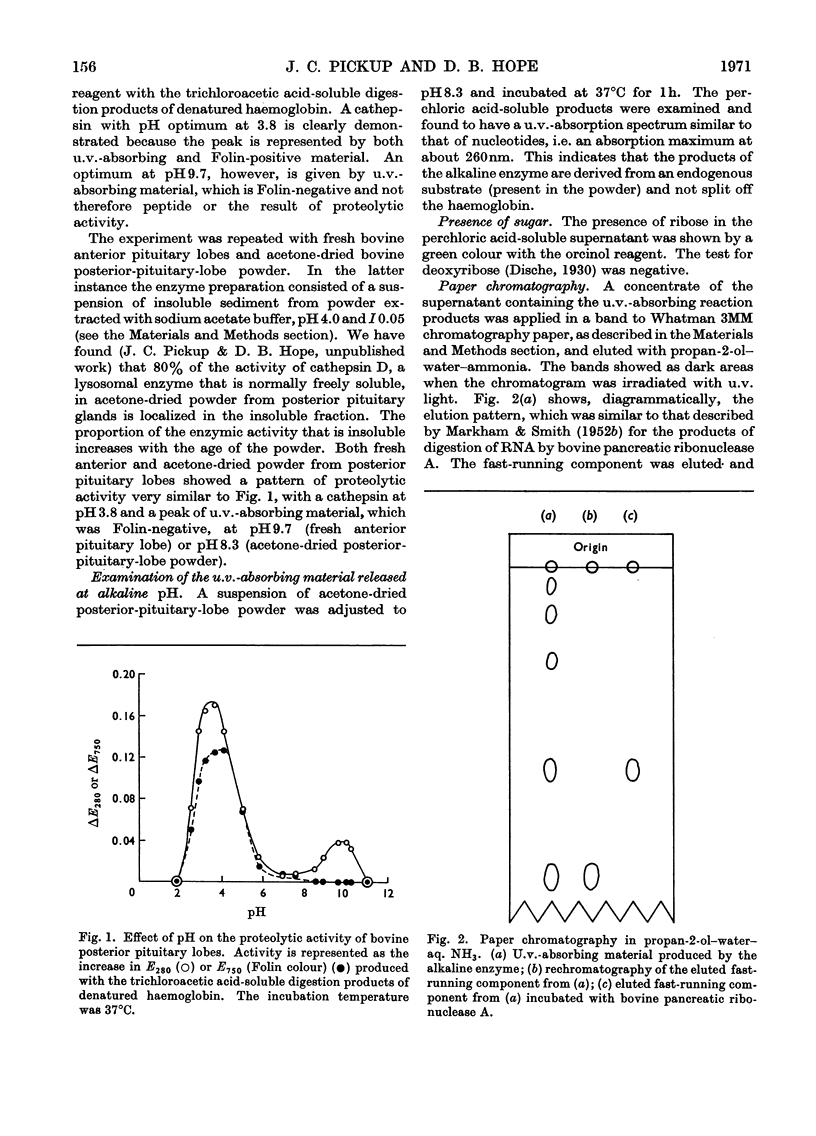
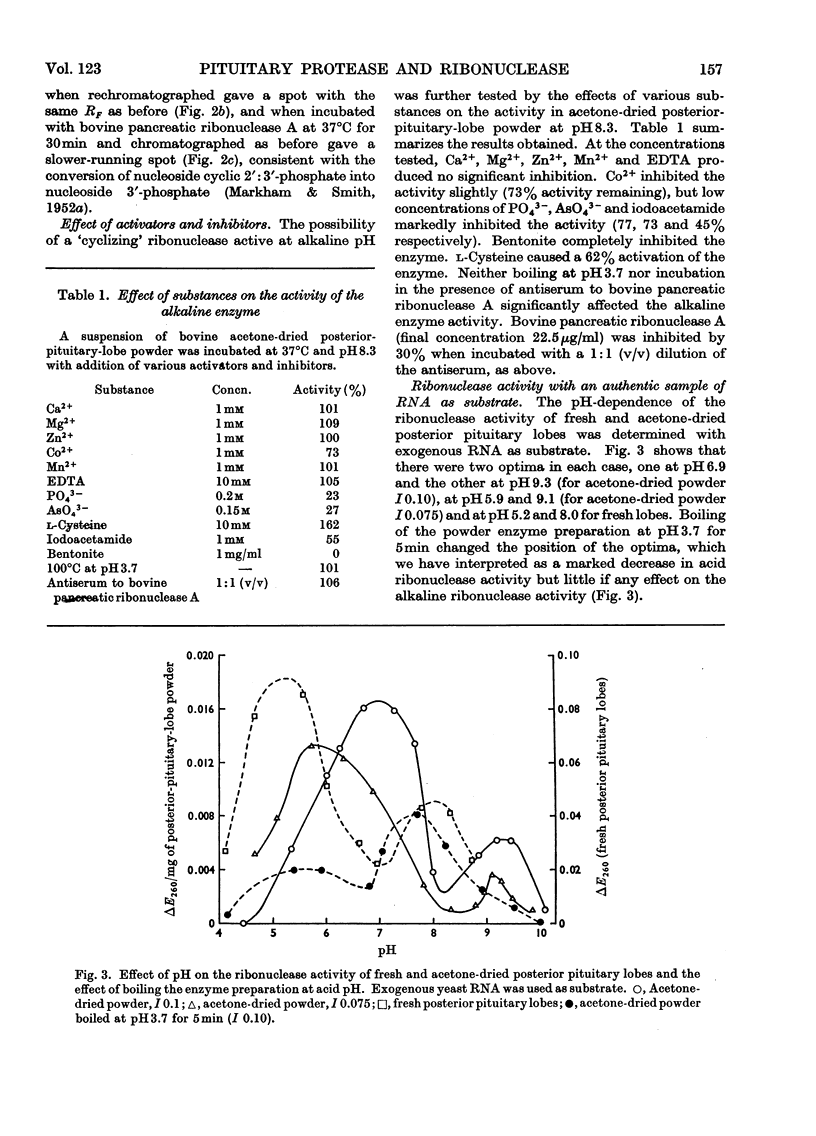
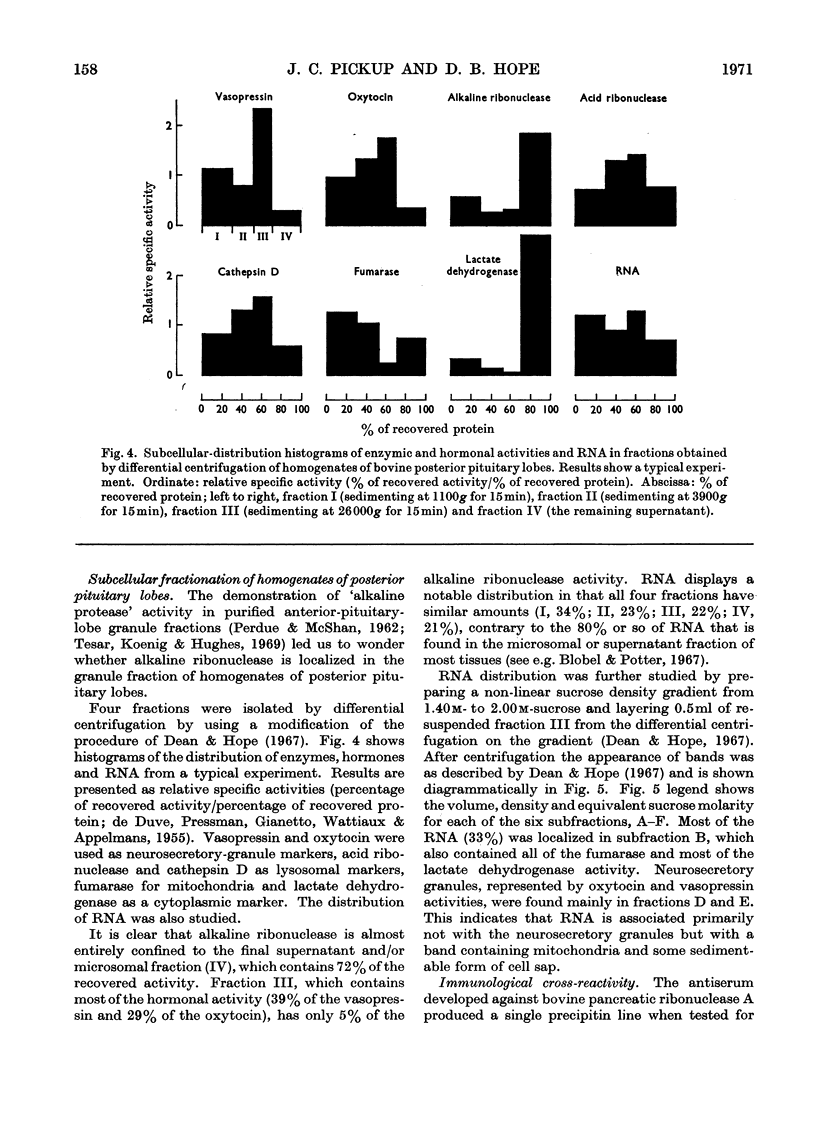
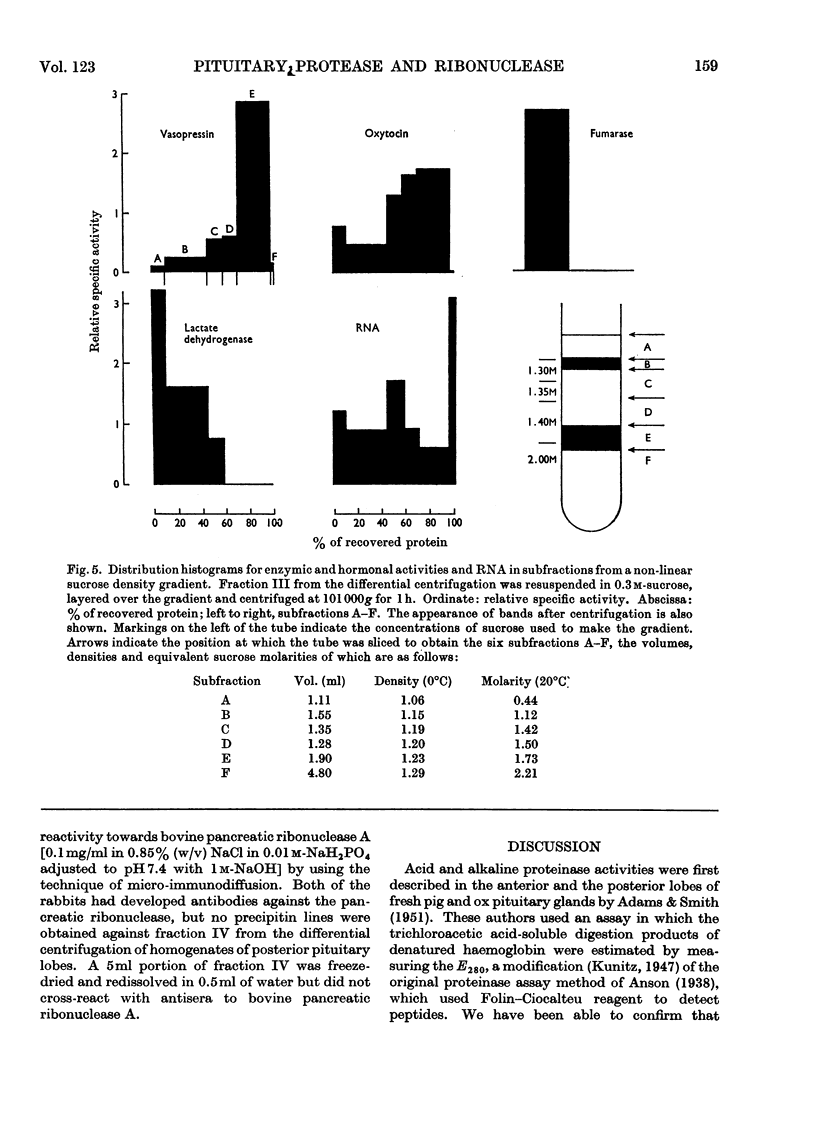
Abstract
1. Acid and alkaline protease activities in bovine anterior and posterior pituitary lobes were reinvestigated by measurement of u.v. and Folin–Ciocalteu colour values of trichloroacetic acid-soluble digestion products of denatured haemoglobin. 2. Both lobes of the pituitary gland contain a cathepsin with a pH optimum at 3.8. 3. When release of u.v.-absorbing material was used as the assay there was also an optimum at pH8.3–9.7, but this proved to be due to the release of nucleosides from an endogenous substrate. 4. The presence of a `cyclizing' ribonuclease active at alkaline pH on endogenous RNA was confirmed by the inhibitory effects of phosphate, arsenate and bentonite. The activity was unaffected by heat, EDTA or metal ions. The enzyme also acted on exogenous RNA. 5. A purified preparation of neurosecretory granules from fresh bovine posterior pituitary lobes was free from alkaline ribonuclease activity. Most of the activity present in the tissue was recovered in the supernatant plus microsomal material. 6. The distribution of RNA did not follow that of the alkaline ribonuclease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS E., SMITH E. L. Proteolytic activity of pituitary extracts. J Biol Chem. 1951 Aug;191(2):651–664. [PubMed] [Google Scholar]
- Arora D. J., de Lamirande G. Ribonuclease activity in the hepatic ribonucleoprotein particles of growing rat. Arch Biochem Biophys. 1968 Feb;123(2):416–417. doi: 10.1016/0003-9861(68)90153-7. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- Barnard E. A. Ribonucleases. Annu Rev Biochem. 1969;38:677–732. doi: 10.1146/annurev.bi.38.070169.003333. [DOI] [PubMed] [Google Scholar]
