Full text
PDF

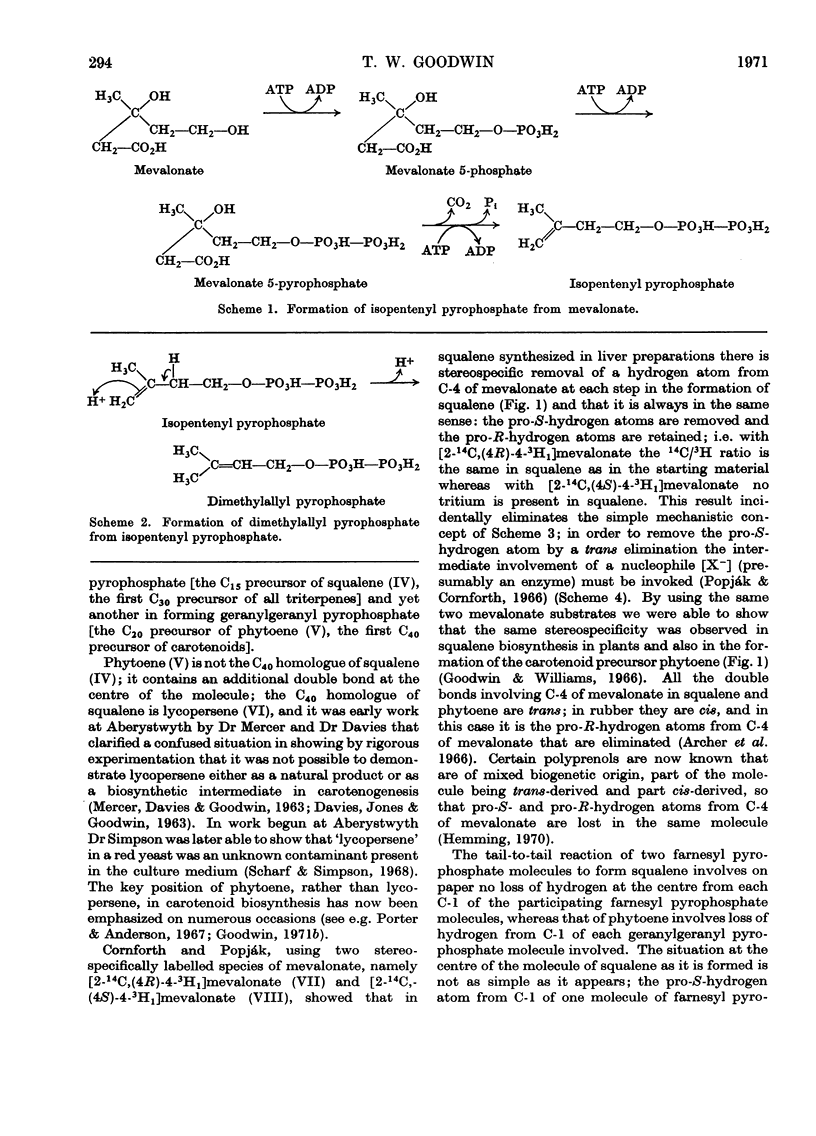
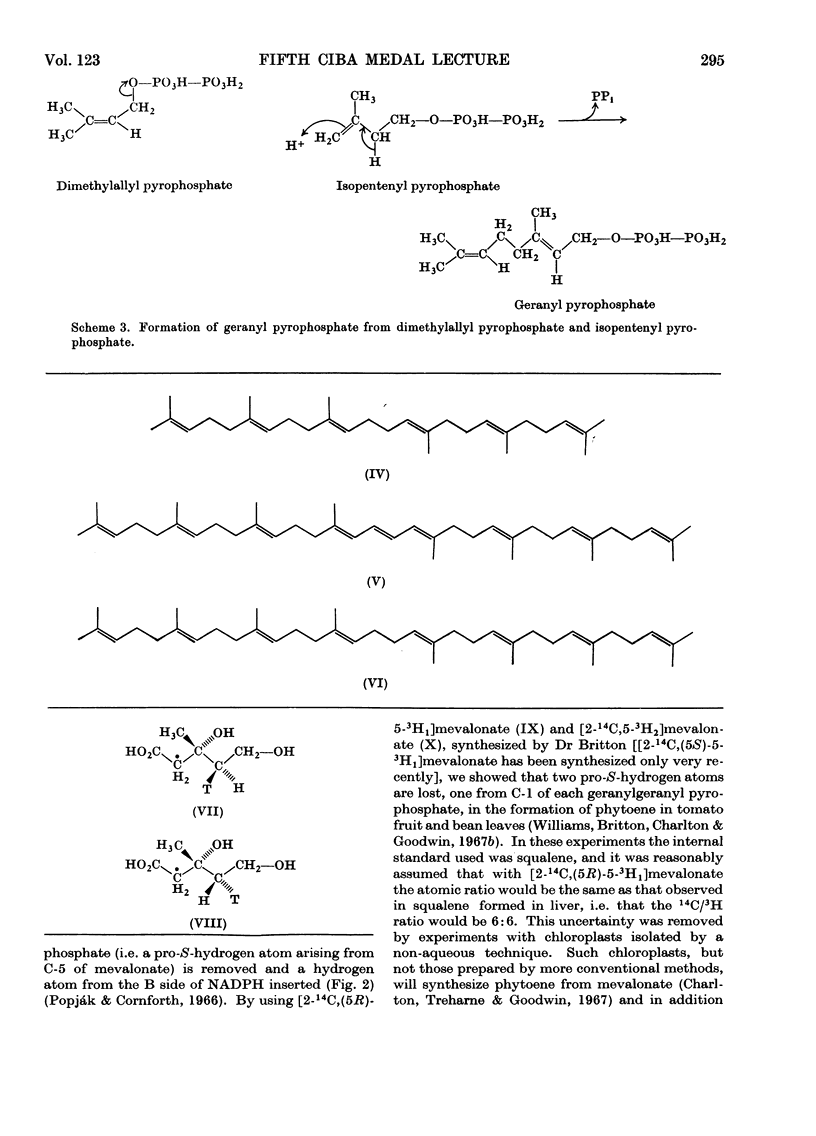
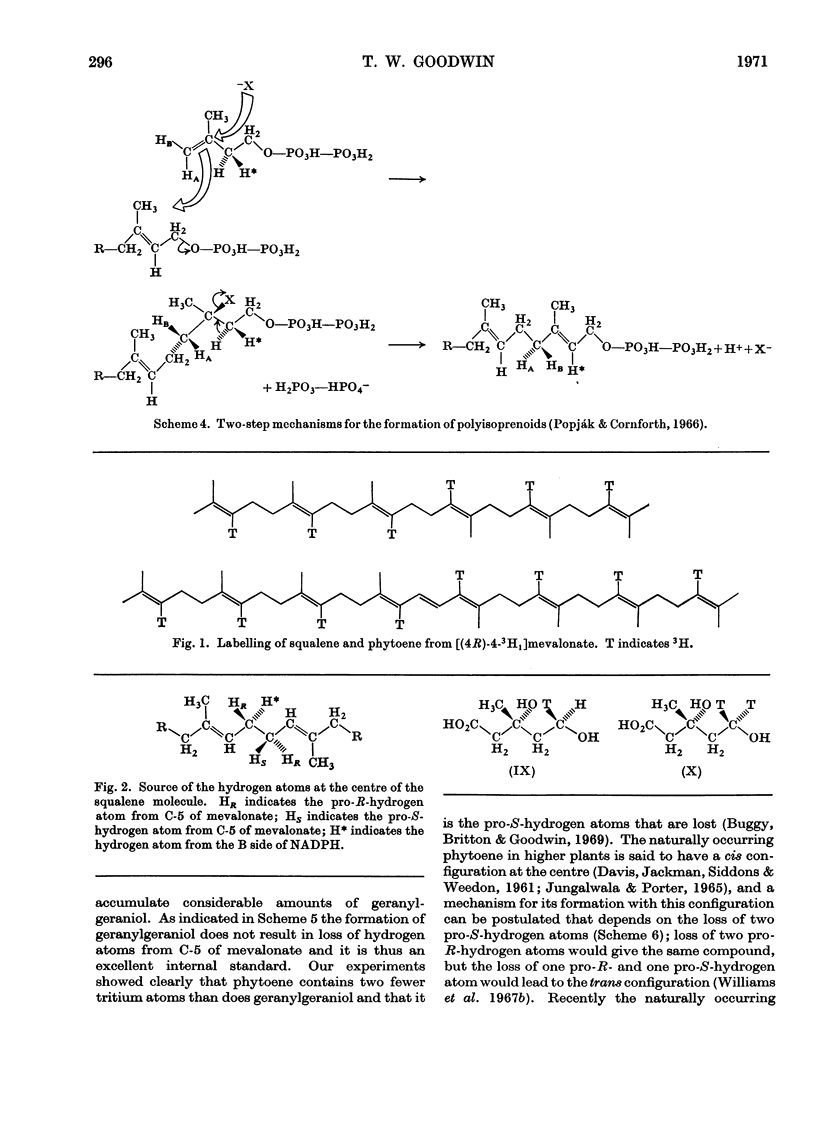
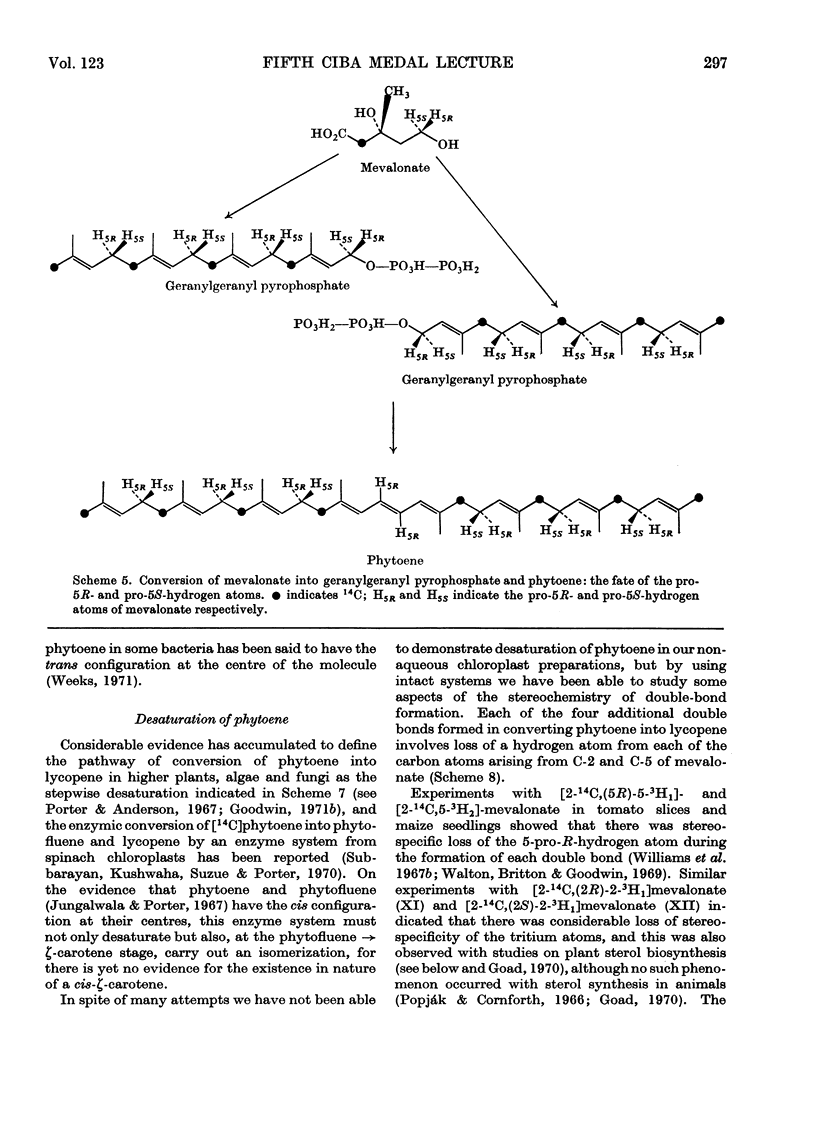

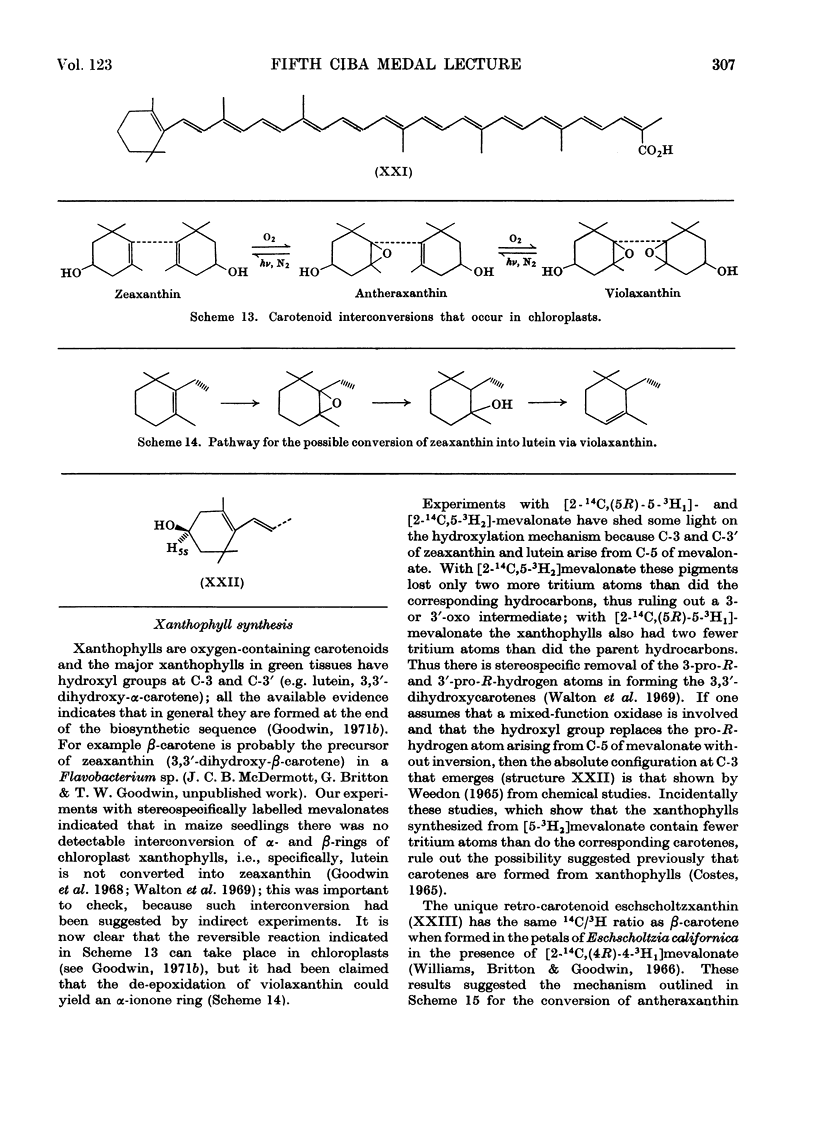
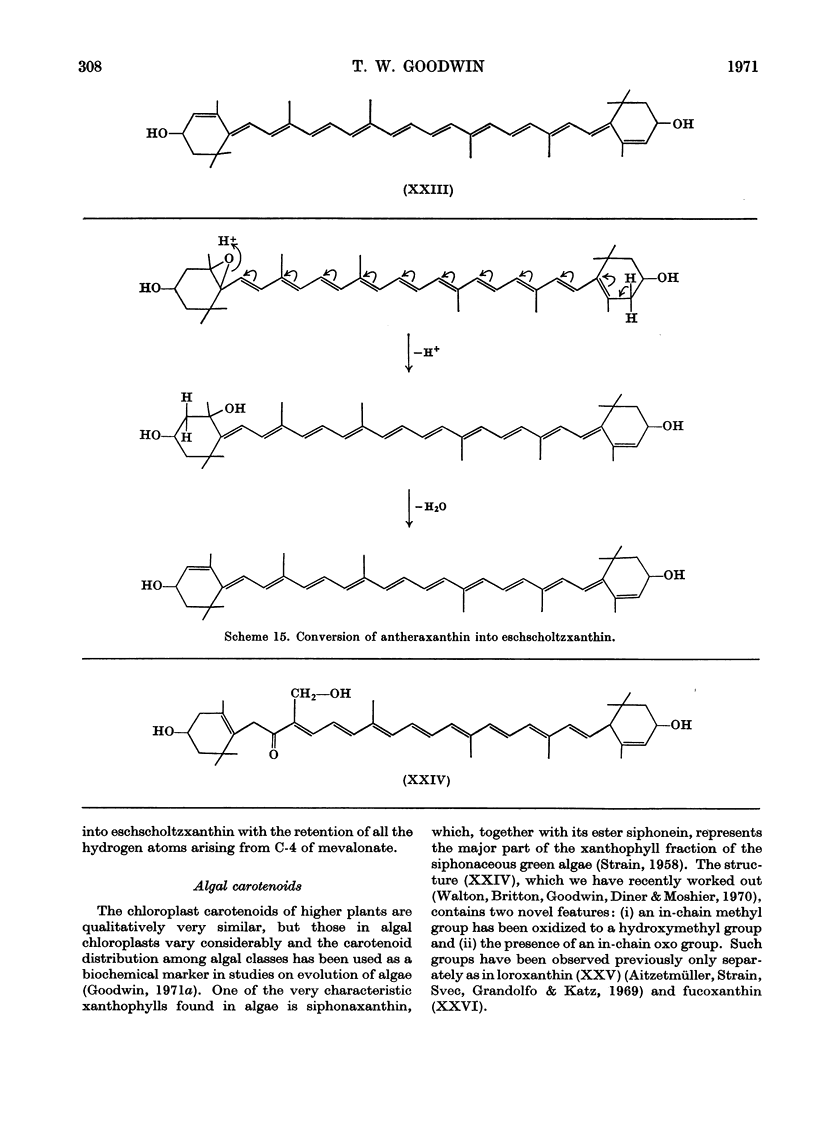
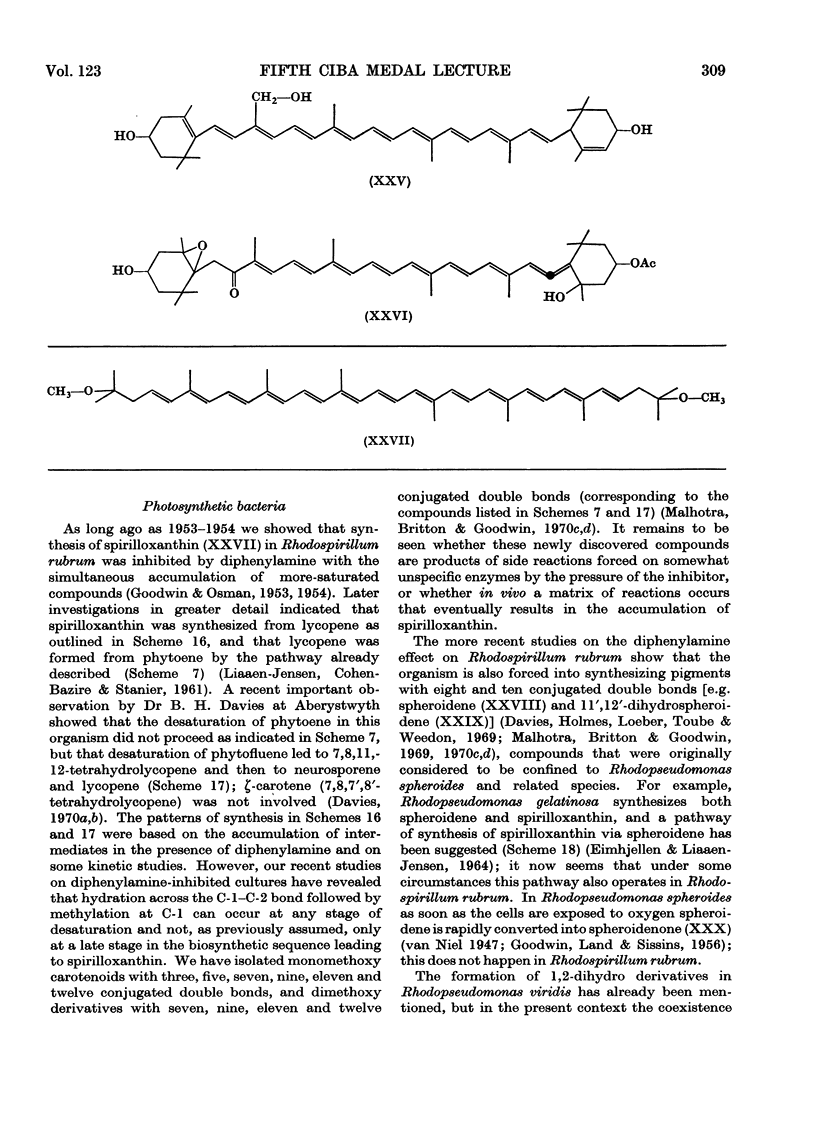
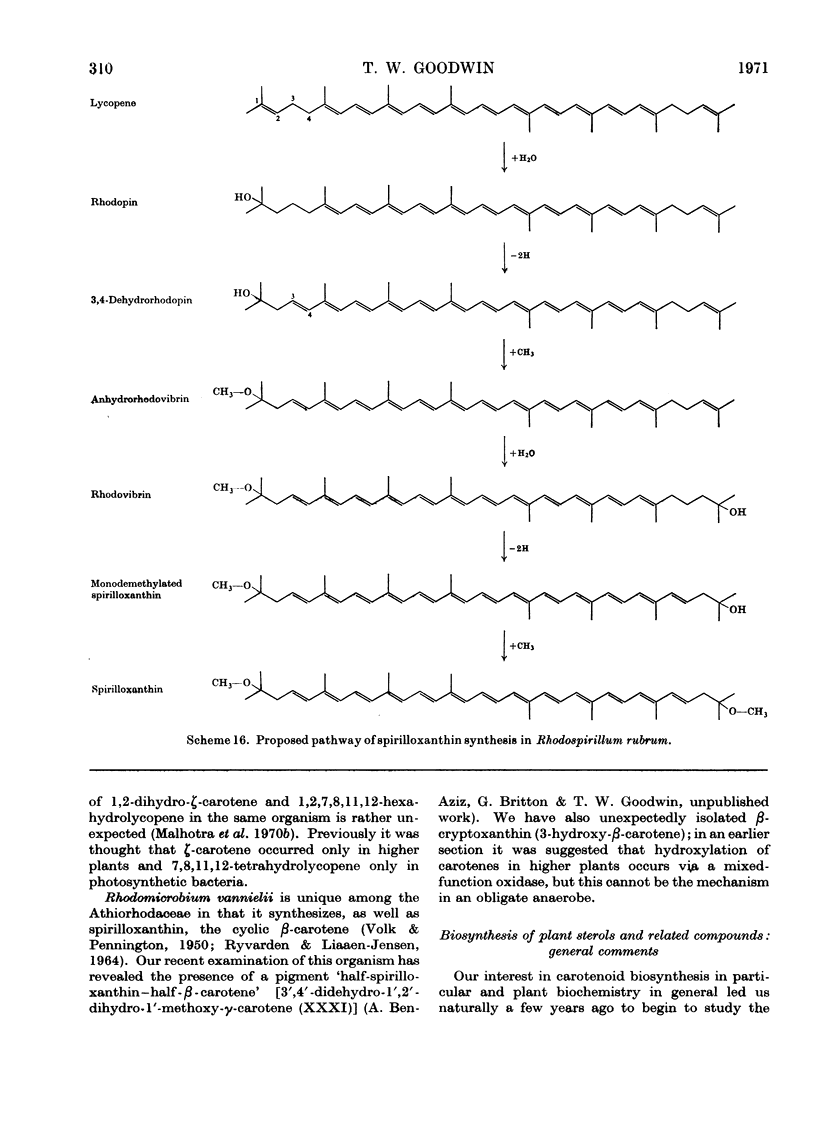




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhtar M., Marsh S. The stereochemistry of the hydrogen elimination in the biological conversion of cholest-7-en-3-beta-ol into cholesterol. Biochem J. 1967 Feb;102(2):462–467. doi: 10.1042/bj1020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Watkinson I. A., Rahimtula A. D., Wilton D. C., Munday K. A. The role of a cholesta-8,14-dien-3-beta-ol system in cholesterol biosynthesis. Biochem J. 1969 Mar;111(5):757–761. doi: 10.1042/bj1110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimpson T., Goad L. J., Goodwin T. W. The stereochemistry of hydrogen elimination at C-7,C-22 and C-23 during the conversion of cholesterol (cholest-5-en-3 beta-ol) into cholesta-5,7,22-trien-3 beta-ol by Tetrahymena pyriformis. Biochem J. 1969 Dec;115(4):857–858. doi: 10.1042/bj1150857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger L. M., Rees H. H., Ghisalberti E. L., Goad L. J., Goodwin T. W. Biosynthesis of 24-ethylcholesta-5,22,25-trien-3-beta-ol, a new sterol from Clerodendrum campbellii. Biochem J. 1970 Jun;118(1):197–200. doi: 10.1042/bj1180197. [DOI] [PMC free article] [PubMed] [Google Scholar]
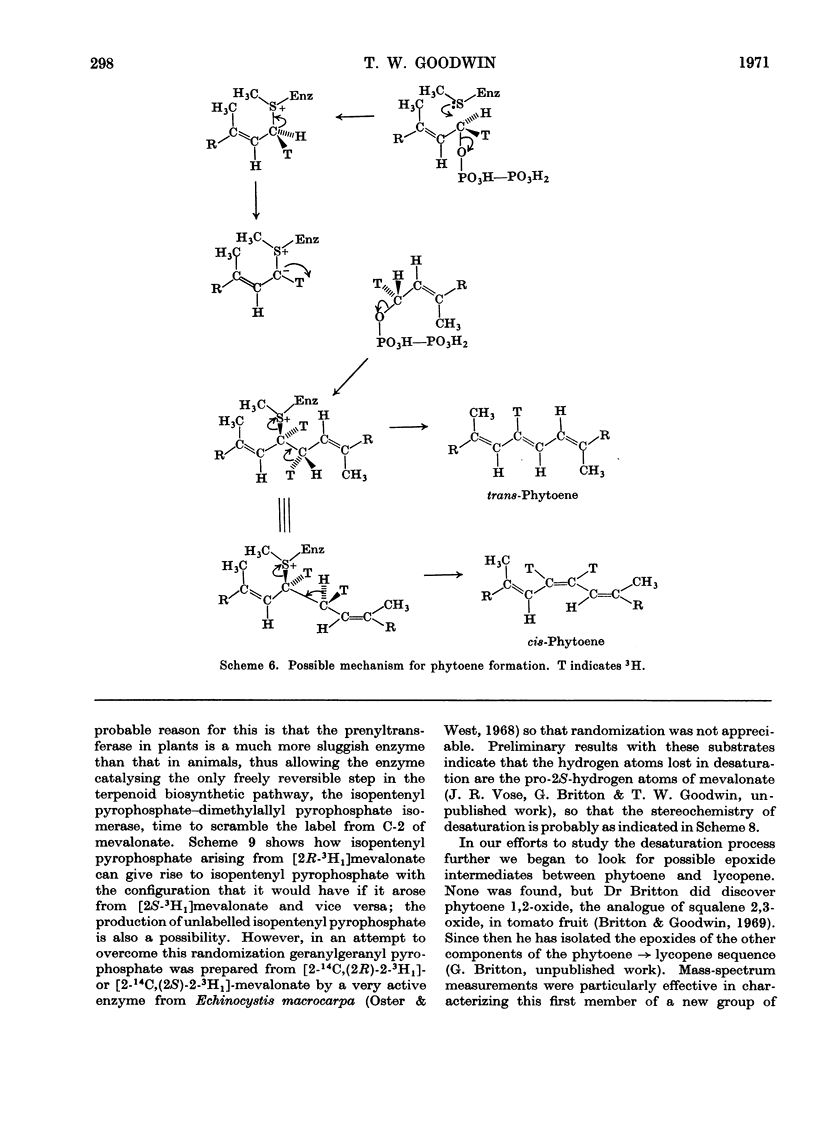
- Buggy M. J., Britton G., Goodwin T. W. Stereochemistry of phytoene biosynthesis by isolated chloroplasts. Biochem J. 1969 Sep;114(3):641–643. doi: 10.1042/bj1140641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonica L., Fiecchi A., Galli Kienle M., Scala A. The stereochemistry of hydrogen elimination in the biological conversion of 5 alpha-cholest-8-en-3 beta-ol to 5 alpha-cholest-7-en-3 beta-ol. Steroids. 1968 Jun;11(6):749–753. doi: 10.1016/s0039-128x(68)80091-1. [DOI] [PubMed] [Google Scholar]
