Abstract
In the last years, major progress has occurred in heart failure (HF) management. The 2023 ESC focused update of the 2021 HF guidelines introduced new key recommendations based on the results of the last years of science. First, two drugs, sodium–glucose co‐transporter‐2 (SGLT2) inhibitors and finerenone, a novel nonsteroidal, selective mineralocorticoid receptor antagonist (MRA), are recommended for the prevention of HF in patients with diabetic chronic kidney disease (CKD). Second, SGLT2 inhibitors are now recommended for the treatment of HF across the entire left ventricular ejection fraction spectrum. The benefits of quadruple therapy in patients with HF with reduced ejection fraction (HFrEF) are well established. Its rapid and early up‐titration along with a close follow‐up with frequent clinical and laboratory re‐assessment after an episode of acute HF (the so‐called ‘high‐intensity care’ strategy) was associated with better outcomes in the STRONG‐HF trial. Patients experiencing an episode of worsening HF might require a fifth drug, vericiguat. In the STEP‐HFpEF‐DM and STEP‐HFpEF trials, semaglutide 2.4 mg once weekly administered for 1 year decreased body weight and significantly improved quality of life and the 6 min walk distance in obese patients with HF with preserved ejection fraction (HFpEF) with or without a history of diabetes. Further data on safety and efficacy, including also hard endpoints, are needed to support the addition of acetazolamide or hydrochlorothiazide to a standard diuretic regimen in patients hospitalized due to acute HF. In the meantime, PUSH‐AHF supported the use of natriuresis‐guided diuretic therapy. Further options and most recent evidence for the treatment of HF, including specific drugs for cardiomyopathies (i.e., mavacamten in hypertrophic cardiomyopathy and tafamidis in transthyretin cardiac amyloidosis), device therapies, cardiac contractility modulation and percutaneous treatment of valvulopathies, with the recent finding from the TRILUMINATE Pivotal trial, are also reviewed in this article.
Keywords: comorbidities, finerenone, heart failure, prevention, prognosis, SGLT2 inhibitors, treatment
Introduction
Heart failure (HF) is defined as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality, corroborated by elevated natriuretic peptides and/or objective evidence of pulmonary or systemic congestion. 1 It remains a leading global cause of mortality, morbidity and poor quality of life (QoL) with high use of resources and healthcare costs. 2 Therefore, it is an area of active research. 3 This article aims to highlight the most recent findings of the last years (Figure 1).
Figure 1.
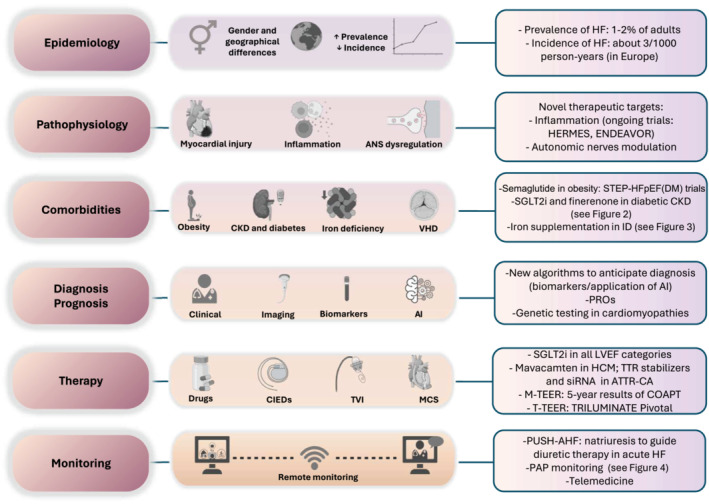
Main topics summarized in this review and key new evidence. AI, artificial intelligence; ANS, autonomic nervous system; ATTR‐CA, transthyretin cardiac amyloidosis; CIEDs, cardiac implantable electronic devices; CKD, chronic kidney disease; HCM, hypertrophic cardiomyopathy; HF, heart failure; ID, iron deficiency; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; M‐TEER, mitral transcatheter edge‐to‐edge repair; PAP, pulmonary artery pressure; PROs, patient‐reported outcomes; SGLT2i, sodium–glucose co‐transporter‐2 inhibitor; siRNA, small interference RNA; T‐TEER, tricuspid transcatheter edge‐to‐edge repair; TTR, transthyretin; TVI, transcatheter valve intervention; VHD, valvular heart disease.
