Abstract
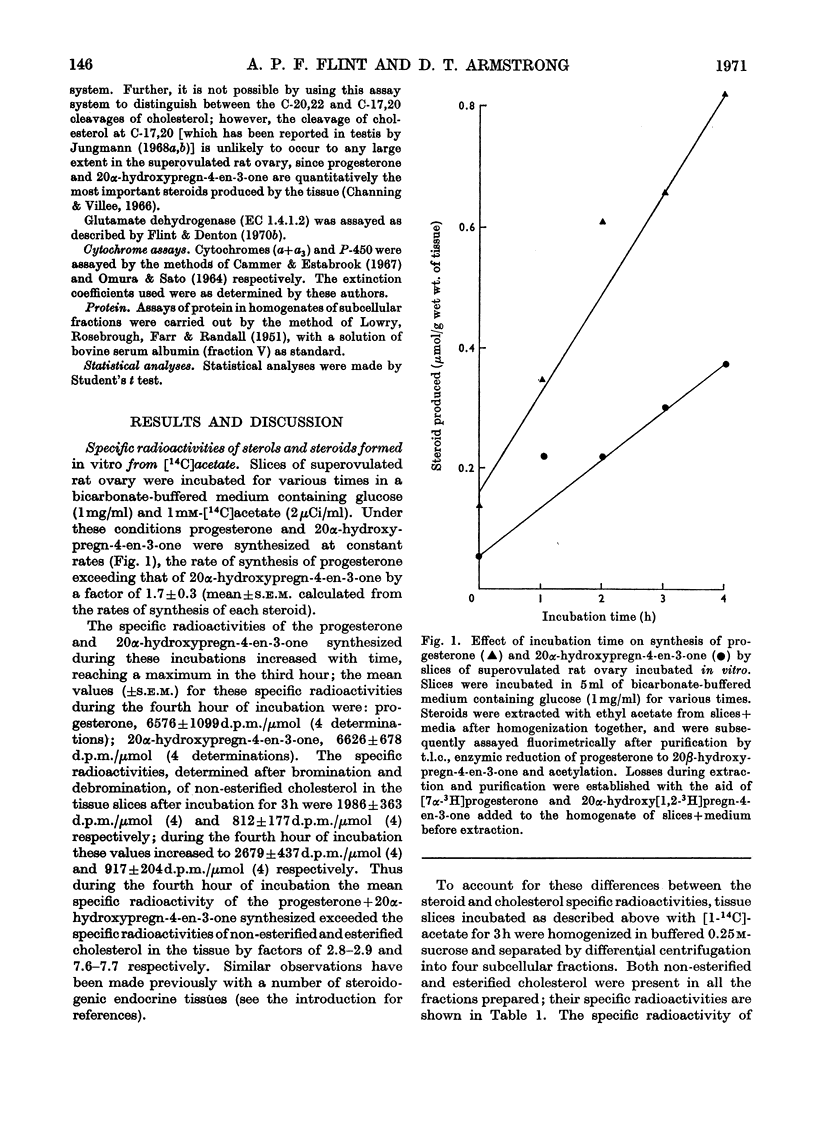
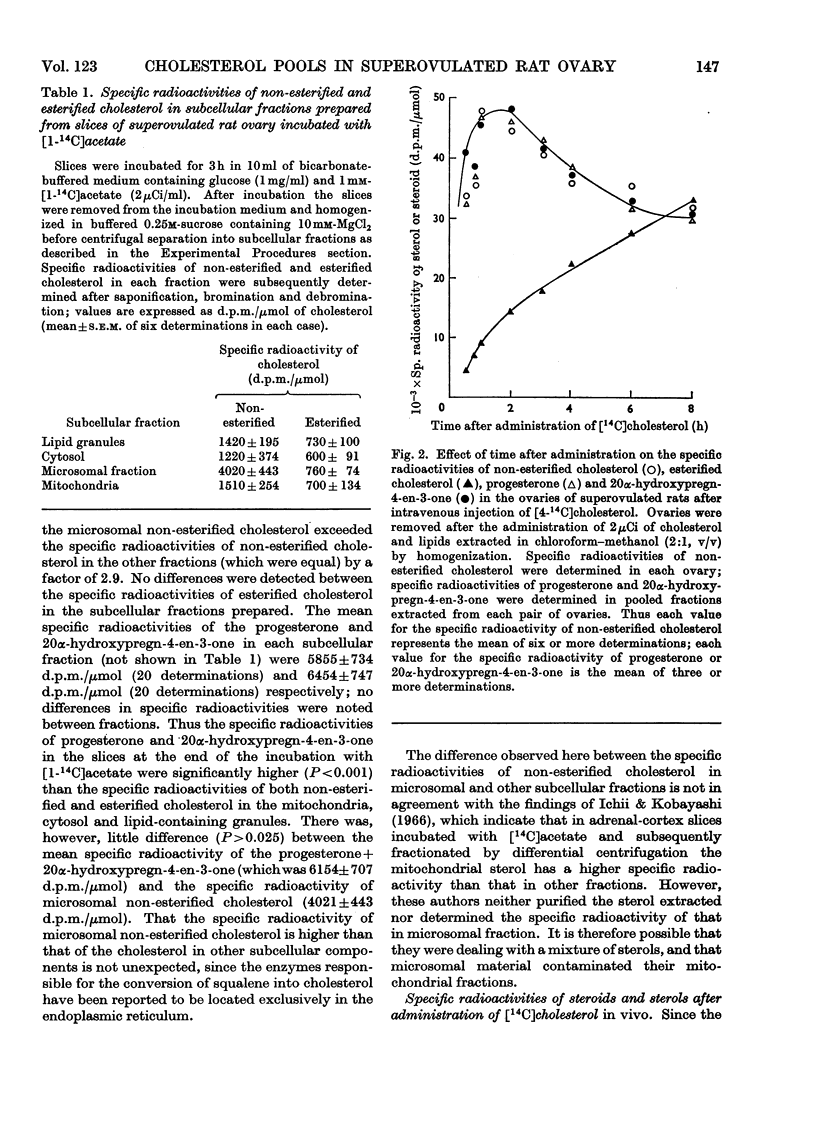
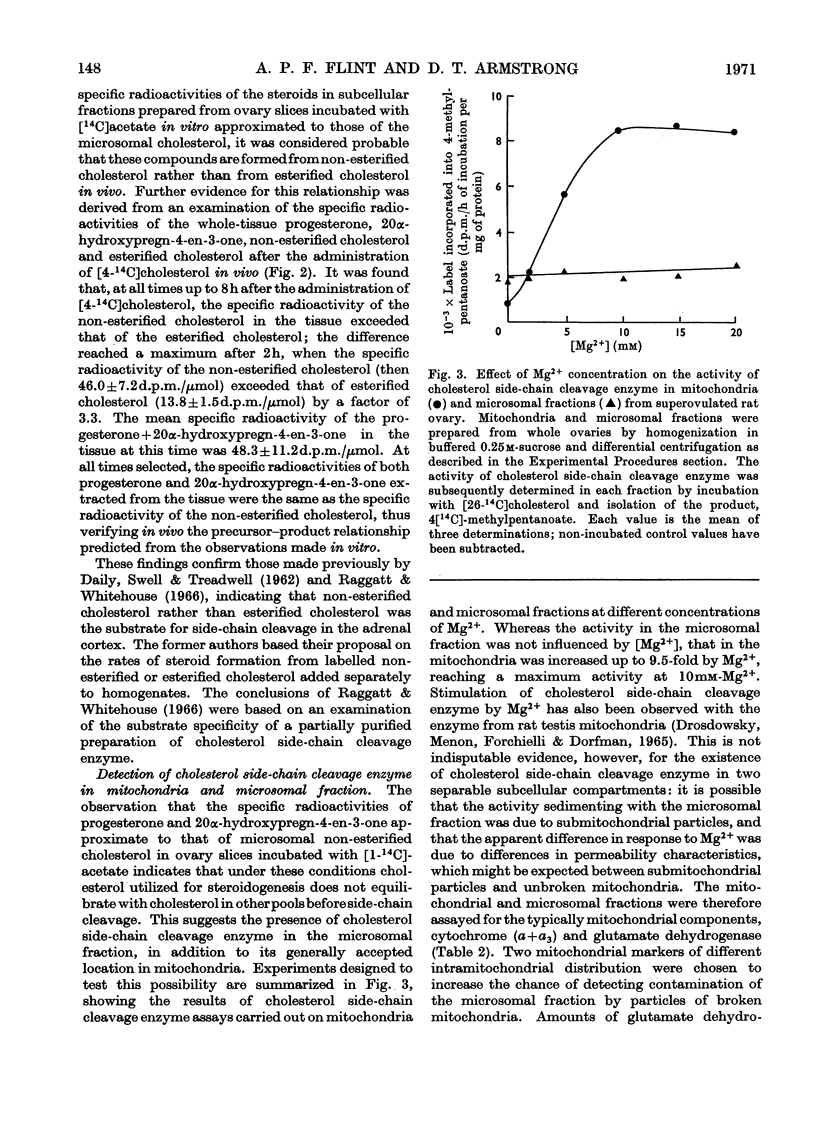
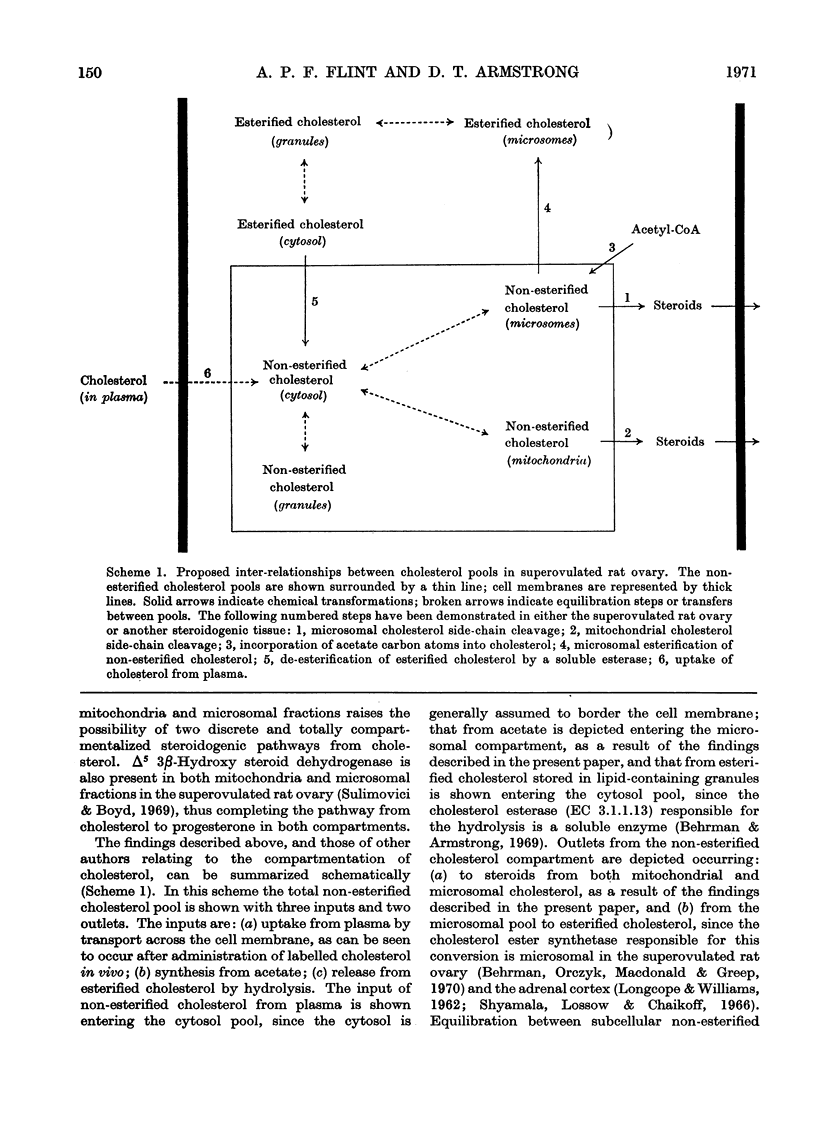
1. The specific radioactivities of non-esterified and esterified cholesterol, progesterone and 20α-hydroxypregn-4-en-3-one were determined in slices of superovulated rat ovary after incubation with [1-14C]acetate in vitro for various times. The specific radioactivities of progesterone and 20α-hydroxypregn-4-en-3-one were equal, and (during the fourth hour of incubation) exceeded those of the non-esterified cholesterol and the esterified cholesterol by factors of 2.8 and 7.6 respectively. 2. After separation of homogenates of superovulated rat ovary slices previously incubated with [14C]acetate into subcellular fractions by differential centrifugation, the specific radioactivities of non-esterified cholesterol in the cytosol, mitochondria, lipid-containing storage granules and microsomal fraction were 1220, 1510, 1420 and 4020d.p.m./μmol respectively; the corresponding values for the specific radioactivity of the esterified cholesterol were 600, 700, 730 and 760d.p.m./μmol. The specific radioactivities of progesterone and 20α-hydroxypregn-4-en-3-one were equal in all fractions; the corresponding mean specific radioactivity of progesterone+20α-hydroxypregn-4-en-3-one was 6150d.p.m./μmol. 3. By using glutamate dehydrogenase and cytochrome (a+a3) as mitochondrial markers, the presence of cholesterol side-chain cleavage enzyme was demonstrated in microsomal fraction free of mitochondrial contamination. 