- Canonica L., Fiecchi A., Kienle M. G., Scala A., Galli G., Paoletti E. G., Paoletti R. The fate of the 15-beta hydrogen of lanosterol in cholesterol biosynthesis. J Am Chem Soc. 1968 Jun 19;90(13):3597–3598. doi: 10.1021/ja01015a074. [DOI] [PubMed] [Google Scholar]
- Caspi E., Greig J. B., Ramm P. J., Varma K. R. Stereochemistry of tritium at C-1 and C-7 in cholesterol derived from (3R,2R)-2T-mevalonic acid. Tetrahedron Lett. 1968 Jul;(35):3829–3832. doi: 10.1016/s0040-4039(01)99112-9. [DOI] [PubMed] [Google Scholar]
- Charlton J. M., Treharne K. J., Goodwin T. W. Incorporation of 2-[14C]mevalonic acid into phytoene by isolated chloroplasts. Biochem J. 1967 Oct;105(1):205–212. doi: 10.1042/bj1050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
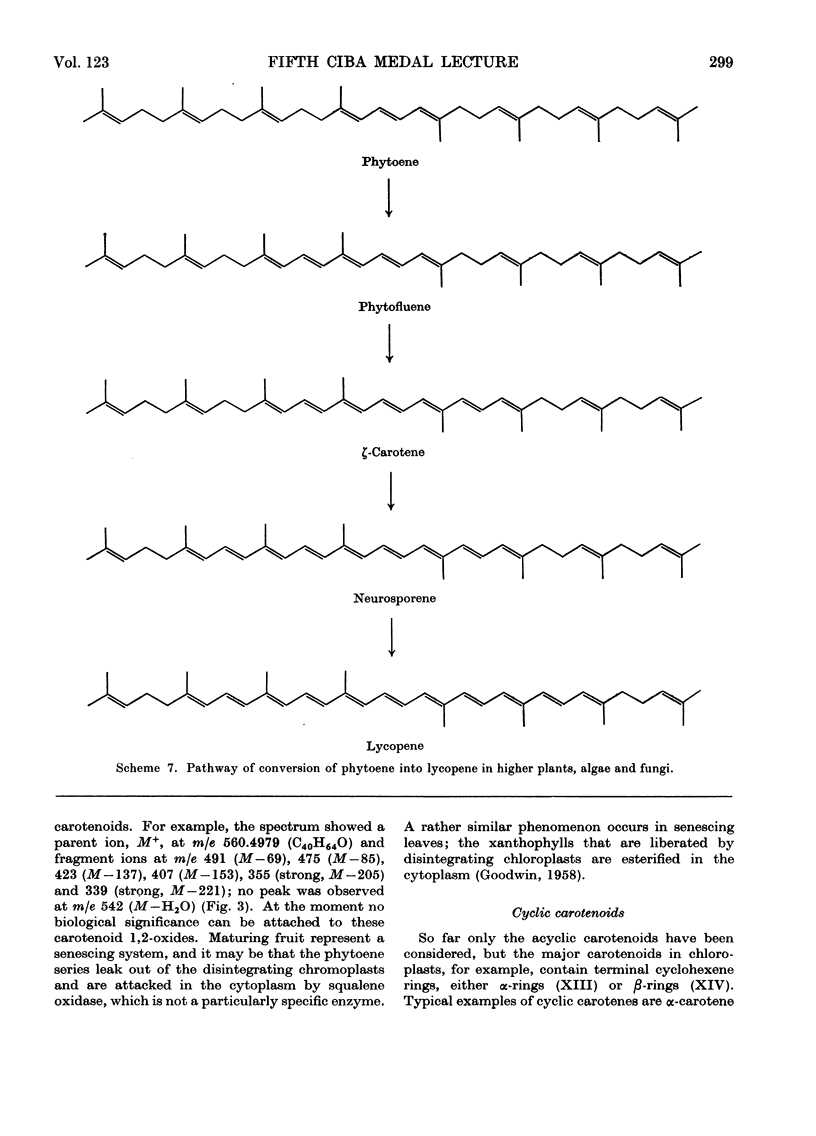
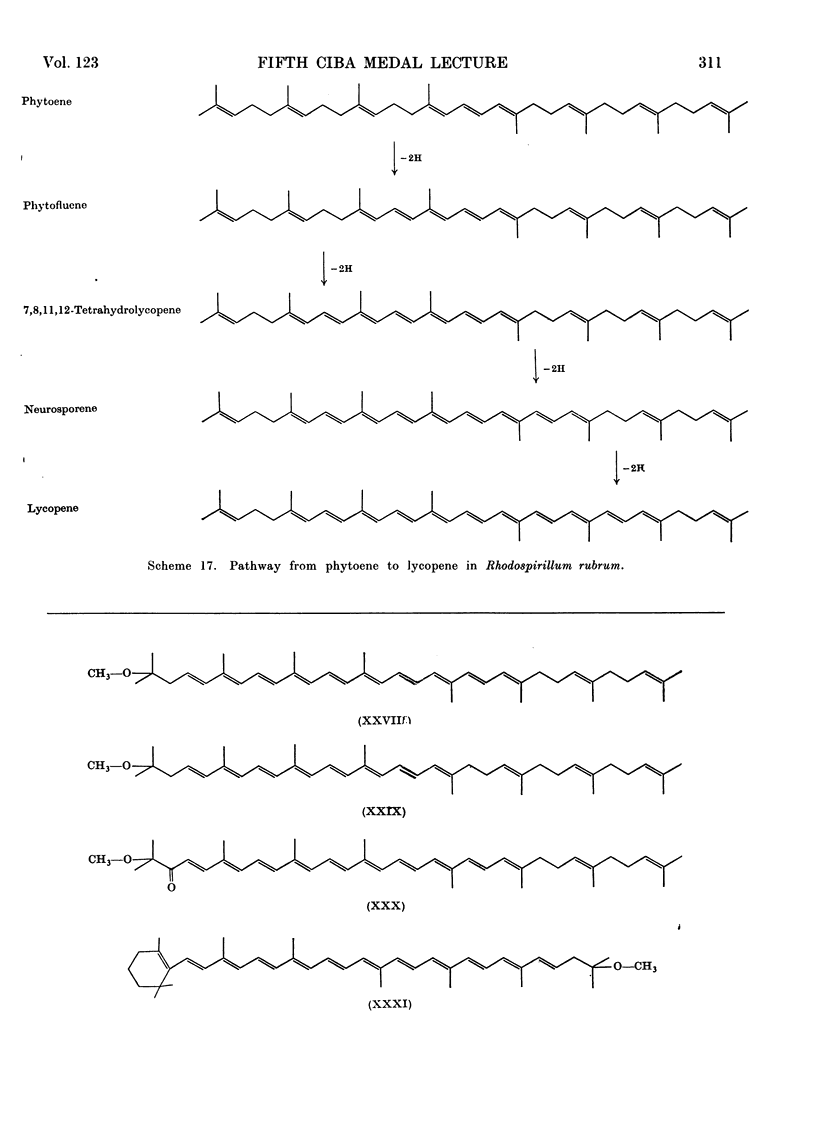
- Davies B. H. A novel sequence for phytoene dehydrogenation in Rhodospirillum rubrum. Biochem J. 1970 Jan;116(1):93–99. doi: 10.1042/bj1160093. [DOI] [PMC free article] [PubMed] [Google Scholar]
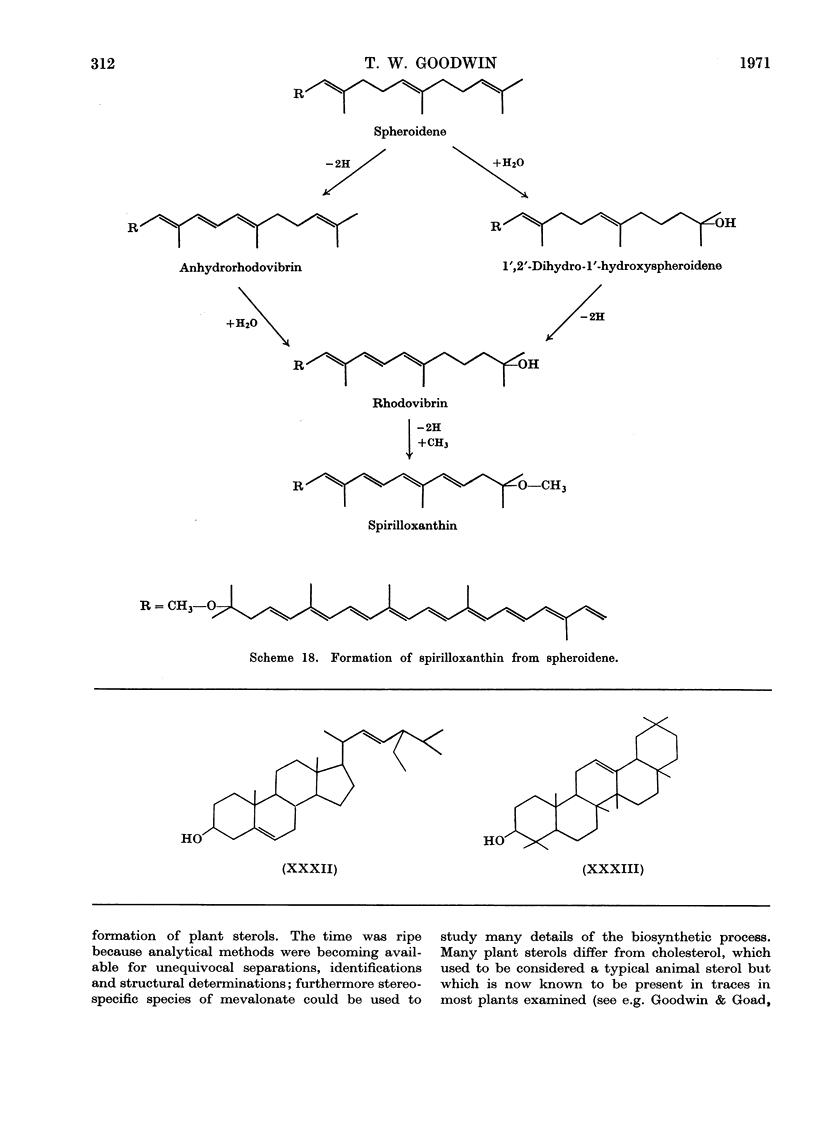
- Davies B. H. Alternative pathways of spirilloxanthin biosynthesis in Rhodospirillum rubrum. Biochem J. 1970 Jan;116(1):101–110. doi: 10.1042/bj1160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. H., Jones D., Goodwin T. W. Studies in carotenogenesis. 30. The problem of lycopersene formation in Neurospora crassa. Biochem J. 1963 May;87(2):326–329. doi: 10.1042/bj0870326. [DOI] [PMC free article] [PubMed] [Google Scholar]
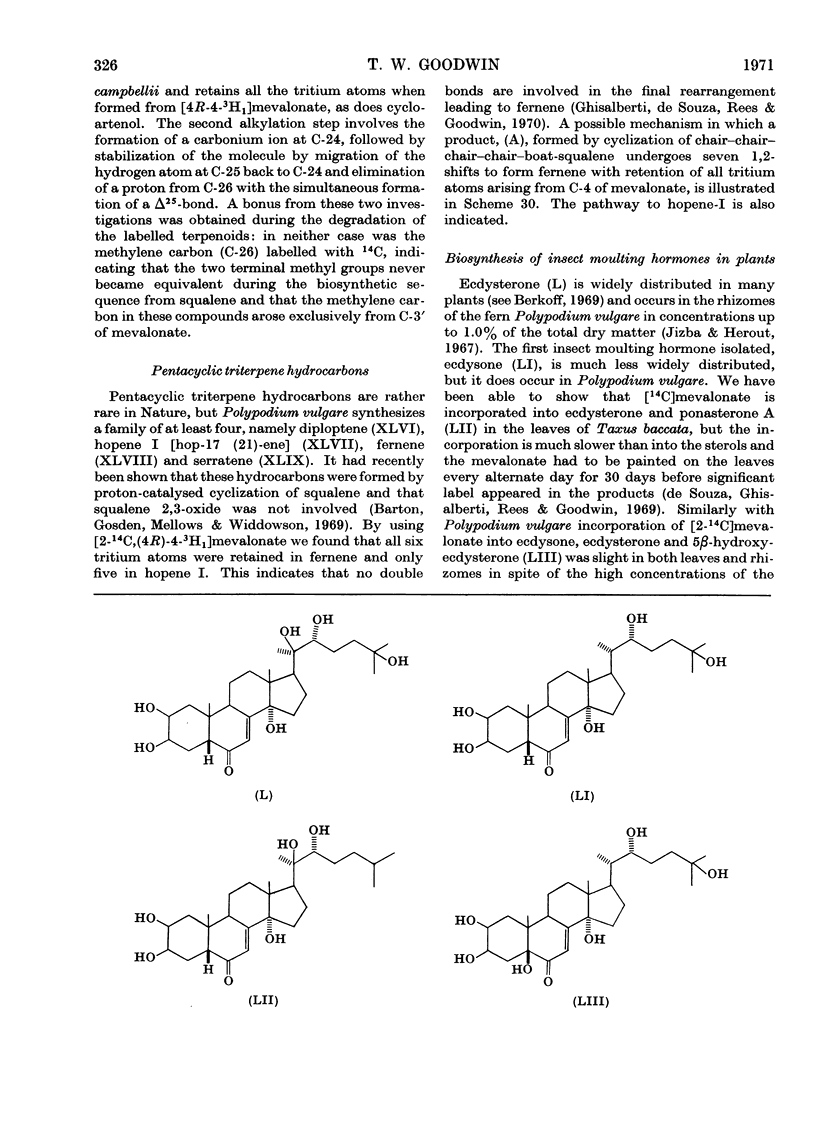
- De Souza N. J., Ghisalberti E. L., Rees H. H., Goodwin T. W. Studies on insect moulting hormones: biosynthesis of ponasterone A and ecdysterone from [2-14C] mevalonate in Taxus baccata. Biochem J. 1969 Oct;114(4):895–896. doi: 10.1042/bj1140895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppenberger U., Hirth L., Ourisson G. Anaerobische Cyclisierung von Squalen-2,3-epoxyd zu Cycloartenol in Gewebekulturen von Nicotiana tabacum L. Eur J Biochem. 1969 Mar;8(2):180–183. doi: 10.1111/j.1432-1033.1969.tb00512.x. [DOI] [PubMed] [Google Scholar]