- Barrett A. J. Lysosomal acid proteinase of rabbit liver. Biochem J. 1967 Aug;104(2):601–608. doi: 10.1042/bj1040601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Brewer E. N., Foster L. B., Sells B. H. A possible role for ribonuclease in the regulation of protein synthesis in normal and hypophysectomized rats. J Biol Chem. 1969 Mar 25;244(6):1389–1392. [PubMed] [Google Scholar]
- CINADER B. Antibody to enzymes--a three-component system. Introduction: immunochemistry of enzymes. Ann N Y Acad Sci. 1963 May 8;103:495–548. doi: 10.1111/j.1749-6632.1963.tb53717.x. [DOI] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELSON D. Latent enzymic activity of a ribonucleoprotein isolated from Escherichia coli. Biochim Biophys Acta. 1959 Dec;36:372–386. doi: 10.1016/0006-3002(59)90179-9. [DOI] [PubMed] [Google Scholar]
- Hellsing K. Immune reactions in polysaccharide media. Polysaccharide-enhanced precipitin reactions with antigens of various sizes. Biochem J. 1969 Aug;114(1):145–149. doi: 10.1042/bj1140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman E., Peterson E. R. Uptake of protein by mammalian neurons. J Cell Biol. 1969 Mar;40(3):863–869. doi: 10.1083/jcb.40.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALNITSKY G., HUMMEL J. P., RESNICK H., CARTER J. R., BARNETT L. B., DIERKS C. The relation of structure to enzymatic activity in ribonuclease. Ann N Y Acad Sci. 1959 Sep 4;81:542–569. doi: 10.1111/j.1749-6632.1959.tb49336.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LaBella F., Vivian S., Queen G. Abundance of cystathionine in the pineal body. Free amino acids and related compounds of bovine pineal, anterior and posterior pituitary, and brain. Biochim Biophys Acta. 1968 May;158(2):286–288. doi: 10.1016/0304-4165(68)90143-8. [DOI] [PubMed] [Google Scholar]
- Lande S., Lerner A. B., Upton G. V. Pituitary peptides. Isolation of new peptides related to beta-melanocyte-stimulating hormone. J Biol Chem. 1965 Nov;240(11):4259–4263. [PubMed] [Google Scholar]