4. The specific radioactivities of ovarian non-esterified and esterified cholesterol, progesterone and 20α-hydroxypregn-4-en-3-one were determined up to 8h after the intravenous injection of [4-14C]cholesterol into superovulated rats. At all times the specific radioactivities of progesterone and 20α-hydroxypregn-4-en-3-one were equal to the specific radioactivity of non-esterified cholesterol and exceeded, by up to 3.3-fold, that of the esterified cholesterol. 5. It is concluded that non-esterified cholesterol formed from [14C]acetate in the endoplasmic reticulum equilibrates slowly with non-esterified cholesterol in other subcellular fractions, and is preferentially converted into steroids. Such a mechanism presupposes the operation of a microsomal cholesterol side-chain cleavage enzyme using non-esterified cholesterol as its substrate. Unrelated evidence is presented in support of the existence of such an enzyme. The results are discussed in the light of other biochemical and electron-microscopic findings relating to the compartmentation of cholesterol in steroidogenic tissues.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG D. T., O'BRIEN J., GREEP R. O. EFFECTS OF LUTEINIZING HORMONE ON PROGESTIN BIOSYNTHESIS IN THE LUTEINIZED RAT OVARY. Endocrinology. 1964 Oct;75:488–500. doi: 10.1210/endo-75-4-488. [DOI] [PubMed] [Google Scholar]
- Angel A. Studies on the compartmentation of lipid in adipose cells. I. Subcellular distribution, composition, and transport of newly synthesized lipid: liposomes. J Lipid Res. 1970 Sep;11(5):420–432. [PubMed] [Google Scholar]