- GOODWIN T. W., LAND D. G., SISSINS M. E. Studies in carotenogenesis. 23. The nature of the carotenoids in the photosynthetic bacterium Rhodopseudomonas spheroides (athiorhodaceae). Biochem J. 1956 Nov;64(3):486–492. doi: 10.1042/bj0640486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W., OSMAN H. G. Studies in carotenogenesis. 10. Spirilloxanthin synthesis by washed cells of Rhodospirillum rubrum. Biochem J. 1954 Feb;56(2):222–230. doi: 10.1042/bj0560222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W., OSMAN H. G. Studies in carotenogenesis. 9. General cultural conditions controlling carotenoid (spirilloxanthin) synthesis in the photosynthetic bacterium Rhodospirillum rubrum. Biochem J. 1953 Mar;53(4):541–546. doi: 10.1042/bj0530541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODWIN T. W. Studies in carotenogenesis. 24. The changes in carotenoid and chlorophyll pigments in the leaves of deciduous trees during autumn necrosis. Biochem J. 1958 Mar;68(3):503–511. doi: 10.1042/bj0680503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Smith A. R., Goulston G., Goad L. J., Goodwin T. W., Haines T. H. The sterols of Ochromonas danica and Ochromonas malhamensis. Biochemistry. 1968 May;7(5):1698–1706. doi: 10.1021/bi00845a012. [DOI] [PubMed] [Google Scholar]
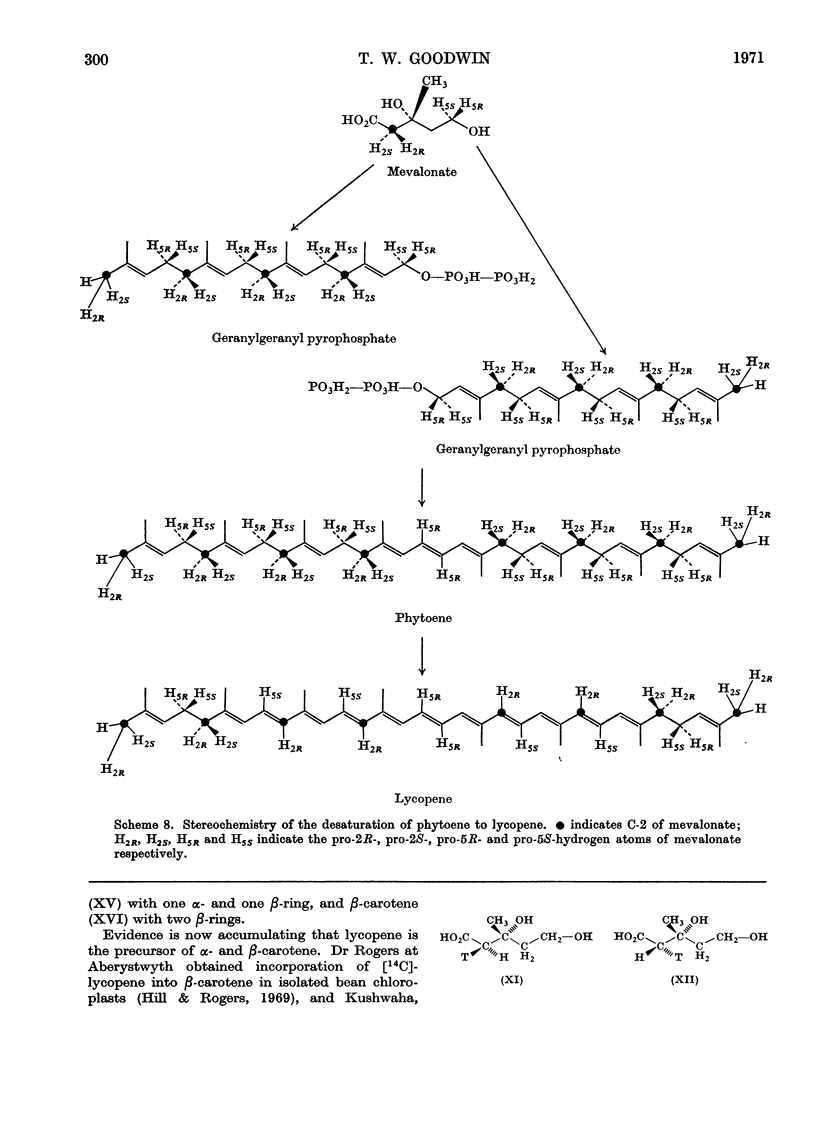
- Goad L. J., Gibbons G. F., Bolger L. M., Rees H. H., Goodwin T. W. Incorporation of (2-14C, (5r)-5-3H1) mevalonic acid into cholesterol by a rat liver homogenate and into beta-sitosterol and 28-isofucosterol by larix decidua leaves. Biochem J. 1969 Oct;114(4):885–892. doi: 10.1042/bj1140885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. Studies in phytosterol biosynthesis: observations on the biosynthesis of fucosterol in the marine brown alga Fucus spiralis. Eur J Biochem. 