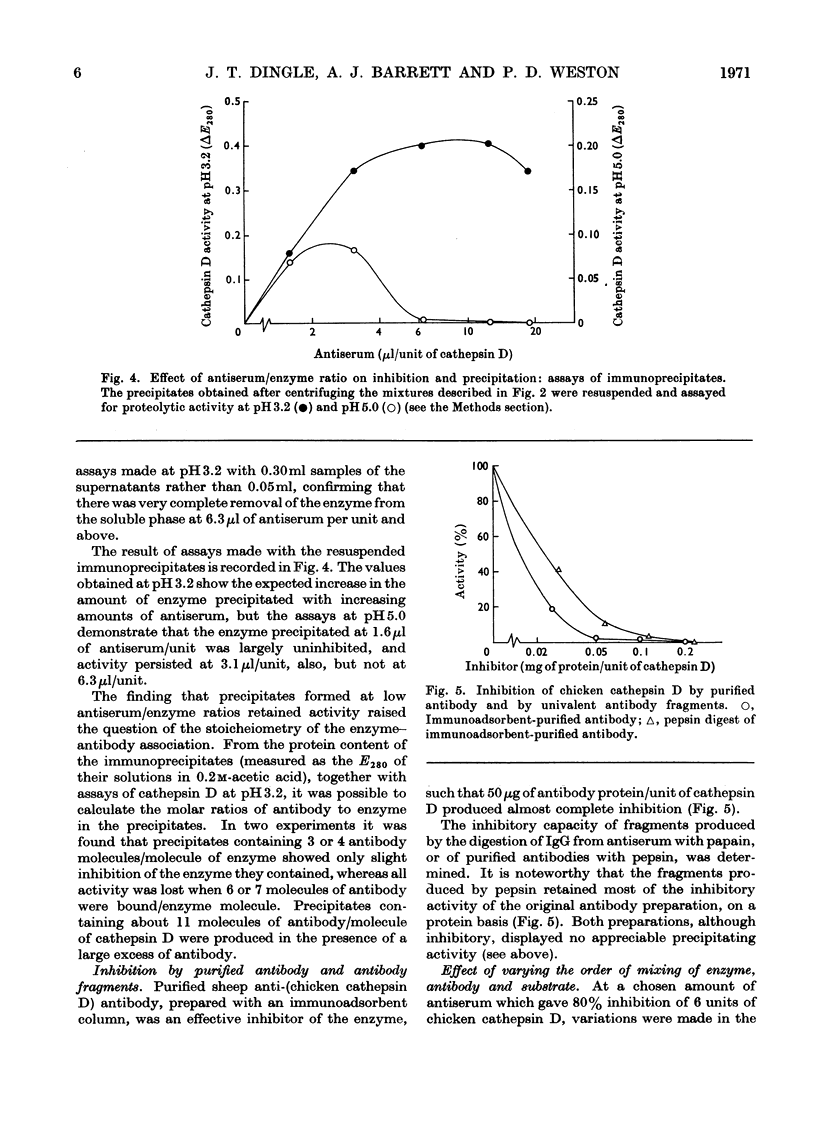
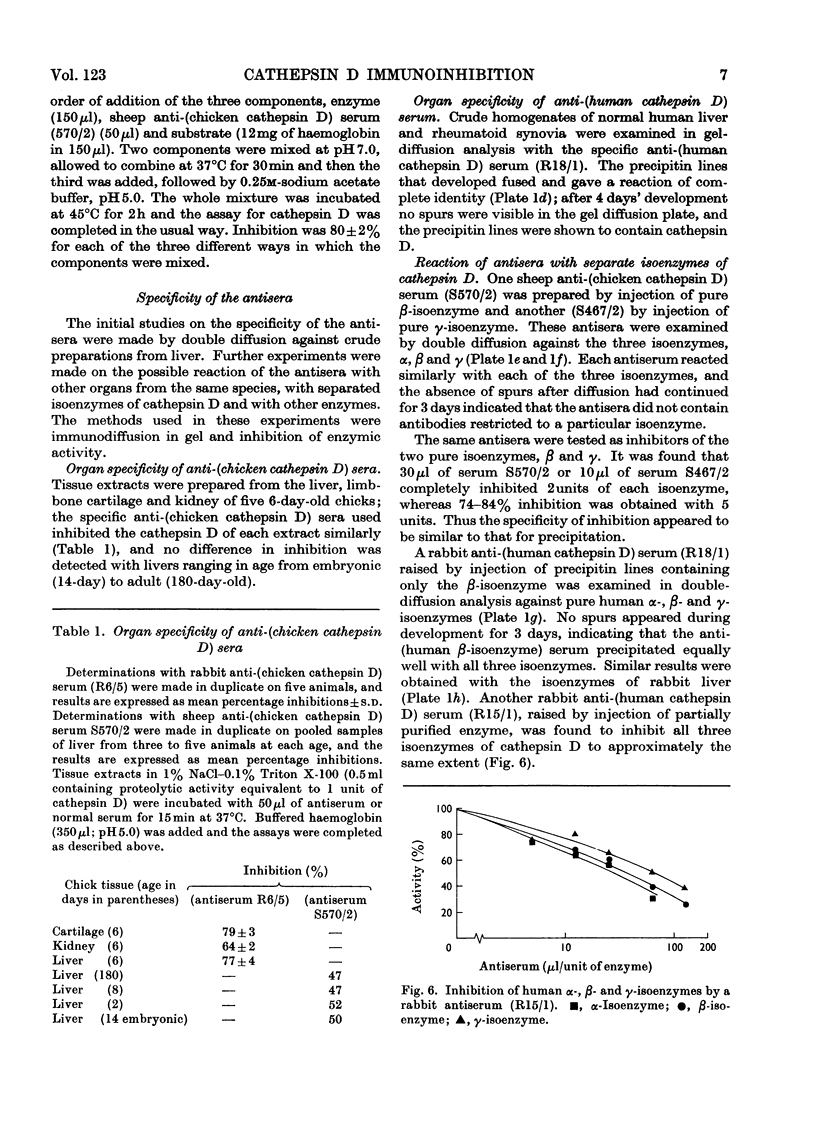
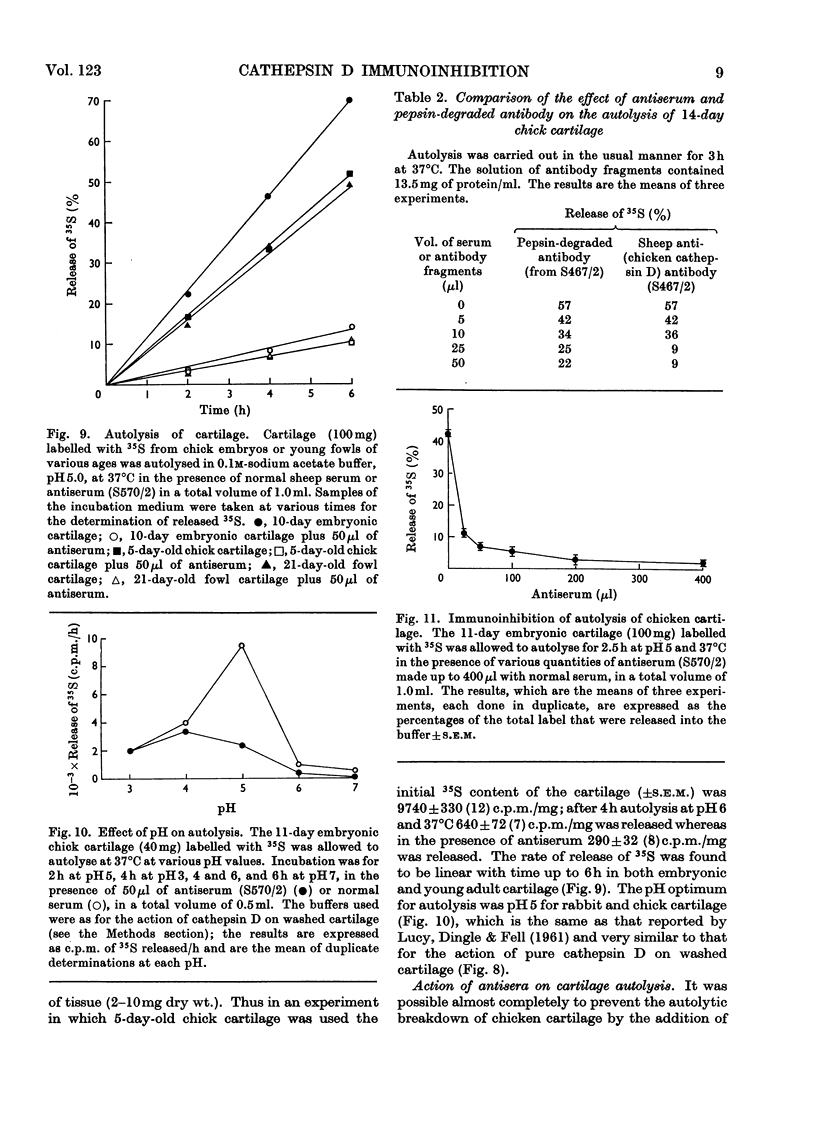
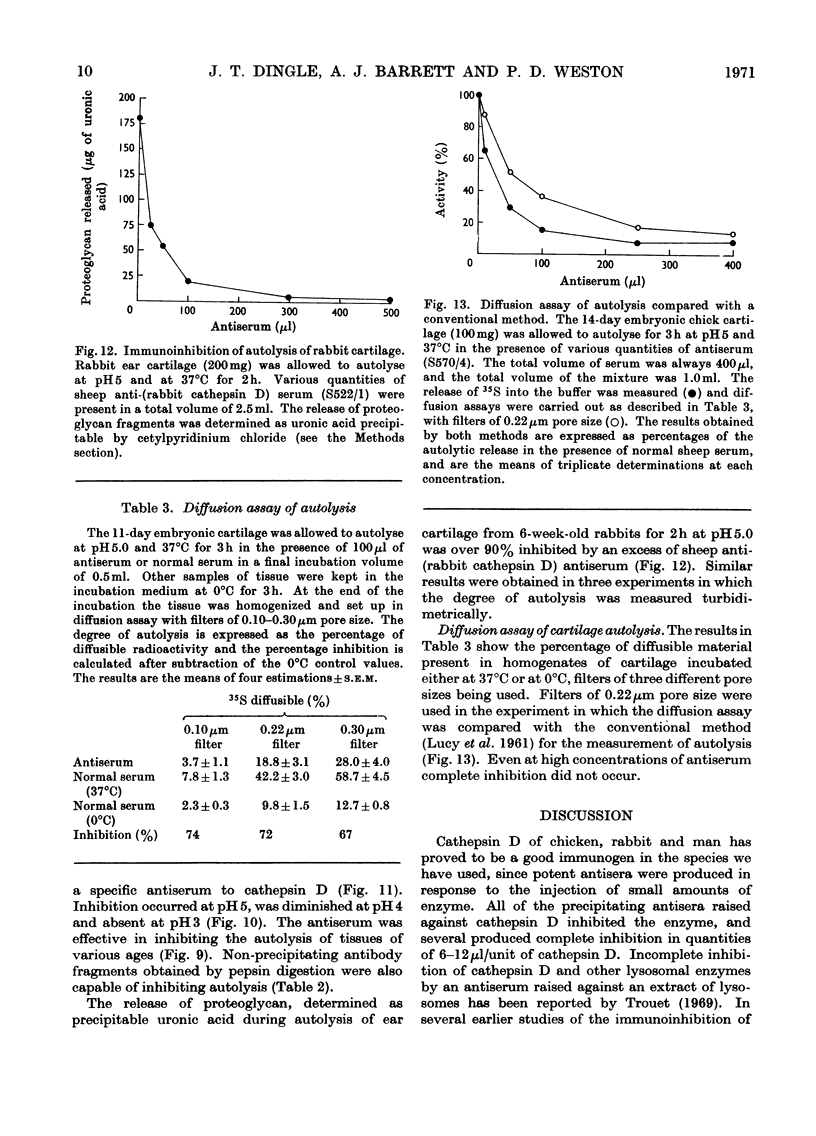
1. Antisera were raised against lysosomal cathepsin D of man, chicken and rabbit. 2. The antisera were found to be specific and potent inhibitors of cathepsin D activity. 3. The immunological nature of the inhibition was established. 4. The inhibitory effect was studied by varying pH, antiserum/enzyme ratio, time of incubation, concentration of components and order of mixing, and by using purified antibody and univalent antibody fragments. 5. The specificities of the antisera were examined with respect to other enzymes, isoenzymes of cathepsin D and cathepsin D from different organs. 6. The antisera prevented the action of cathepsin D on isolated proteoglycans and on cartilage. 7. The antisera produced up to 90% inhibition of the autolysis of cartilage from chicks and rabbits, indicating that cathepsin D is the enzyme mainly responsible for the breakdown of proteoglycans in this system.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon R. A selective fractionation of anti-lysozyme antibodies of different determinant specificities. Eur J Biochem. 1968 Sep 24;5(4):583–589. doi: 10.1111/j.1432-1033.1968.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Arnon R., Shapira E. Antibodies to papain. A selective fractionation according to inhibitory capacity. Biochemistry. 1967 Dec;6(12):3942–3950. doi: 10.1021/bi00864a041. [DOI] [PubMed] [Google Scholar]