- Armstrong D. T., Lee T. P., Miller L. S. Stimulation of progesterone biosynthesis in bovine corpora lutea by luteinizing hormone in the presence of an inhibitor of cholesterol synthesis. Biol Reprod. 1970 Feb;2(1):29–36. doi: 10.1095/biolreprod2.1.29. [DOI] [PubMed] [Google Scholar]
- Armstrong D. T., Miller L. V., Knudsen K. A. Regulation of lipid metabolism and progesterone production in rat corpora lutea and ovarian interstitial elements by prolactin and luteinizing hormone. Endocrinology. 1969 Sep;85(3):393–401. doi: 10.1210/endo-85-3-393. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Amstrong D. T. Cholesterol esterase stimulation by luteinizing hormone in luteinized rat ovaries. Endocrinology. 1969 Sep;85(3):474–480. doi: 10.1210/endo-85-3-474. [DOI] [PubMed] [Google Scholar]
- Behrman H. R., Orczyk G. P., Macdonald G. J., Greep R. O. Prolactin induction of enzymes controlling luteal cholesterol ester turnover. Endocrinology. 1970 Dec;87(6):1251–1256. doi: 10.1210/endo-87-6-1251. [DOI] [PubMed] [Google Scholar]
- Bjersing L. On the ultrastructure of granulosa lutein cells in porcine corpus luteum. With special reference to endoplasmic reticulum and steroid hormone synthesis. Z Zellforsch Mikrosk Anat. 1967;82(2):187–211. doi: 10.1007/BF01901701. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Villee C. A. Stimulation of cholesterol metabolism in the luteinized rat ovary by luteinizing hormone. Biochim Biophys Acta. 1966 Sep 26;127(1):1–17. doi: 10.1016/0304-4165(66)90469-7. [DOI] [PubMed] [Google Scholar]