1969 Feb;7(4):502–508. doi: 10.1111/j.1432-1033.1969.tb19636.x. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. Studies on phytosterol biosynthesis: the sterols of Larix decidua leaves. Eur J Biochem. 1967 May;1(3):357–362. doi: 10.1007/978-3-662-25813-2_49. [DOI] [PubMed] [Google Scholar]
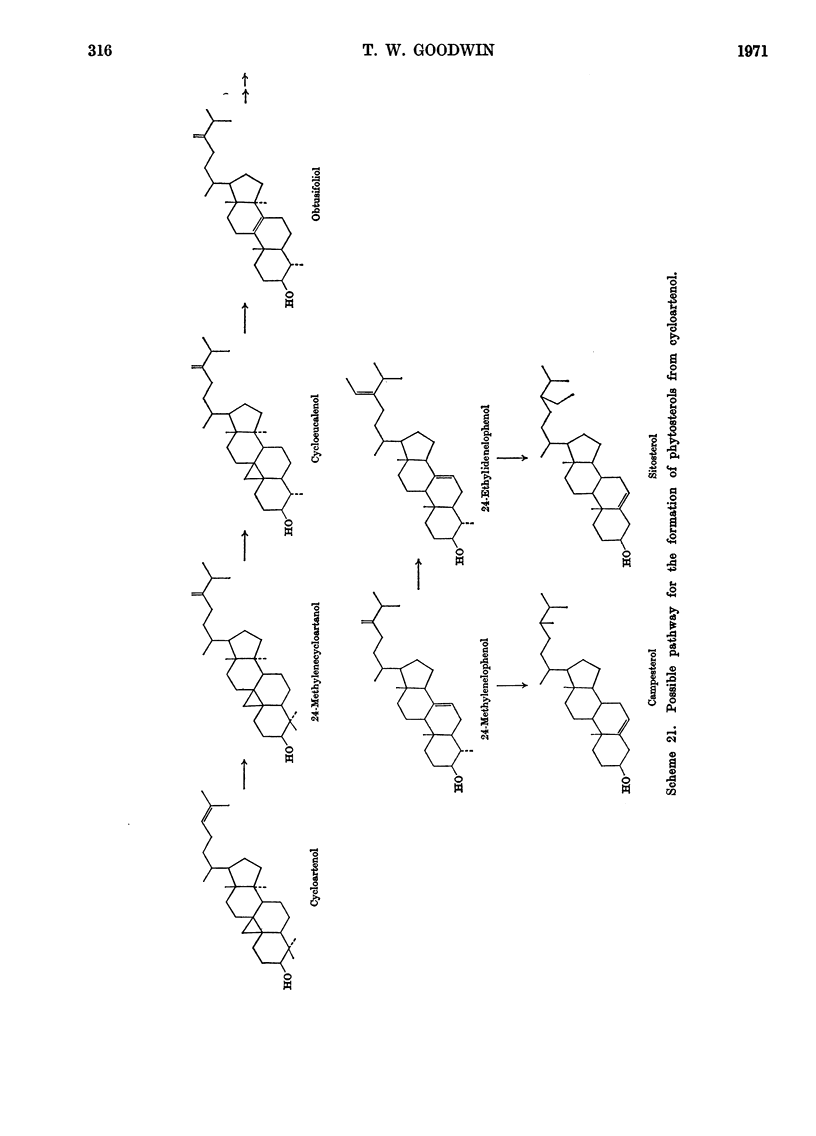
- Goad L. J., Goodwin T. W. The biosynthesis of sterols in higher plants. Biochem J. 1966 Jun;99(3):735–746. doi: 10.1042/bj0990735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Hammam A. S., Dennis A., Goodwin T. W. Biosynthesis of the phytosterol side chain. Nature. 1966 Jun 25;210(5043):1322–1324. doi: 10.1038/2101322a0. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Williams B. L., Goodwin T. W. Studies on phytosterol biosynthesis. The presence of 4-alpha,14-alpha-dimethyl-delta-8,24(28)-ergostadien-3-beta-ol in grapefruit peel and its co-occurrece with cycloeucalenol in higher plant tissues. Eur J Biochem. 1967 Dec;3(2):232–236. doi: 10.1111/j.1432-1033.1967.tb19521.x. [DOI] [PubMed] [Google Scholar]
- Hall J., Smith A. R., Goad L. J., Goodwin T. W. The conversion of lanosterol, cycloartenol and 24-methylenecycloartanol into poriferasterol by Ochromonas malhamensis. Biochem J. 1969 Mar;112(1):129–130. doi: 10.1042/bj1120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming F. W. Polyprenols. Biochem Soc Symp. 1970;29:105–117. [PubMed] [Google Scholar]
- Hewlins M. J., Ehrhardt J. D., Hirth L., Ourisson G. The conversion of [14C]cycloartenol and [14C)lanosterol into phytosterols by cultures of Nicotiana tabacum. Eur J Biochem. 1969 Mar;8(2):184–188. doi: 10.1111/j.1432-1033.1969.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Howes C. D., Batra P. P. Accumulation of lycopene and inhibition of cyclic carotenoids in Mycobacterium in the presence of nicotine. Biochim Biophys Acta. 1970 Oct 27;222(1):174–179. doi: 10.1016/0304-4165(70)90362-4. [DOI] [PubMed] [Google Scholar]