Epidemiology
Although the incidence of HF slightly declined over time, its prevalence is increasing due to improved HF treatments and longer life expectancy in the population. 2 In European countries, the median incidence of HF was 3.20 cases per 1000 person‐years, and the median HF prevalence was 17.20 cases per 1000 people. 4 , 5
Significant geographical and socio‐demographic variations and different temporal trends in HF burden have been described. Among patients with an acute myocardial infarction (AMI) enrolled in the PARADISE‐MI trial, rates of incident HF varied almost six‐fold among regions, with the lowest rate in South Asia (1.0/100 person‐years) and the highest in Northern Europe (5.9/100 person‐years). 6
Although ischaemic heart disease remains one of the most common causes of HF, the improvements in the management of AMI and secondary prevention have reduced the risk of HF hospitalization (HFH) following the first AMI. 7
Gender differences
Gender differences in prevalence, pathophysiological pathways, HF phenotypes, rates of morbidity and mortality, as well as in treatment prescription, have been described. 8 , 9 , 10 Comparing the gene expression of 363 biomarkers, Ravera et al. observed distinct molecular patterns, underlying gender differences in patients with HF: biomarkers associated with lipid metabolic pathways were mainly observed in women, while biomarkers associated with neuro‐inflammatory response were more active in men. 11
In a retrospective study including 155 670 US patients hospitalized for HF from the GWTG‐HF Registry, females, when compared with males, had lower adjusted mortality but experienced significantly greater loss of survival time compared with the median US population matched for age and sex and had a higher risk of rehospitalization at 5 years. 12 In a pre‐specified secondary analysis of the GALACTIC trial, early intensive and sustained vasodilatation with rapid up‐titration of renin–angiotensin–aldosterone system (RAAS) inhibitors during acute HFH was less successful in women versus men. 10 On the other hand, in the STRONG‐HF trial, a similar average percentage of the optimal dose of guideline‐directed medical therapies (GDMTs) was reached in both sexes. Also, there was no significant treatment‐by‐sex interaction in the occurrence of the primary endpoint as well as in QoL improvement or in adverse events. 9
Gender differences were also described in a large population of patients with transthyretin (TTR) cardiac amyloidosis (ATTR‐CA) referred to the UK National Amyloidosis Centre (NAC). 13 Non‐indexed wall thickness measurements may have contributed to both under‐representation and delays in diagnosis for affected females. Aimo et al. confirmed in a different cohort of ATTR‐CA patients that interventricular septum thickness and posterior wall thickness were smaller in women than men; therefore, the use of lower cut‐off values in women or indexed echocardiographic parameters has been proposed for a more accurate assessment at diagnosis and for disease prognostic stratification. 14
Women are still under‐represented in HF clinical trials. 15
Pathophysiology
Cardiac injury can lead to HF. 16 Packer reviewed the intrinsic molecular pathways of cardiac injury, during which the heart recapitulates the foetal signalling programme, which (although advantageous in the short term) is highly deleterious if sustained for long; these changes lead to a marked increase in protein O‐GlcNAcylation that is associated with impaired calcium kinetics, contractile derangements, mitochondrial dysfunction, fibrosis and maladaptive hypertrophy. 17 Clonal haematopoiesis of indeterminate potential was associated with biomarkers and risk factors of HF as well as with incident HF in patients aged under 65 years. 18
Inflammation
Inflammation plays a central role in HF pathophysiology. 19 , 20 , 21 , 22 , 23 Twenty‐four inflammatory biomarkers were collected in 1231 patients from the CASABLANCA study. These patients were stratified into three levels of inflammation (low, medium and high). The high inflammation group was at increased adjusted risk of HF events across all the stages of HF. 24
Among unselected patients presenting to the emergency department with acute dyspnoea, those diagnosed with acute HF had higher interleukin‐6 (IL‐6) concentrations. IL‐6 was elevated (>4.45 ng/L) in 83.7% of acute HF patients and was a strong and independent predictor of 1 year mortality. 25 A double‐blind, randomized placebo‐controlled trial with a human monoclonal antibody directed against the IL‐6 ligand (ziltivekimab) in patients with HF and left ventricular (LV) ejection fraction (LVEF) ≥40% is ongoing (HERMES trial, NCT05636176).