- Drosdowsky M., Menon K. M., Forchielli E., Dorfman R. I. Requirements of the cholesterol side-chain-cleaving enzyme system of rat-testis mitochondria. Biochim Biophys Acta. 1965 Jun 15;104(1):229–236. doi: 10.1016/0304-4165(65)90240-0. [DOI] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Glucose metabolism in the superovulated rat ovary in vitro. Effects of luteinizing hormone and the role of glucose metabolism in steroidogenesis. Biochem J. 1969 Apr;112(2):243–254. doi: 10.1042/bj1120243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. Metabolism of endogenous sterol ester by the superovulated rat ovary in vitro. Biochem J. 1970 Jan;116(1):79–82. doi: 10.1042/bj1160079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint A. P., Denton R. M. The role of nicotinamide-adenine dinucleotide phosphate-dependent malate dehydrogenase and isocitrate dehydrogenase in the supply of reduced nicotinamide-adenine dinucleotide phosphate for steroidogenesis in the superovulated rat ovary. Biochem J. 1970 Mar;117(1):73–83. doi: 10.1042/bj1170073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HECHTER O., SOLOMON M. M., ZAFFARONI A., PINCUS G. Transformation of cholesterol and acetate to adrenal cortical hormones. Arch Biochem Biophys. 1953 Sep;46(1):201–214. doi: 10.1016/0003-9861(53)90182-9. [DOI] [PubMed] [Google Scholar]
- HENNING H. D., ZANDER J. [Use of Huebener's 20 beta-hydroxysteroid dehydrogenase in the microchemical identification and separation of steroids]. Hoppe Seylers Z Physiol Chem. 1962 Dec 15;330:31–37. doi: 10.1515/bchm2.1962.330.1.31. [DOI] [PubMed] [Google Scholar]