- Lerner M. P., Johnson T. C. Regulation of protein synthesis in developing mouse brain tissue. Alteration in ribosomal activity. J Biol Chem. 1970 Mar 25;245(6):1388–1393. [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acid. I. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem J. 1952 Dec;52(4):552–557. doi: 10.1042/bj0520552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKHAM R., SMITH J. D. The structure of ribonucleic acids. II. The smaller products of ribonuclease digestion. Biochem J. 1952 Dec;52(4):558–565. doi: 10.1042/bj0520558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAVER M. E., GRECO A. E. The chromatographic separation and characterization of the acid and alkaline ribonucleases of bovine spleen and liver. J Biol Chem. 1962 Mar;237:736–741. [PubMed] [Google Scholar]
- MEYER R. K., CLIFTON K. H. Effect of diethylstilbestrol on the quantity and intracellular distribution of pituitary proteinase activity. Arch Biochem Biophys. 1956 May;62(1):198–209. doi: 10.1016/0003-9861(56)90103-5. [DOI] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- Marrink J., Gruber M. Use of casein in assays for proteolytic activity in tissue extracts: a warning. Biochim Biophys Acta. 1966 May 5;118(2):438–439. doi: 10.1016/s0926-6593(66)80058-9. [DOI] [PubMed] [Google Scholar]
- Morikawa S. Studies on alkaline and acid ribonucleases in mammalian tissues. Immunohistochemical localization and immunochemical properties. J Histochem Cytochem. 1967 Aug;15(11):662–673. doi: 10.1177/15.11.662. [DOI] [PubMed] [Google Scholar]
- OSINCHAK J. ELECTRON MICROSCOPIC LOCALIZATION OF ACID PHOSPHATASE AND THIAMINE PYROPHOSPHATASE ACTIVITY IN HYPOTHALAMIC NEUROSECRETORY CELLS OF THE RAT. J Cell Biol. 1964 Apr;21:35–47. doi: 10.1083/jcb.21.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERDUE J. F., McSHAN W. H. Isolation and biochemical study of secretory granules from rat pituitary glands. J Cell Biol. 1962 Nov;15:159–172. doi: 10.1083/jcb.15.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippu A., Schümann H. J. Ribonucleaseaktivität isolierter Nebennierenmarkgranula. Experientia. 1964 Oct 15;20(10):547–548. doi: 10.1007/BF02150281. [DOI] [PubMed] [Google Scholar]