- JENSEN S. L., COHEN-BAZIRE G., STANIER R. Y. Biosynthesis of carotenoids in purple bacteria: a reevaluation based on considerations of chemical structure. Nature. 1961 Dec 23;192:1168–1172. doi: 10.1038/1921168a0. [DOI] [PubMed] [Google Scholar]
- JUNGALWALA F. B., PORTER J. W. THE CONFIGURATION OF PHYTOENE. Arch Biochem Biophys. 1965 May;110:291–299. doi: 10.1016/0003-9861(65)90121-9. [DOI] [PubMed] [Google Scholar]
- Jungalwala F. B., Porter J. W. Biosynthesis of phytoene from isopentenyl and farnesyl pyrophosphates by a partially purified tomato enzyme system. Arch Biochem Biophys. 1967 Mar;119(1):209–219. doi: 10.1016/0003-9861(67)90448-1. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Subbarayan C., Beeler D. A., Porter J. W. The conversion of lycopene-15,15'-3H to cyclic carotenes by soluble extracts of higher plant plastids. J Biol Chem. 1969 Jul 10;244(13):3635–3642. [PubMed] [Google Scholar]
- Malhotra H. C., Britton G., Goodwin T. W. A novel series of 1,2-dihydro carotenoids. Int Z Vitaminforsch. 1970;40(3):315–322. [PubMed] [Google Scholar]
- Malhotra H. C., Britton G., Goodwin T. W. The occurrence of hydroxy-derivatives of phytoene and phytofluene in diphenylamine-inhibited cultures of Rhodospirillum rubrum. FEBS Lett. 1970 Feb 25;6(4):334–336. doi: 10.1016/0014-5793(70)80091-6. [DOI] [PubMed] [Google Scholar]
- Mercer E. I., Davies B. H., Goodwin T. W. Studies in carotenogenesis. 29. Attempts to detect lycopersene in higher plants. Biochem J. 1963 May;87(2):317–325. doi: 10.1042/bj0870317. [DOI] [PMC free article] [PubMed] [Google Scholar]
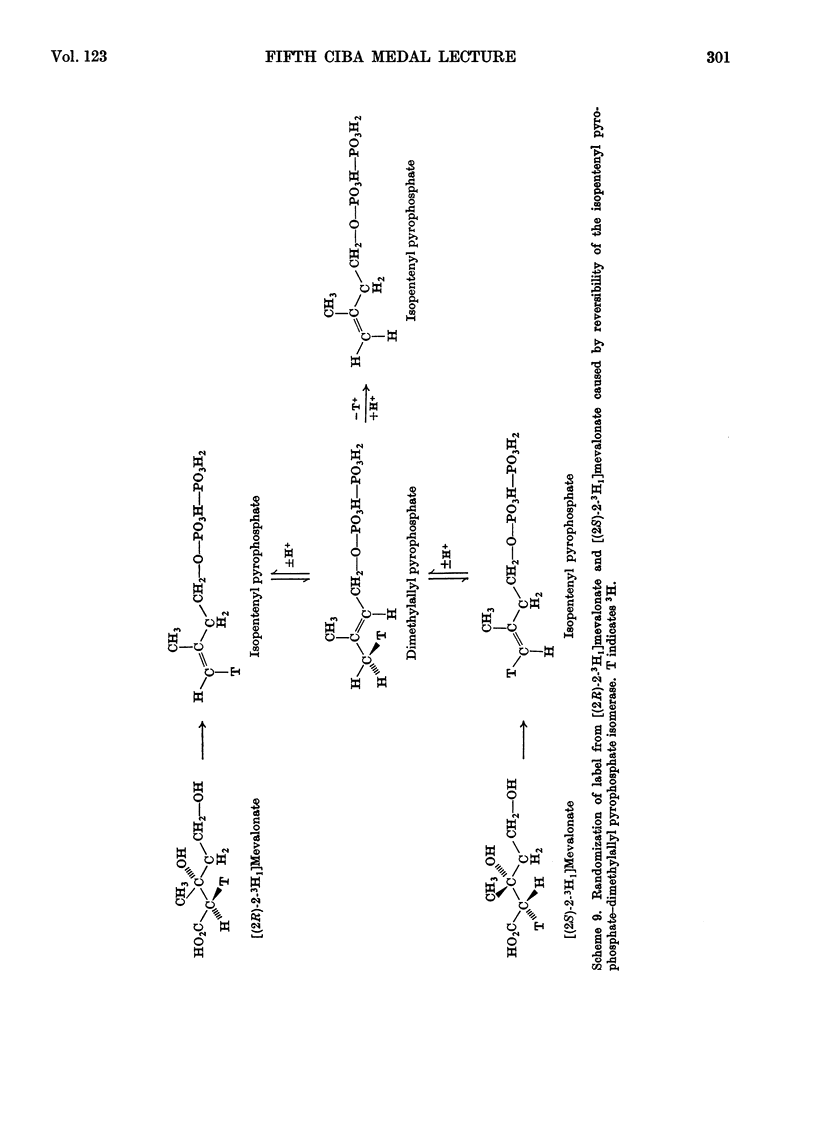
- Oster M. O., West C. A. Biosynthesis of trans-geranylgeranyl pyrophosphate in endosperm of Echinocystis macrocarpa Greene. Arch Biochem Biophys. 1968 Sep 20;127(1):112–123. doi: 10.1016/0003-9861(68)90207-5. [DOI] [PubMed] [Google Scholar]