Seven neutrophil activity‐related plasma proteins have been associated with the risk of incident HF and with adverse cardiac remodelling. 26 ENDEAVOR is a combined, seamless phase 2b–3 study investigating the efficacy and safety of mitiperstat, a novel selective myeloperoxidase inhibitor, in patients with HF with mildly reduced ejection fraction (HFmrEF) or with preserved ejection fraction (HFpEF). 27
Of note, levels of circulating immune checkpoint ligands are increased in HF patients and correlate with disease severity or prognosis. These data underscore the involvement of adaptive immune response in the pathophysiology of HF. 28
Autonomic nervous system
A further driver of HF progression is autonomic nervous system dysregulation. Volume recruitment from the splanchnic compartment is a physiological response to stressors such as physical activity and blood loss. Recently, the regulation of sympathetic stimulation through splanchnic nerve modulation has become a target of interventions. 29 , 30 Analogically, the modulation of pulmonary artery (PA) autonomic nerves may help rebalance the pulmonary pressure in selected patients. 31
Badrov et al. assessed determinants of augmented muscle sympathetic nerve activity (MSNA) in 177 patients with HF versus 658 healthy volunteers. MSNA was higher among HF patients, especially in men with ischaemic heart disease and with sleep apnoea; burst frequency was directly associated with norepinephrine and peripheral vascular resistance and inversely associated with stroke volume, cardiac output and peak oxygen consumption. 32 However, this sympathetic overdrive was detected only in a subgroup of patients with HF (ranging from 32% to 51%); therefore, a selective setup of sympatho‐modulatory interventions should be implemented in HF.
Comorbidities
Patients with HF often suffer from several comorbidities that may affect their health status, management and outcome and are themselves therapeutic targets, particularly in HFpEF. 33 , 34 , 35 , 36 Of the 91 463 patients enrolled in the Swedish HF Registry (median age 76 years), 98% had at least one among the 17 explored comorbidities [94% at least one cardiovascular (CV) and 85% at least one non‐CV comorbidity]. All comorbidities, except for coronary artery disease (CAD), were more frequent in HFpEF. 36 Among patients with HF with reduced ejection fraction (HFrEF) from the PARADIGM‐HF and ATMOSPHERE trials, patients with coexistent peripheral artery disease (PAD) and stroke were at greatest individual risk for all‐cause death, whereas coexistent chronic kidney disease (CKD) and hypertension displayed the highest population attributable fractions and, thus, mattered most from a population perspective. 37
Hypertension
Hypertension is a major cause and a common comorbidity of HF. Pugliese et al. investigated the haemodynamic and prognostic correlates of hypertensive response to exercise. A steeper systolic blood pressure/workload slope was associated with impaired functional capacity across the HF spectrum and could be a more sensitive predictor of adverse events than absolute systolic blood pressure values, mainly in patients in stages A and B and HFpEF. 38
Pharmacological or surgical modulation of dysfunctional chemoreceptors is emerging as a potential therapeutic option for patients with hypertension and HF. A recent review summarized the chemoreflex physiology and pathophysiology and its correlation with ventilation and sympathetic drive, focusing on the importance of careful selection of patients that would benefit the most from chemoreflex modulation strategies. 39
Diabetes and kidney dysfunction
Diabetic cardiomyopathy is a form of stage B HF at high risk for progression to overt disease. The ARISE‐HF is a phase 3 randomized, placebo‐controlled, double‐blind clinical study to investigate the efficacy of a novel investigational highly specific aldose reductase inhibitor in patients with diabetic cardiomyopathy at high risk of progression to overt HF. 40
Diabetic kidney disease is also a crucial risk factor for developing HF. 41 , 42 , 43 Sodium–glucose co‐transporter‐2 (SGLT2) inhibitors and finerenone, a nonsteroidal and selective mineralocorticoid receptor antagonist (MRA), are now recommended [Class of Recommendation (CoR) I, Level of Evidence (LoE) A] for the prevention of HF in patients with type 2 diabetes mellitus (T2DM) and CKD (Figure 2). 44 SGLT2 inhibitors also reduced CV mortality in these patients. The indication for SGLT2 inhibitors is based on the results of the DAPA‐CKD and EMPA‐KIDNEY trials and a subsequent meta‐analysis of 13 major randomized controlled trials (RCTs), including also CREDENCE, SCORED and HF trials. 45 , 46 FIDELIO‐DKD and FIGARO‐DKD trials and a pre‐specified individual patient‐level, pooled analysis of these two trials (FIDELITY pooled analysis) demonstrated the benefits of finerenone on CV and kidney outcomes versus placebo across the entire spectrum of CKD in patients with T2DM (Figure 2). 47 , 48 , 49 , 50
Figure 2.