- Pickup J. C., Hope D. B. Protease and ribonuclease activities in bovine pituitary lobes. Biochem J. 1971 Mar;122(1):31P–32P. doi: 10.1042/bj1220031pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preddie E. C. Structure of a large polypeptide of bovine posterior pituitary tissue. J Biol Chem. 1965 Nov;240(11):4194–4203. [PubMed] [Google Scholar]
- RAMACHANDRAN L. K., WINNICK T. Peptides of neuro-intermediate lobe of hog pituitary gland. Biochim Biophys Acta. 1957 Mar;23(3):533–540. doi: 10.1016/0006-3002(57)90373-6. [DOI] [PubMed] [Google Scholar]
- REICHERT L. E., Jr Proteinases of the pituitaries of several fish and of a whale. Gen Comp Endocrinol. 1962 Jun;2:283–285. doi: 10.1016/0016-6480(62)90062-x. [DOI] [PubMed] [Google Scholar]
- SACHS H., TAKABATAKE Y. EVIDENCE FOR A PRECURSOR IN VASOPRESSIN BIOSYNTHESIS. Endocrinology. 1964 Dec;75:943–948. doi: 10.1210/endo-75-6-943. [DOI] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B., HARRINGTON W. F. The correlation of ribonuclease activity with specific aspects of tertiary structure. Biochim Biophys Acta. 1957 Dec;26(3):502–512. doi: 10.1016/0006-3002(57)90096-3. [DOI] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B. Some spectrophotometric and polarimetric experiments with ribonuclease. Biochim Biophys Acta. 1957 May;24(2):229–235. doi: 10.1016/0006-3002(57)90186-5. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Barrett J. F. Isolation and structure of a hexapeptide from pig neurohypophysis. Biochim Biophys Acta. 1968 Apr 9;154(3):595–597. doi: 10.1016/0005-2795(68)90023-8. [DOI] [PubMed] [Google Scholar]