- Arnon R. The reaction of papain with antipapain. Immunochemistry. 1965 Jun;2(2):107–114. doi: 10.1016/0019-2791(65)90012-1. [DOI] [PubMed] [Google Scholar]
- BOLLET A. J., BONNER W. M., Jr, NANCE J. L. THE PRESENCE OF HYALURONIDASE IN VARIOUS MAMMALIAN TISSUES. J Biol Chem. 1963 Nov;238:3522–3527. [PubMed] [Google Scholar]
- BOLLET A. J., HANDY J. R., STURGILL B. C. Chondroitin sulfate concentration and protein-polysaccharide composition of articular cartilage in osteoarthritis. J Clin Invest. 1963 Jun;42:853–859. doi: 10.1172/JCI104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Cathepsin D. Purification of isoenzymes from human and chicken liver. Biochem J. 1970 Apr;117(3):601–607. doi: 10.1042/bj1170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Sledge C. B., Dingle J. T. Effect of cortisol on the synthesis of chondroitin sulphate by embryonic cartilage. Nature. 1966 Jul 2;211(5044):83–84. doi: 10.1038/211083a0. [DOI] [PubMed] [Google Scholar]
- CINADER B., LAFFERTY K. J. Antibody as inhibitor of ribonuclease: the role of steric hindrance, aggregate formation, and specificity. Ann N Y Acad Sci. 1963 May 8;103:653–673. doi: 10.1111/j.1749-6632.1963.tb53725.x. [DOI] [PubMed] [Google Scholar]