- PETZOLD E. N., QUACKENBUSH F. W., McQUISTAN M. Zeacarotenes, new provitamins A from corn. Arch Biochem Biophys. 1959 May;82(1):117–124. doi: 10.1016/0003-9861(59)90096-7. [DOI] [PubMed] [Google Scholar]
- PORTER J. W., ANDERSON D. G. The biosynthesis of carotenes. Arch Biochem Biophys. 1962 Jun;97:520–528. doi: 10.1016/0003-9861(62)90116-9. [DOI] [PubMed] [Google Scholar]
- Paliokas A. M., Schroepfer G. J., Jr Enzymatic stereospecificity in the conversion of delta-7-cholesten-3-beta-ol to 7-dehydrocholesterol. Biochem Biophys Res Commun. 1967 Mar 21;26(6):736–741. doi: 10.1016/s0006-291x(67)80135-9. [DOI] [PubMed] [Google Scholar]
- Paliokas A. M., Schroepfer G. J., Jr Stereospecificity in the enzymatic conversion of delta-7-cholesten-3-beta-ol to 7-dehydrocholesterol. J Biol Chem. 1968 Feb 10;243(3):453–464. [PubMed] [Google Scholar]
- Popják G., Cornforth J. W. Substrate stereochemistry in squalene biosynthesis: The first Ciba medal lecture. Biochem J. 1966 Dec;101(3):553.b4–553568. [PMC free article] [PubMed] [Google Scholar]
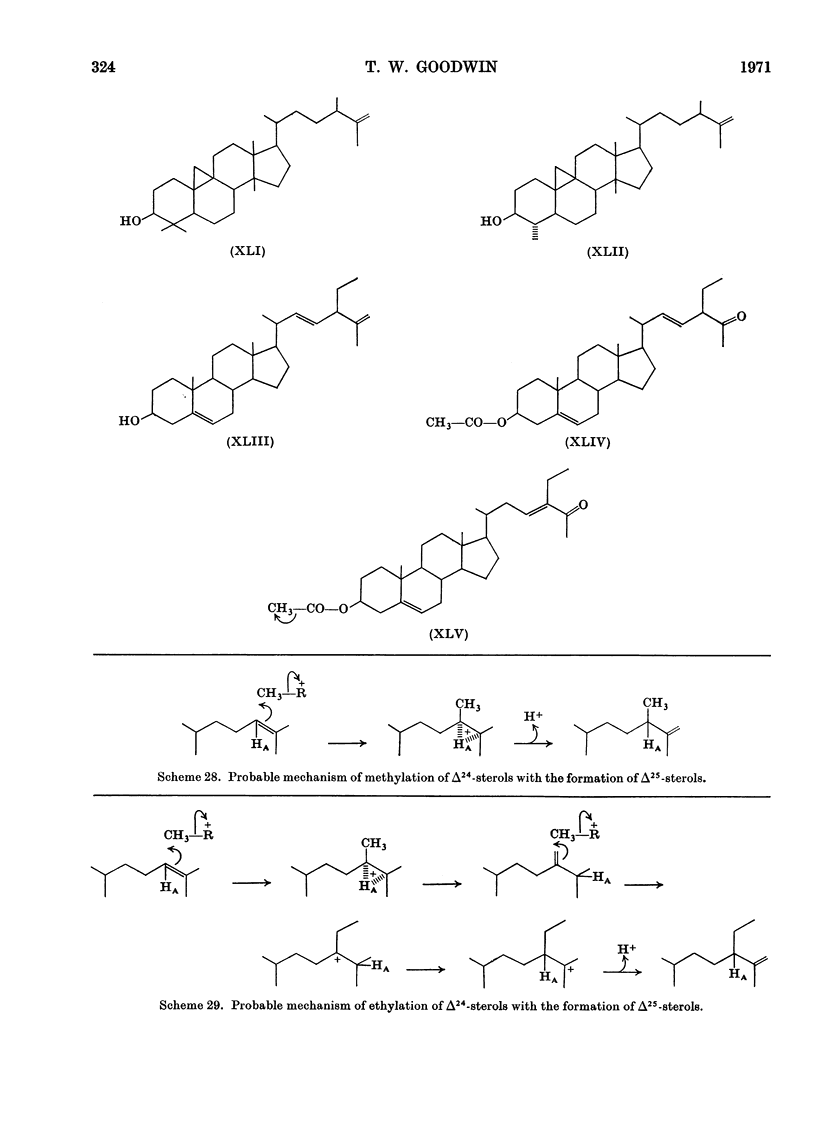
- Raab K. H., De Souza N. J., Nes W. R. The H-migration in the alkylation of sterols at C-24. Biochim Biophys Acta. 1968 Jul 1;152(4):742–748. doi: 10.1016/0005-2760(68)90120-3. [DOI] [PubMed] [Google Scholar]
- Ramm P. J., Caspi E. The stereochemistry of tritium at carbon atoms 1, 7, and 15 in cholesterol derived from (3R,2R)-(2-3H)-mevalonic acid. J Biol Chem. 1969 Nov 25;244(22):6064–6073. [PubMed] [Google Scholar]
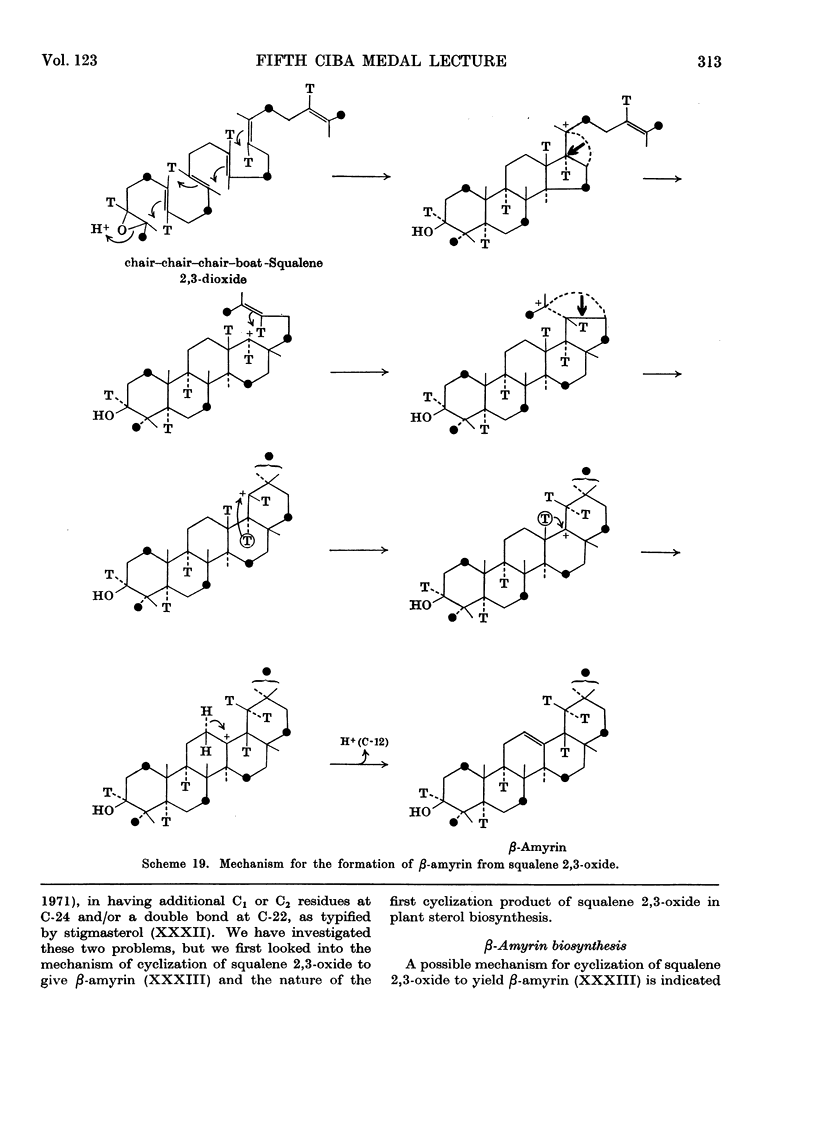
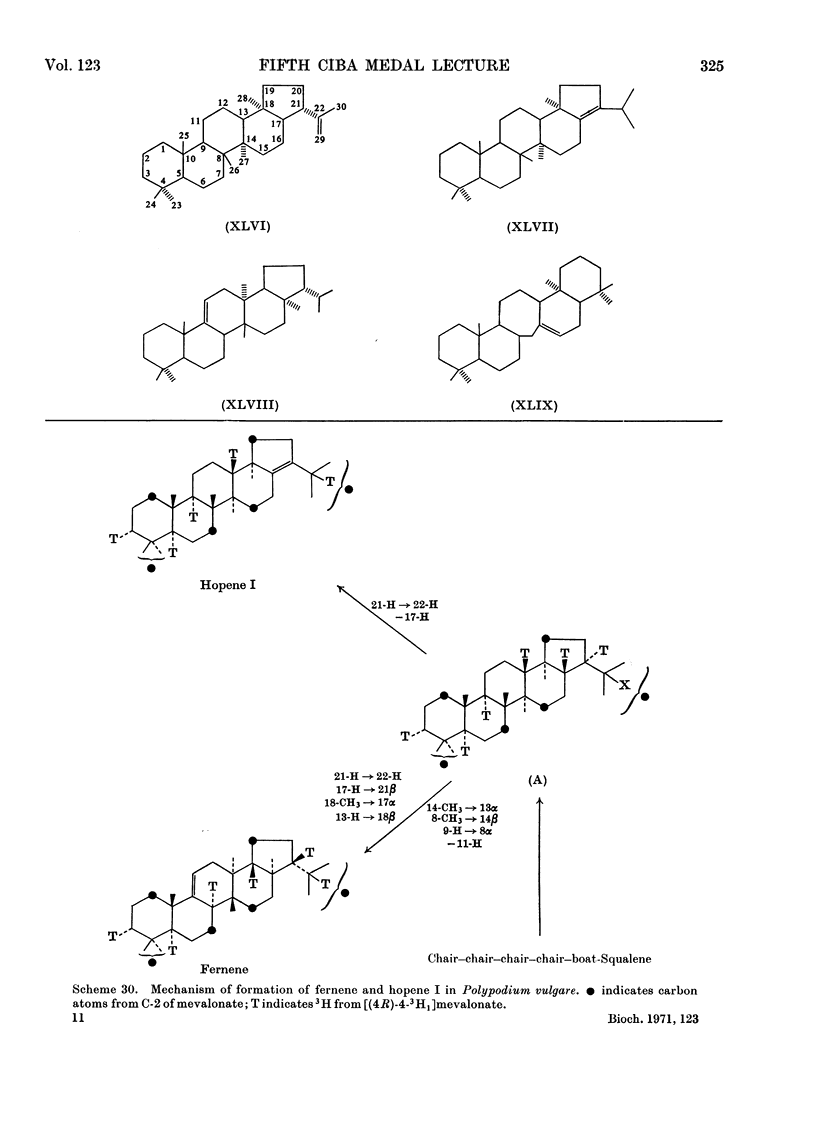
- Rees H. H., Britton G., Goodwin T. W. The biosynthesis of beta-amyrin. Mechanism of squalene cyclization. Biochem J. 1968 Feb;106(3):659–665. doi: 10.1042/bj1060659. [DOI] [PMC free article] [PubMed] [Google Scholar]
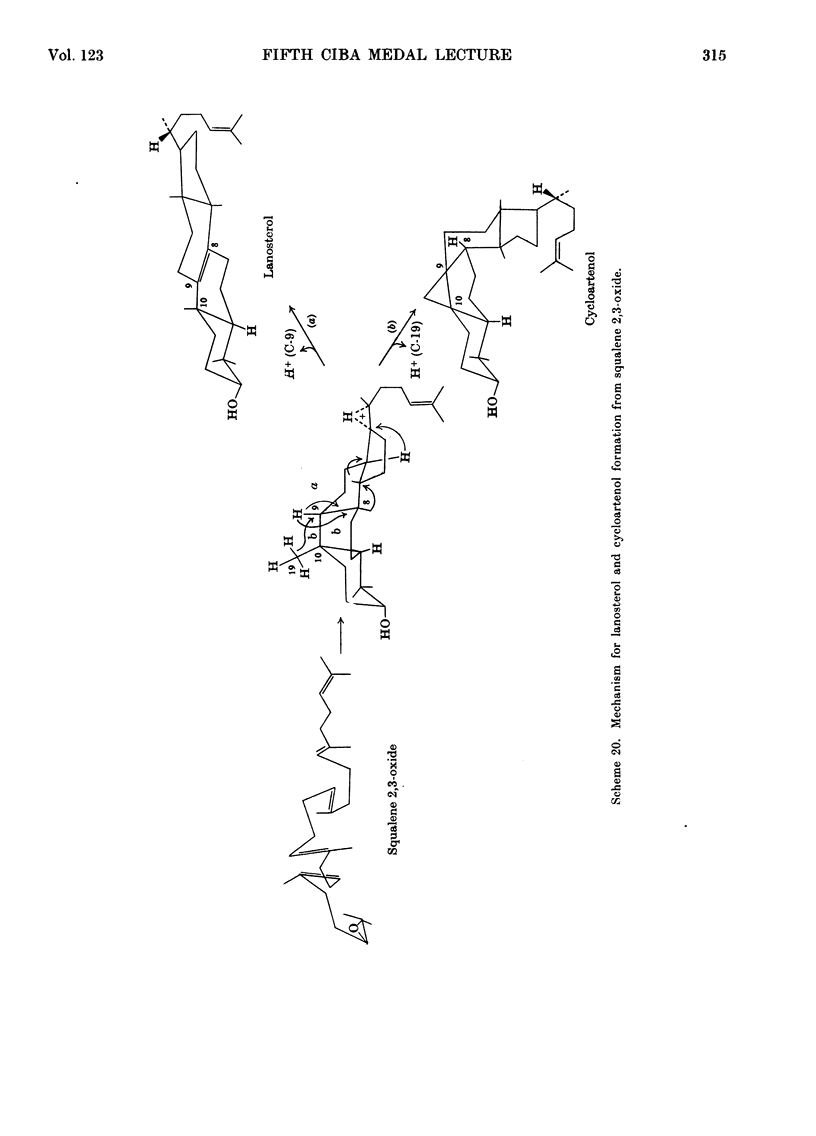