Cardiovascular (CV) and kidney outcomes with sodium–glucose co‐transporter‐2 inhibitor (SGLT2i) and finerenone in patients with chronic kidney disease (CKD). ACR, albumin‐to‐creatinine ratio; CI, confidence interval; eGFR, estimated glomerular filtration rate; ESKD, end‐stage kidney disease; HF, heart failure; HFH, heart failure hospitalization; HR, hazard ratio; MI, myocardial infarction; RR, rate ratio; T2DM, type 2 diabetes mellitus. *ESKD, sustained decrease in eGFR to <10 mL/min/1.73 m2, sustained decrease in eGFR of ≥40% from baseline or death from renal causes.
Coronary artery disease (CAD)
Among patients hospitalized due to acute decompensated HF, obstructive CAD was more prevalent in HFrEF than in HFpEF. 51 The role of percutaneous or surgical coronary revascularization remains uncertain in patients with HFrEF and chronic coronary syndromes. 4 , 52 Percutaneous coronary intervention (PCI) was not superior in reducing the incidence of death from any cause or HFH compared with GDMT alone in the REVIVED‐BCIS2 trial. 52 Iaconelli et al. conducted a meta‐analysis of five RCTs (some of them not blinded) with a total of 2842 patients to investigate the effects of coronary revascularization on morbidity and mortality in patients with chronic HF due to CAD. Compared with GDMT alone, coronary revascularization was associated with a lower risk of all‐cause mortality [hazard ratio (HR) 0.88, 95% confidence interval (CI) 0.79–0.99] and CV mortality (HR 0.80, 95% CI 0.70–0.93) but not the composite of hospitalization for HF or all‐cause mortality. 53
Aortic valve disease
Of the 15 216 patients from the European Society of Cardiology (ESC) Heart Failure Association (HFA) EURObservational Research Programme (EORP) Heart Failure Long‐Term Registry, ~10% had aortic valve disease (AVD), with a higher prevalence in HFpEF. Severe aortic stenosis (AS), but not severe aortic regurgitation (AR), was independently associated with an increased risk of CV death and HFH, regardless of LVEF. 54 Both patients with HFpEF and severe AS had impaired functional capacity with similarly reduced peak oxygen consumption, peak cardiac output and peak arteriovenous oxygen. 55 Novel data on the 5 year outcomes of patients with severe symptomatic AS at low surgical risk undergoing transcatheter aortic valve replacement (TAVR) as compared with those undergoing surgery have been published. 56
Atrial fibrillation
Atrial fibrillation (AF) can coexist, cause or exacerbate HF. 57 , 58 AF transcatheter ablation in HF is currently reserved for patients with arrhythmia‐induced cardiomyopathy and those in whom the worsening HF symptoms are clearly related to AF. 4 , 59 , 60 Several ongoing studies might expand the latter indication.