- CINADER B., LAFFERTY K. J. MECHANISM OF ENZYME INHIBITION BY ANTIBODY. A STUDY OF THE NEUTRALIZATION OF RIBONUCLEASE. Immunology. 1964 Jul;7:342–362. [PMC free article] [PubMed] [Google Scholar]
- CONCHIE J., LEVVY G. A. Inhibition of glycosidases by aldonolactones of corresponding configuration. Biochem J. 1957 Feb;65(2):389–395. doi: 10.1042/bj0650389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGLE J. T. Aetiological factors in the collagen diseases. Lysosomal enzymes and the degradation of cartilage matrix. Proc R Soc Med. 1962 Feb;55:109–111. [PubMed] [Google Scholar]
- DINGLE J. T., LUCY J. A., FELL H. B. Studies on the mode of action of excess of vitamin A. 1. Effect of excess of vitamin A on the metabolism and composition of embryonic chick-limb cartilage grown in organ culture. Biochem J. 1961 Jun;79:497–500. doi: 10.1042/bj0790497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein A., Hobart M. J. Structural relationship and complement fixing activity of sheep and other ruminant immunoglobulin G subclasses. Nature. 1969 Aug 30;223(5209):950–952. doi: 10.1038/223950a0. [DOI] [PubMed] [Google Scholar]
- Fisher D., Whitehouse M. W., Kent P. W. Beta-xylosidase and beta-galactosidase activities of mammalian connective tissues and other sources. Nature. 1967 Jan 14;213(5072):204–205. doi: 10.1038/213204a0. [DOI] [PubMed] [Google Scholar]