- Ichii S., Kobayashi S. Studies on the biosynthesis of sterol and corticosterone in rat adrenal gland. Endocrinol Jpn. 1966 Mar;13(1):39–45. doi: 10.1507/endocrj1954.13.39. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A. Androgen biosynthesis. I. Enzymatic cleavage of the cholesterol side-chain to dehydroepiandrosterone and 2-methylheptan-6-one. Biochim Biophys Acta. 1968 Sep 2;164(1):110–123. doi: 10.1016/0005-2760(68)90077-5. [DOI] [PubMed] [Google Scholar]
- Koritz S. B. The energy linked synthesis of pregnenolone in beef adrenal cortex mitochondria. Biochem Biophys Res Commun. 1966 May 25;23(4):485–489. doi: 10.1016/0006-291x(66)90754-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Major P. W., Armstrong D. T., Greep R. O. Effect of luteinizing hormone in vivo and in vitro on cholesterol conversion to progestins in rat corpus luteum tissue. Endocrinology. 1967 Jul;81(1):19–28. doi: 10.1210/endo-81-1-19. [DOI] [PubMed] [Google Scholar]
- Raggatt P. R., Whitehouse M. W. Substrate and inhibitor specificity of the cholesterol oxidase in bovine adrenal cortex. Biochem J. 1966 Dec;101(3):819–830. doi: 10.1042/bj1010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyamala G., Lossow W. J., Chaikoff I. L. Esterification of cholesterol by rat adrenal gland homogenates and subcellular components. Biochim Biophys Acta. 1966 Jun 1;116(3):543–554. doi: 10.1016/0005-2760(66)90124-x. [DOI] [PubMed] [Google Scholar]
- Sulimovici S., Boyd G. S. The delta-5-3-beta-hydroxysteroid dehydrogenase of rat ovarian tissue. The effect of adenosine 3',5'-cyclic-monophosphoric acid. Eur J Biochem. 1969 Feb;7(4):549–558. [PubMed] [Google Scholar]