Real‐world data from the Swedish Heart Failure Registry showed that first‐time catheter ablation for AF was associated with a lower risk of all‐cause mortality or first HFH compared with medical therapy alone, regardless of LVEF; in HFpEF patients, catheter ablation also resulted in a reduction of recurrent HFH. 61 The ARC‐HF and CAMTAF randomized trials compared early routine catheter ablation and pharmacological rate control in patients with persistent AF and HF; after trial completion, delayed selective catheter ablation was performed when clinically indicated in the rate control group. No differences in long‐term outcomes were reported between the early and delayed catheter ablation groups. However, the early catheter ablation group showed greater symptom improvement compared with the rate control group. Furthermore, analyses according to received treatment suggested an association between catheter ablation and improved outcomes as compared with rate control. 62 The single‐centre, open‐label CASTLE‐HTx trial showed that catheter ablation was safe and effective even in patients with symptomatic AF and end‐stage HF (patients referred for heart transplantation evaluation) with a significant reduction in the primary endpoint of death from any cause, implantation of an LV assist device (LVAD) or urgent heart transplantation. 63
Pulmonary hypertension
Quality indicators for the assessment of care and outcomes of adults with pulmonary hypertension (PH) were developed by ESC. 64 Treatment of PH has improved dramatically in the last years. 65 In a post hoc analysis of the GRIPHON study, the selective prostacyclin receptor agonist selexipag reduced morbidity and mortality versus placebo regardless of concomitant CV comorbidities. 66 Tadalafil did not improve right ventricular (RV) systolic function in adults with congenital heart disease and systemic right ventricles (SERVE trial). 67
In HF patients, PH is mainly present in isolated post‐capillary form because of volume and/or pressure overload; however, chronic isolated post‐capillary can lead to vascular remodelling, resulting in the development of combined post‐capillary and pre‐capillary forms; in addition, pre‐capillary forms due to pulmonary arterial hypertension (PAH) can coexist. 4
The Sildenafil in Heart Failure (SilHF) trial randomly assigned patients with HFrEF and PA systolic pressure (PASP) ≥40 mmHg measured by echocardiography in a 2:1 ratio to receive sildenafil (up to 40 mg three times/day) or placebo. Compared with placebo, sildenafil did not improve symptoms, QoL or exercise capacity. 68 The SPHERE‐HF trial investigated the effect of mirabegron (a selective β3 adrenoreceptor agonist) on patients with left heart disease and combined post‐capillary and pre‐capillary PH compared with placebo. The primary outcome of reduction of pulmonary vascular resistance (PVR) was not met, even if mirabegron showed a significant improvement in RV ejection fraction (EF) (secondary endpoint). 69
Cancer
New guidelines on cardio‐oncology and management of cardiotoxicity were published in 2022. 70 Nouhravesh et al. examined the 1 year prognosis following new‐onset HF stratified by cancer status in patients with breast, gastrointestinal or lung cancer. In total, 193 359 Danish patients with HF were included. Cancer status was categorized as history of cancer (no cancer‐related contact within 5 years of HF diagnosis), non‐active cancer (curative intended procedure administered) and active cancer. Standardized 1 year all‐cause mortality was comparable for patients with a history of cancer and non‐active cancer regardless of cancer type but varied comprehensively for active cancers. 71 Tomasoni et al. found that a history of cancer (within 5 years) was associated with a higher independent risk of all‐cause and non‐CV mortality but not with CV mortality. 36 The results were consistent with a previous analysis by Dobbin et al. 72 Age‐ and sex‐adjusted incidence of new cancer in the HFrEF and HFpEF trials was 1.09 (95% CI 0.83–1.36) and 1.07 (95% CI 0.81–1.32) per 100 person‐years, respectively. 72
Anaemia and iron deficiency