- GERBER B. R., FRANKLIN E. C., SCHUBERT M. Ultracentrifugal fractionation of bovine nasal chondromucoprotein. J Biol Chem. 1960 Oct;235:2870–2875. [PubMed] [Google Scholar]
- GREENBAUM L. M., FRUTON J. S. Purification and properties of beef spleen cathepsin B. J Biol Chem. 1957 May;226(1):173–180. [PubMed] [Google Scholar]
- HENION W. F., SUTHERLAND E. W. Immunological differences of phosphorylases. J Biol Chem. 1957 Jan;224(1):477–488. [PubMed] [Google Scholar]
- Hall T. C., Cocking E. C. High-efficiency liquid-scintillation counting of 14C-labelled material in aqueous solution and determination of specific activity of labelled proteins. Biochem J. 1965 Sep;96(3):626–633. doi: 10.1042/bj0960626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman D., Barland P. Structure and function of the synovial membrane. Bull Rheum Dis. 1966 Jan;16(5):396–399. [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Omenn G. S., Ontjes D. A., Anfinsen C. B. Fracionation of antibodies against staphylococcal nuclease on 'sepharose' immunoadsorbents. Nature. 1970 Jan 10;225(5228):189–190. doi: 10.1038/225189a0. [DOI] [PubMed] [Google Scholar]
