Abstract
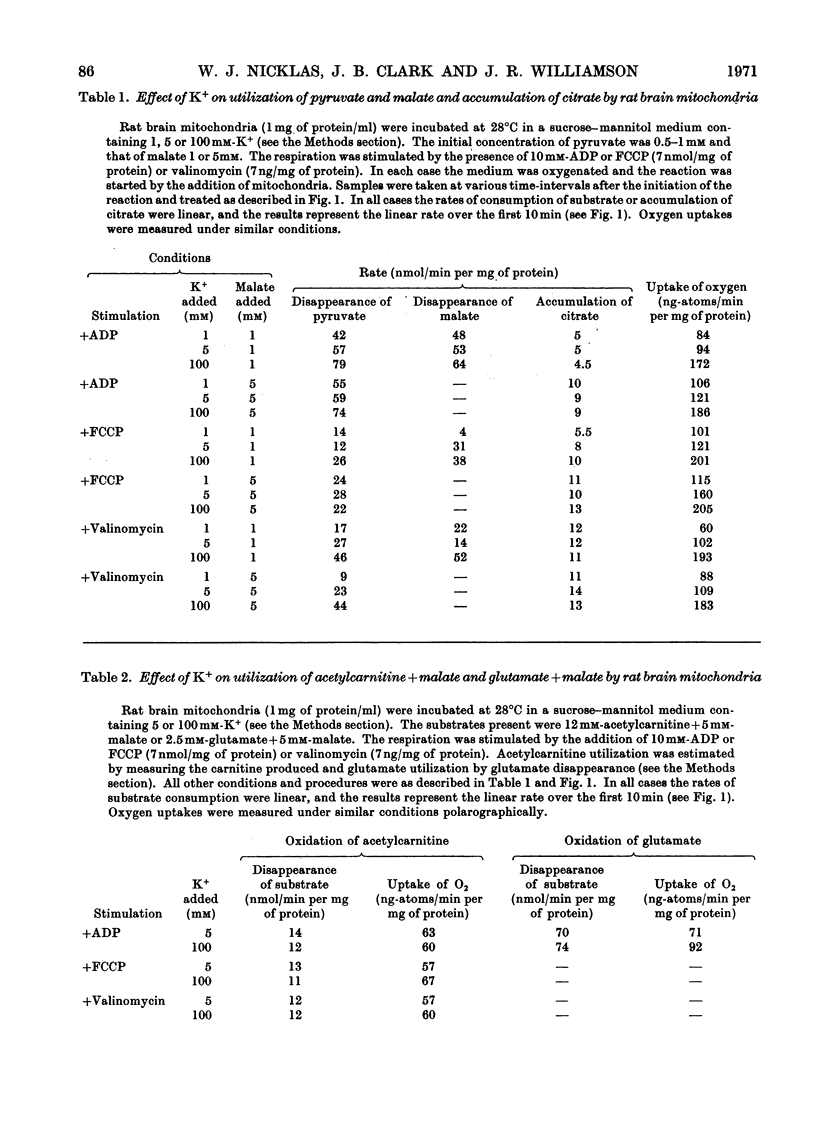
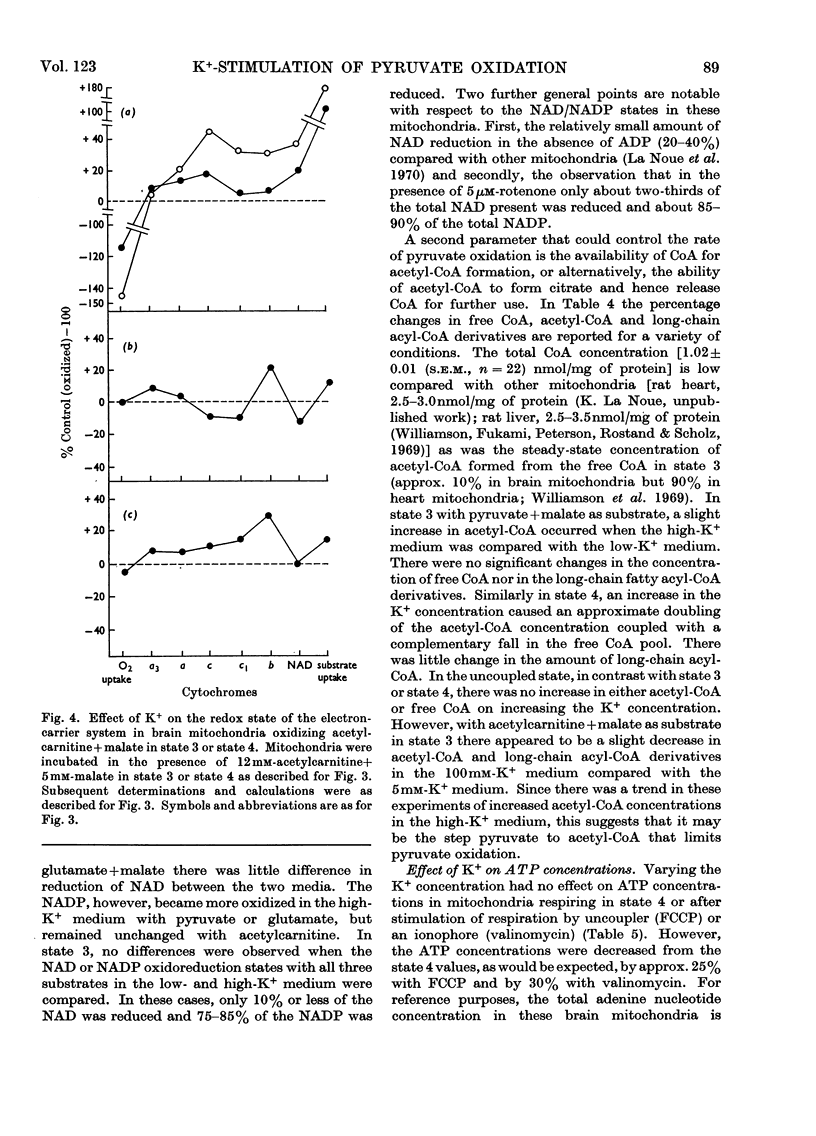
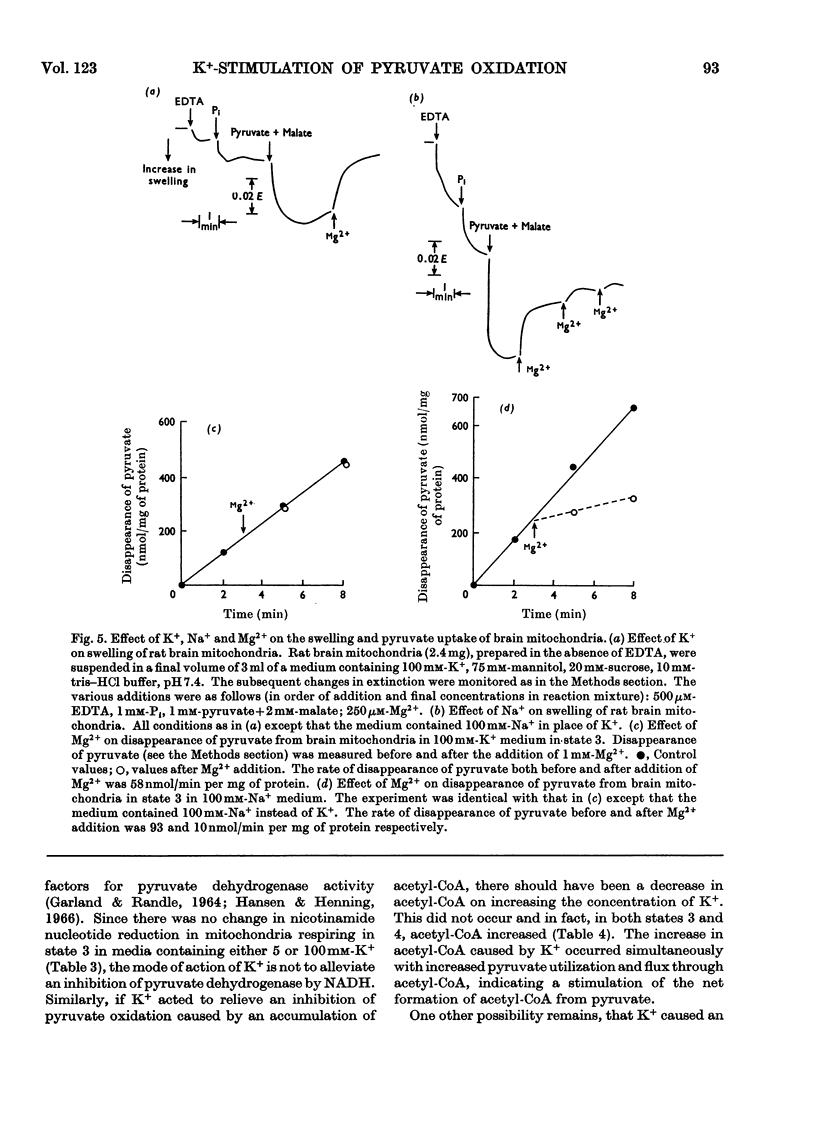
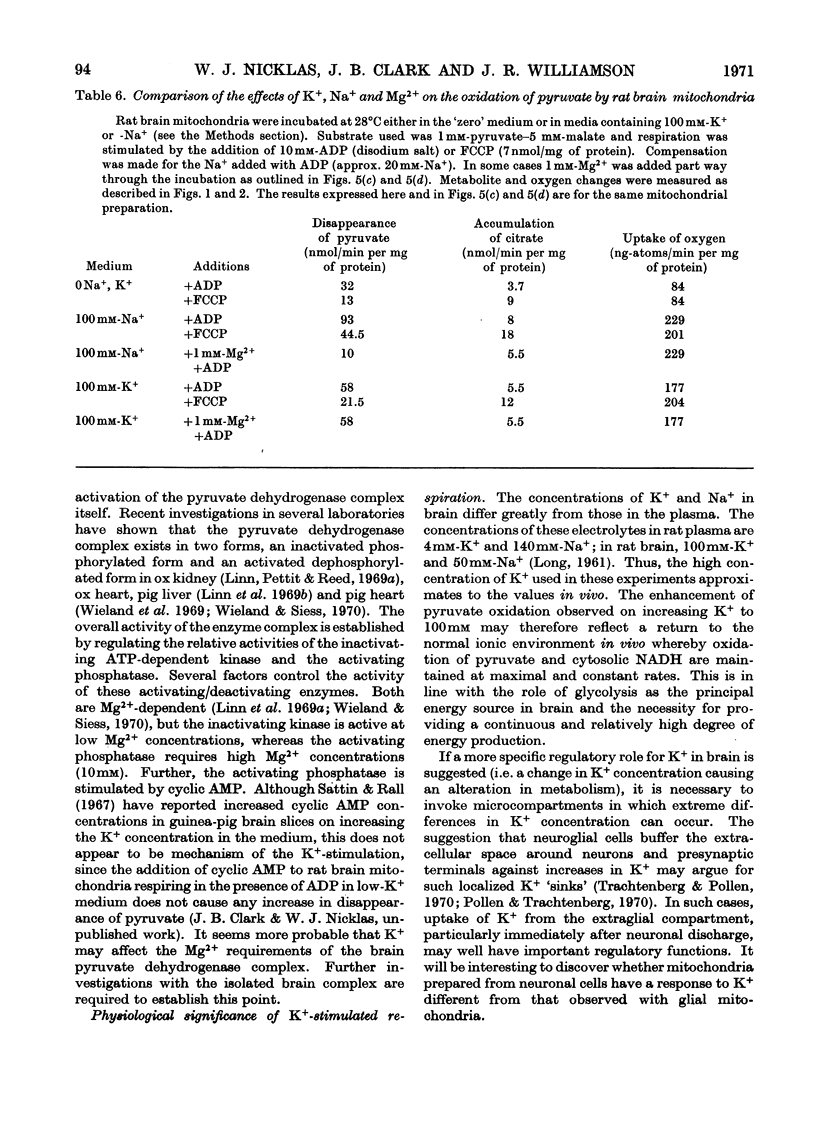
1. Rat brain-cortex mitochondria were incubated in media containing 1, 5 or 100mm-K+ in the presence of ADP, uncoupler (FCCP, carbonyl cyanide p-trifluoro-methoxyphenylhydrazone) or valinomycin while metabolizing pyruvate and malate, or acetylcarnitine and malate or glutamate and malate as substrates. Both the uptake of oxygen and disappearance of substrate were measured under these conditions. 2. With pyruvate and malate as substrate in the presence of both ADP and valinomycin, both the uptake of oxygen and disappearance of pyruvate increased markedly on increasing the K+ content of the incubation medium from 5 to 100mm-K+. However, in the presence of uncoupler (FCCP), although the oxygen uptake doubled little change was observed in the rate of disappearance of pyruvate on increasing the K+ concentration. 