The prognostic significance of anaemia and iron deficiency (ID) is well known. 73 Intravenous (IV) iron supplementation has proven to alleviate HF symptoms, improve QoL and exercise capacity, and reduce the risk of HFH in patients with HFrEF/HFmrEF and ID. Secondary analyses of the AFFIRM‐AHF trial showed similar benefits with IV ferric carboxymaltose (FCM) compared with placebo regardless of baseline haemoglobin levels 74 and slightly greater effectiveness in patients with ischaemic compared with non‐ischaemic aetiology. 75 Furthermore, in a pre‐defined analysis of the IRON‐CRT trial, treatment with IV FCM was associated with improvement in RV function 76 ; an improvement in hypercapnic ventilatory response and sleep‐related breathing disorders was also observed. 77 The HEART‐FID double‐blind, randomized trial enrolled 3065 ambulatory patients with symptomatic HFrEF [New York Heart Association (NYHA) II–IV], ID, and a recent HFH or elevated natriuretic peptide levels. Patients were randomized to FCM or placebo. The unmatched win ratio for the hierarchical composite of death, HFH or change from baseline in the 6 min walk distance was 1.10 (99% CI 0.99–1.23) (Figure 3). 78 In the pre‐specified analysis of the IRONMAN trial censoring follow‐up on September 2020 due to coronavirus disease 2019 (COVID‐19) pandemic, IV ferric derisomaltose showed a significant reduction of the primary outcome of recurrent HFH and CV death. 79 Meta‐analyses of RCTs comparing IV iron supplementation with placebo confirmed a significant reduction of HFH without, however, a benefit on CV or all‐cause mortality. 80 , 81 , 82 , 83 Based on these data, the 2023 ESC focus update of HF guidelines recommended IV iron supplementation in symptomatic HFrEF or HFmrEF patients with ID to alleviate symptoms and improve QoL (Class I, Level A) or to reduce the risk of HFH (Class IIa, Level A) (Figure 3). 44 The concern regarding IV FCM and its potential association with hypophosphataemia continues to be a topic of ongoing debate and has recently come under scrutiny once more. 84
Figure 3.

Novel evidence and indications for the treatment of iron deficiency. 6MWT, 6 min walk test; CI, confidence interval; CoR, Class of Recommendation; CV, cardiovascular; FCM, ferric carboxymaltose; FDM, ferric derisomaltose; HF, heart failure; HFH, heart failure hospitalization; HFmrEF, heart failure with mildly reduced ejection fraction; HFrEF, heart failure with reduced ejection fraction; ID, iron deficiency; LoE, Level of Evidence; LVEF, left ventricular ejection fraction; PROBE, prospective, randomized, open‐label, blinded endpoint; QoL, quality of life; RCTs, randomized controlled trials; RR, rate ratio.
Despite the strong recommendation by the guidelines and the cost‐effective analysis showing a positive economic impact on healthcare systems, 4 , 44 , 85 ID screening and FCM treatment are still underused in clinical practice. 86
The use of SGLT2 inhibitors has been associated with an increase in haemoglobin and haematocrit levels, even in patients with ID. This benefit might result mainly from an anti‐inflammatory effect and a reduction in oxidative stress, which results in a reduction in hepcidin, thus promoting the mobilization of iron from intracellular stores and an increase in erythropoietin. 87 , 88 A retrospective, single‐centre analysis among 160 HFrEF patients showed a greater increase in haemoglobin and haematocrit with IV iron and SGLT2 inhibitors combined treatment compared with IV iron only. 89
Infections and COVID‐19
The COVID‐19 pandemic had enormous consequences on the global healthcare system because of complications of the infection itself, including CV complications, but also because of reduced access to hospitals by patients. 90 , 91 , 92 , 93 , 94 , 95 , 96 From January 2019 to December 2021, there were fewer hospitalizations, diagnostic and interventional procedures and outpatient consultations across all CV diseases. The COVID‐19 pandemic also had a major impact on clinical trials with reduced enrolment and missed visits at follow‐up. 97 The DELIVER trial was one of the most affected, with >75% of follow‐up time occurring during the pandemic; nevertheless, treatment benefits of dapagliflozin persisted when censoring at COVID‐19 diagnosis and pandemic onset. 98
COVID‐19 vaccination is indicated in all patients with HF, including frail or heart transplant patients. 94 , 99 Although generally safe, rare post‐vaccine myocarditis was observed with mostly mild and transient forms and greater involvement of the female sex. 100 Mid‐term follow‐up with cardiac magnetic resonance showed that patients who experienced acute myocarditis after the mRNA COVID‐19 vaccine had generally preserved biventricular function. 101
Diagnosis and prognosis
Early diagnosis
The HFA of the ESC developed a consensus statement addressed to non‐cardiology physicians to facilitate the early diagnosis of HF, including screening through the measurement of natriuretic peptides. 102 By making this simple laboratory test readily available, there is significant potential to improve the early diagnosis of HF, resulting in better patient outcomes and reduced healthcare costs. 103 In addition, because brain natriuretic peptide (BNP) and N‐terminal pro‐BNP (NT‐proBNP) levels are lower in obese patients, adjusting NT‐proBNP concentrations in such patients seems to further increase its clinical utility in the rapid detection of HF. 104 Practical algorithms for early diagnosis of HF using NT‐proBNP, with different cut‐offs depending on patient characteristics and diagnostic likelihood, have been recently published by HFA of ESC. 105 Interestingly, results of standard 12‐lead electrocardiograms, when analysed by a deep learning machine learning (ML) process, might also prevent underdiagnosing of HF or cardiomyopathies. 106 Nevertheless, this novel method still requires further verification. 107