- PORTER R. R. The hydrolysis of rabbit y-globulin and antibodies with crystalline papain. Biochem J. 1959 Sep;73:119–126. doi: 10.1042/bj0730119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin A. L., Karpati G., Bulcke J. A. Immunohistochemical localization of creatine phosphokinase in skeletal muscle. Proc Natl Acad Sci U S A. 1969 Sep;64(1):171–175. doi: 10.1073/pnas.64.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman H. H., Small P. A., Jr, Cotlove E. Human alkaline phosphatase. Immunochemical identification of organ-specific isoenzymes. J Biol Chem. 1968 Jan 10;243(1):160–166. [PubMed] [Google Scholar]
- Vaerman J. P., Lebacq-Verheyden A. M., Scolari L., Heremans J. F. Further studies on single radial immunodiffusion. II. The reversed system: diffusion of antibodies in antigen-containing gels. Immunochemistry. 1969 Mar;6(2):287–293. doi: 10.1016/0019-2791(69)90165-7. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Spilberg I. Breakdown of cartilage proteinpolysaccharide by lysosomes. Arthritis Rheum. 1968 Apr;11(2):162–169. doi: 10.1002/art.1780110206. [DOI] [PubMed] [Google Scholar]
- Weston P. D. A specific antiserum to lysosomal cathepsin D. Immunology. 1969 Sep;17(3):421–428. [PMC free article] [PubMed] [Google Scholar]
- Weston P. D., Barrett A. J., Dingle J. T. Specific inhibition of cartilage breakdown. Nature. 1969 Apr 19;222(5190):285–286. doi: 10.1038/222285b0. [DOI] [PubMed] [Google Scholar]