- Rees H. H., Goad L. J., Goodwin T. W. 2,3-oxidosqualene cycloartenol cyclase from Ochromonas malhamensis. Biochim Biophys Acta. 1969 Jun 10;176(4):892–894. [PubMed] [Google Scholar]
- Rees H. H., Goad L. J., Goodwin T. W. Cyclization of 2,3-oxidosqualene to cycloartenol in a cell-free system from higher plants. Tetrahedron Lett. 1968 Feb;6:723–725. doi: 10.1016/s0040-4039(00)75620-6. [DOI] [PubMed] [Google Scholar]
- Rees H. H., Goad L. J., Goodwin T. W. Studies in phytosterol biosynthesis. Mechanism of biosynthesis of cycloartenol. Biochem J. 1968 Apr;107(3):417–426. doi: 10.1042/bj1070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees H. H., Mercer E. I., Goodwin T. W. The stereospecific biosynthesis of plant sterols and alpha- and beta-amyrin. Biochem J. 1966 Jun;99(3):726–734. doi: 10.1042/bj0990726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMPSON K. L., NAKAYAMA T. O., CHICHESTER C. O. BIOSYNTHESIS OF YEAST CAROTENOIDS. J Bacteriol. 1964 Dec;88:1688–1694. doi: 10.1128/jb.88.6.1688-1694.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf S. S., Simpson K. L. Attempts to detect lycopersene formation in yeast. Biochem J. 1968 Jan;106(1):311–315. doi: 10.1042/bj1060311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless K. B., Snyder T. E., Spencer T. A., Maheshwari K. K., Nelson J. A. Biological demethylation of 4,4-dimethyl sterols, Evidence for enzymic epimerization of the 4beta-methyl group prior to its oxidative removal. J Am Chem Soc. 1969 Jun 4;91(12):3394–3396. doi: 10.1021/ja01040a065. [DOI] [PubMed] [Google Scholar]
- Subbarayan C., Kushwaha S. C., Suzue G., Porter J. W. Enzymatic conversion of isopentenyl pyrophosphate-4-14C and phytoene-14C to acyclic carotenes by an ammonium sulfate-precipitated spinach enzyme system. Arch Biochem Biophys. 1970 Apr;137(2):547–557. doi: 10.1016/0003-9861(70)90472-8. [DOI] [PubMed] [Google Scholar]