Clinical assessment
Signs and symptoms of HF include elevated jugular venous pressure, hepatojugular reflux, peripheral oedema, breathlessness, orthopnoea, reduced exercise tolerance and fatigue. 4
Bendopnoea is related to advanced HF, but its prognostic significance remains uncertain. Including 440 patients with advanced HF, de la Espriella et al. showed that a reduction of more than 3% in oxygen saturation when bending forward was associated with the risk of worsening HF compared with those with no change or improvement in oxygen saturation when bending. 108
QoL and health‐related QoL (HRQL) are among the endpoints for clinical trials and are influenced by multiple variables 109 , 110 , 111 ; symptom severity was the main determinant of HRQL rather than social factors such as country income level. 112
Analysing the prognostic implications of longitudinal NYHA class changes (i.e., stable, improving or worsening) in 13 535 patients from the Swedish HF Registry, Lindberg et al. showed that a single‐point assessment of NYHA class itself predicted morbidity and mortality on top of its trajectory, suggesting that the one‐time NYHA class assessment might be the preferable approach for clinical trials' design and in clinical practice. 113
Patient‐reported outcomes (PROs) are relevant outcomes that directly assess the patient's experience, health behaviours, and the impact of the disease and its treatment on the patient's health status. PROs can be assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ) or with other questionnaires. The methodology and use of PROs need to be standardized and implemented in clinical practice. 114 , 115
Biomarkers
Biomarkers remain a milestone for the diagnosis, management and prognosis of HF. 103 , 116 , 117 , 118 In patients with AF, higher baseline and increasing or persistently elevated values of NT‐proBNP, high‐sensitivity troponin T (hs‐TnT) and growth differentiation factor‐15 (GDF‐15) over 1 year were associated with higher risk of HF outcomes regardless of HF history or LVEF. 119 Prognostic models in chronic HFpEF, based on NT‐proBNP and hs‐TnT, along with a few readily available clinical variables, provided effective risk discrimination for both morbidity and mortality. 120 Among 1559 HF patients from the PARADIGM‐HF trial, McDowell et al. examined whether 11 biomarkers, individually or collectively, improved the performance of the PREDICT‐HF prognostic model, which includes clinical, routine laboratory and BNP data. None of the studied biomarkers (including urinary albumin‐to‐creatinine ratio, hs‐TnT and aldosterone) led to a meaningful improvement in the prediction of outcomes. 121
Biomarkers are also used as inclusion criteria and surrogate or safety endpoints in clinical trials. 122 , 123 , 124
MicroRNAs (miRNAs), small circulating non‐coding RNAs, might have the potential to rule out HF or differentiate HF phenotypes. 125 , 126 , 127 Moreover, proteomic signatures of circulating plasma proteins may also aid our understanding of HF‐specific signalling, and thereby, they can support new therapeutic and diagnostic efforts for chronic HF. 128
Imaging
Multimodality imaging is a key tool for diagnosis, identification of the cause, proper management and monitoring of therapeutic response in HF. 4 , 129 , 130 Global longitudinal strain (GLS) is a reproducible and well‐validated echocardiographic parameter that presents a high prognostic value, even higher than LVEF (especially in patients with LVEF > 45%). 131 In a retrospective cohort study including 311 patients with HFpEF, abnormal GLS was a strong predictor for clinical events and future deterioration in LVEF. 132 Left atrial (LA) compliance (ratio of LA reservoir strain to E/e′) during exercise versus resting LA compliance or exercise E/e′ ratio alone showed superior diagnostic ability in HFpEF patients. 133 Increased LA volume was associated with PVR, and reduced LA function was associated with a disrupted PVR–compliance relationship. 134 Furthermore, LA remodelling and dysfunction provided important prognostic information. 135 Changes in the LA dimension (positive or adverse remodelling) may be a useful marker of response to GDMT and cardiac resynchronization therapy (CRT).