3. Only small changes in uptake of substrate and oxygen were observed in the presence of ADP, uncoupler (FCCP) or valinomycin on increasing the K+ concentration when acetylcarnitine+malate or glutamate+malate were used as substrates by brain mitochondria. 4. Further, increasing the K+ concentration from 1 to 20mm when rat brain mitochondria were oxidizing a mixture of pyruvate and glutamate in the presence of malate and ADP caused a 30% increase in the respiration rate, 50% increase in the rate of disappearance of pyruvate and an 80% decrease in the rate of disappearance of glutamate. 5. Investigation of the redox state of the cytochromes and the nicotinamide nucleotides in various conditions with either pyruvate or acetylcarnitine as substrates suggested that the specific stimulation of metabolism of pyruvate by K+ could not be explained by a general stimulation of the electron-transport system. 6. Low-amplitude high-energy swelling of rat brain mitochondria was investigated in both Na+- and K+-containing media. Swelling of brain mitochondria was much greater in the Na+-containing medium and in this medium, the addition of Mg2+ caused a partial reversal of swelling together with an 85% decrease in the rate of utilization of pyruvate. However, in the K+-containing medium, the addition of Mg2+, although also causing a reversal of swelling, did not affect the rate of disappearance of pyruvate. 7. Measurements of the ATP, NADH/NAD+ and acetyl-CoA/CoA contents were made under various conditions and no evidence that K+ concentrations affected these parameters was obtained. 8. The results are discussed in relationship to the physiological significance of the stimulation of pyruvate metabolism by K+ in rat brain mitochondria. It is proposed that K+ causes its effects by a direct stimulation of the pyruvate dehydrogenase complex.
Full text
PDF

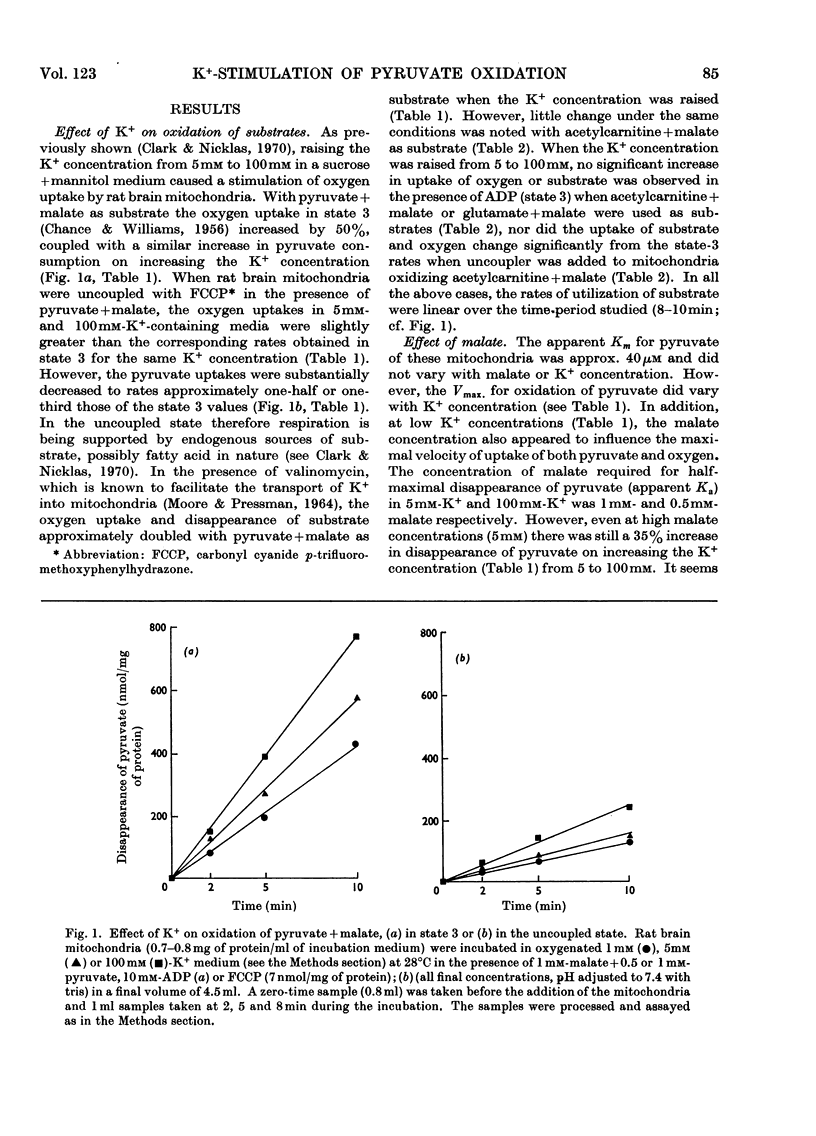
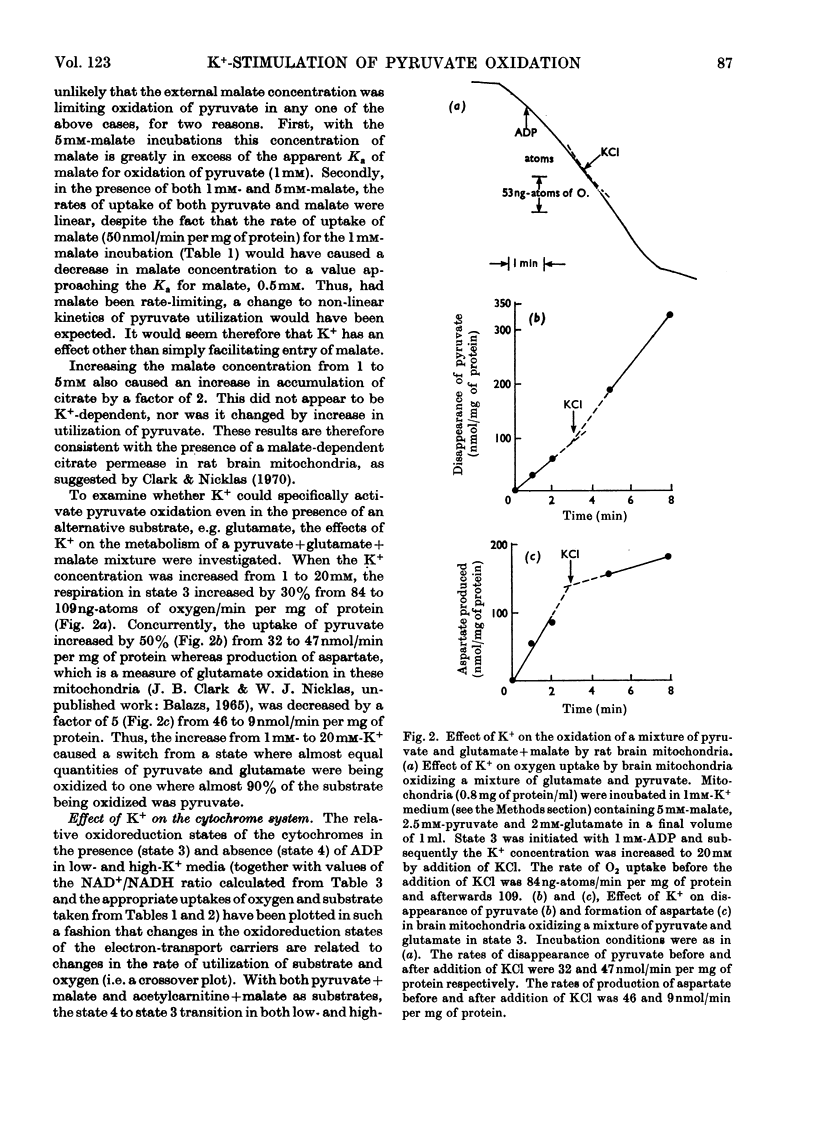




Selected References
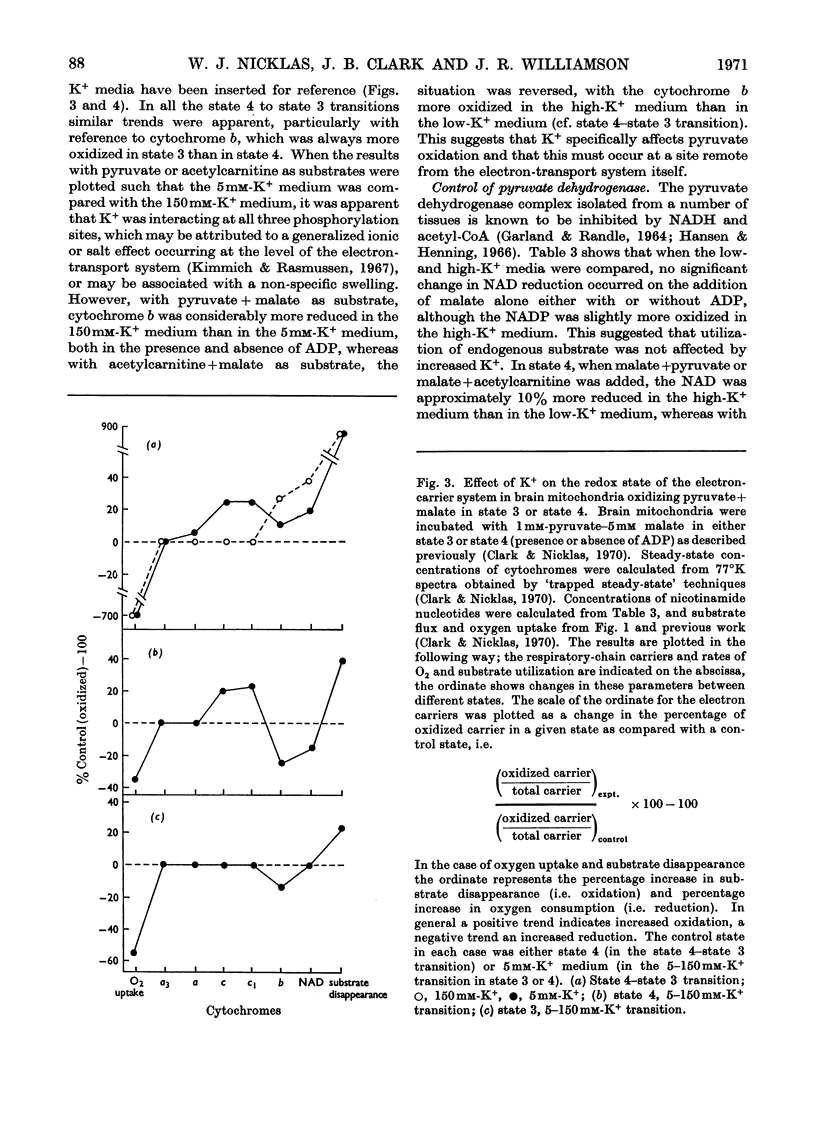