- Tefft R. E., Goodwin T. W., Simpson K. L. Aspects of the stereochemistry of torularhodin biosynthesis. Biochem J. 1970 May;117(5):921–927. doi: 10.1042/bj1170921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornber J. P., Olson J. M., Williams D. M., Clayton M. L. Isolation of the reaction center of Rhodopseudomonas viridis. Biochim Biophys Acta. 1969 Feb 25;172(2):351–354. doi: 10.1016/0005-2728(69)90083-8. [DOI] [PubMed] [Google Scholar]
- VOLK W. A., PENNINGTON D. The pigments of the photosynthetic bacterium Rhodomicrobium vannielii. J Bacteriol. 1950 Feb;59(2):169–170. doi: 10.1128/jb.59.2.169-170.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton T. J., Britton G., Goodwin T. W. Biosynthesis of xanthophylls in higher plants: stereochemistry of hydroxylation at C-3. Biochem J. 1969 Apr;112(3):383–385. doi: 10.1042/bj1120383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Britton G., Charlton J. M., Goodwin T. W. The stereospecific biosynthesis of phytoene and polyunsaturated carotenes. Biochem J. 1967 Sep;104(3):767–777. doi: 10.1042/bj1040767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. J., Britton G., Goodwin T. W. A possible mechanism for the biosynthesis of eschscholtzxanthin. Biochim Biophys Acta. 1966 Jul 27;124(1):200–203. doi: 10.1016/0304-4165(66)90333-3. [DOI] [PubMed] [Google Scholar]
- Williams R. J., Britton G., Goodwin T. W. The biosynthesis of cyclic carotenes. Biochem J. 1967 Oct;105(1):99–105. doi: 10.1042/bj1050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilton D. C., Munday K. A., Skinner S. J., Akhtar M. The biological conversion of 7-dehydrocholesterol into cholesterol and comments on the reduction of double bonds. Biochem J. 1968 Feb;106(4):803–810. doi: 10.1042/bj1060803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Aller R. T., Chikamatsu H., de Souza N. J., John J. P., Nes W. R. The metabolic role of the 24-ethylidenecholesterols. Biochem Biophys Res Commun. 1968 Jun 10;31(5):842–844. doi: 10.1016/0006-291x(68)90640-2. [DOI] [PubMed] [Google Scholar]