- Shin S., LaBella F., Queen G. Characterization of amino acids and low-molecular-- weight peptides bound to cytoplasmic granules from the posterior pituitary. Can J Biochem. 1970 Apr;48(4):455–462. doi: 10.1139/o70-074. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. Acid nucleases of the bovine adrenal medulla. Nature. 1965 Aug 7;207(997):634–634. doi: 10.1038/207634a0. [DOI] [PubMed] [Google Scholar]
- TODD P. E., TRIKOJUS V. M. Purification and properties of adrenal acid proteinase. Biochim Biophys Acta. 1960 Dec;45:234–242. doi: 10.1016/0006-3002(60)91447-5. [DOI] [PubMed] [Google Scholar]
- Tesar J. T., Koenig H., Hughes C. Hormone storage granules in the beef anterior pituitary. I. Isolation, ultrastructure, and some biochemical properties. J Cell Biol. 1969 Jan;40(1):225–235. doi: 10.1083/jcb.40.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umaña R. Reevaluation of the method of Kunitz for the assay of proteolytic activities in liver and brain homogenates. Anal Biochem. 1968 Dec;26(3):430–438. doi: 10.1016/0003-2697(68)90204-2. [DOI] [PubMed] [Google Scholar]
- Upton G. V., Lerner A. B., Lande S. Pituitary peptides. Resolution by gel filtration. J Biol Chem. 1966 Dec 10;241(23):5585–5589. [PubMed] [Google Scholar]
- Vanha-Perttula T. P., Hopsu V. K. Esterolytic and proteolytic enzymes of the rat adenohypophysis. I. Studies with homogenate and its fractions. Histochemie. 1965 Jan 12;4(5):372–387. doi: 10.1007/BF00306249. [DOI] [PubMed] [Google Scholar]
- Vellan E. J., Gjessing L. R., Stalsberg H. Free amino acids in the pineal and pituitary glands of human brain. J Neurochem. 1970 May;17(5):699–701. doi: 10.1111/j.1471-4159.1970.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Zomzely C. E., Roberts S., Gruber C. P., Brown D. M. Cerebral protein synthesis. II. Instability of cerebral messenger ribonucleic acid-ribosome complexes. J Biol Chem. 1968 Oct 25;243(20):5396–5409. [PubMed] [Google Scholar]