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford C. A., Dixon K. C. The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochem J. 1935;29(1):157–168. doi: 10.1042/bj0290157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALAZS R. CONTROL OF GLUTAMATE OXIDATION IN BRAIN AND LIVER MITOCHONDRIAL SYSTEMS. Biochem J. 1965 May;95:497–508. doi: 10.1042/bj0950497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl S., Nicklas W. J., Clarke D. D. Compartmentation of citric acid cycle metabolism in brain: labelling of glutamate, glutamine, aspartate and gaba by several radioactive tracer metabolites. J Neurochem. 1970 Jul;17(7):1009–1015. doi: 10.1111/j.1471-4159.1970.tb02254.x. [DOI] [PubMed] [Google Scholar]
- Berl S., Nicklas W. J., Clarke D. D. Compartmentation of glutamic acid metabolism in brain slices. J Neurochem. 1968 Feb;15(2):131–140. doi: 10.1111/j.1471-4159.1968.tb06184.x. [DOI] [PubMed] [Google Scholar]
- Cerletti P., Giordano M. G., Giovenco M. A., Barra D., Strom R. Role of phospholipids in succinate dehydrogenase. Biochim Biophys Acta. 1966 Aug 10;122(2):352–355. doi: 10.1016/0926-6593(66)90075-0. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Dickens F., Greville G. D. The metabolism of normal and tumour tissue: Neutral salt effects. Biochem J. 1935 Jun;29(6):1468–1483. doi: 10.1042/bj0291468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Passonneau J. V., Lowry O. H. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem. 1966 Sep 10;241(17):3997–4003. [PubMed] [Google Scholar]
- Hanig R. C., Aprison M. H. Determination of calcium, copper, iron, magnesium, manganese, potassium, sodium, zinc, and chloride concentrations in several brain areas. Anal Biochem. 1967 Nov;21(2):169–177. doi: 10.1016/0003-2697(67)90178-9. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Höfer M. P., Pressman B. C. Stimulation of mitochondrial respiration and phosphorylation by transport-inducing antibiotics. Biochemistry. 1967 May;6(5):1348–1360. doi: 10.1021/bi00857a018. [DOI] [PubMed] [Google Scholar]
- KINI M. M., QUASTEL J. H. Carbohydrate--amino-acid inter-relations in brain cortex in vitro. Nature. 1959 Jul 25;184:252–256. doi: 10.1038/184252a0. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Rasmussen H. Inhibition of mitochondrial respiration by loss of intra-mitochondrial K+. Biochim Biophys Acta. 1967 May 9;131(3):413–420. doi: 10.1016/0005-2728(67)90001-1. [DOI] [PubMed] [Google Scholar]
- LaNoue K., Nicklas W. J., Williamson J. R. Control of citric acid cycle activity in rat heart mitochondria. J Biol Chem. 1970 Jan 10;245(1):102–111. [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Hucho F., Reed L. J. Alpha-keto acid dehydrogenase complexes. XI. Comparative studies of regulatory properties of the pyruvate dehydrogenase complexes from kidney, heart, and liver mitochondria. Proc Natl Acad Sci U S A. 1969 Sep;64(1):227–234. doi: 10.1073/pnas.64.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn T. C., Pettit F. H., Reed L. J. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1969 Jan;62(1):234–241. doi: 10.1073/pnas.62.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Seta K., Araki H., Handa H. The dependence of brain mitochondrial respiration on potassium ion. J Biochem. 1967 Mar;61(3):352–358. doi: 10.1093/oxfordjournals.jbchem.a128555. [DOI] [PubMed] [Google Scholar]
- Trachtenberg M. C., Pollen D. A. Neuroglia: biophysical properties and physiologic function. Science. 1970 Feb 27;167(3922):1248–1252. doi: 10.1126/science.167.3922.1248. [DOI] [PubMed] [Google Scholar]
- Wieland O., Siess E. Interconversion of phospho- and dephospho- forms of pig heart pyruvate dehydrogenase. Proc Natl Acad Sci U S A. 1970 Apr;65(4):947–954. doi: 10.1073/pnas.65.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland O., Von Jagow-Westermann B., Stukowski B. Kinetic and regulatory properties of heart muscle pyruvate dehydrogenase. Hoppe Seylers Z Physiol Chem. 1969 Mar;350(3):329–334. doi: 10.1515/bchm2.1969.350.1.329. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Fukami M. H., Peterson M. J., Rostand S. G., Scholz R. Effect of 4-pentenoic acid on coenzyme A metabolites in rat liver. Biochem Biophys Res Commun. 1969 Aug 7;36(3):407–413. doi: 10.1016/0006-291x(69)90579-8. [DOI] [PubMed] [Google Scholar]