The DAPA‐MODA trial, a multicentre, single‐arm, open‐label, prospective and interventional study, evaluated the effect of dapagliflozin on cardiac remodelling parameters [LA volume index (LAVI) and LV geometry] over 6 months among a total of 162 patients with HF and LVEF > 40%. Dapagliflozin administration was associated with a significant reduction of LA dimension and improvement of LV geometry (reduced LV mass index, end‐diastolic volume and end‐systolic volume) in addition to a significant reduction in natriuretic peptide concentrations. 136
Among 625 patients with de novo HF, approximately one third had RV dysfunction (RVD), defined as tricuspid annular plane systolic excursion (TAPSE) <17 mm; during up‐titration of GDMT, RVD recovery occurred in 49% of the patients and was associated with improved clinical outcomes. 137 In patients with HFrEF and secondary mitral regurgitation (SMR), ~40% of patients improved RV function after percutaneous mitral valve repair and RV function improvement was associated with better long‐term survival free from heart transplantation and a lower risk of HFH. 138
Ultrasound monitoring of congestion during HFH through inferior vena cava (IVC) diameter, jugular vein distensibility ratio or number of B‐lines at the lung is widespread in clinical practice. 139 , 140 Pre‐discharge assessment of residual subclinical (echocardiographic) congestion is recommended. 140 , 141
Machine Learning
Artificial intelligence (AI) is used to create mathematical algorithms to better assess and cross‐reference large patient data. 142 , 143 , 144 Khan et al. summarized the applications of machine learning (ML) techniques in the field of HF. 145 ML‐derived risk models have been proposed for takotsubo syndrome 146 and for Asian patients hospitalized for acute HF. 147 A secondary analysis of BLUSHED‐AHF showed that there was a good agreement in B‐line quantification between an AI/ML automated lung US congestion score and expert‐level assessment. 148
Specific causes of HF
Cardiomyopathies
Cardiomyopathies represent an important and heterogeneous cause of HF. 149 , 150 The 2023 ESC guidelines introduced non‐dilated LV cardiomyopathy as a new phenotype of cardiomyopathies, which includes non‐ischaemic LV scarring or fatty replacement in the absence of LV dilatation or isolated global LV hypokinesia without scarring. 151 Two recent documents summarized the optimal management, new advances and future possible therapeutic targets in the treatment of cardiomyopathies. 152 , 153 Sudden cardiac death (SCD) remains a significant cause of mortality in cardiomyopathies, with an incidence of 0.15%–0.7% per year, highlighting the importance of a thorough risk assessment. 154 , 155
Dilated cardiomyopathy
Genetic testing is crucial for diagnosis, prognosis, arrhythmic risk assessment and therapeutic choice, as well as for providing important information for reproductive counselling in patients with cardiomyopathies. 151 , 156
Of 1412 HFrEF patients from the PARADIGM‐HF trial with whole‐exome sequence data, 4.8% had at least one rare predicted loss‐of‐function variant. These patients were younger, had lower LVEF and had a less likely ischaemic aetiology. 157 Among individuals with dilated cardiomyopathy (DCM) and CAD, the presence of rare pathogenic variants in DCM genes was associated with an increased risk of death or major adverse cardiac events. 158
Hypertrophic cardiomyopathy
Mavacamten (cardiac myosin adenosine triphosphatase inhibitor) is now recommended as the second choice in patients with hypertrophic cardiomyopathy (HCM) and symptomatic LV outflow tract (LVOT) obstruction (LVOTO) after beta‐blockers and/or calcium channel blockers (verapamil or diltiazem). 151 , 159 , 160 , 161 The cross‐over VALOR‐HCM trial confirmed the efficacy of mavacamten in patients with HCM and symptomatic LVOTO, with sustained improvements in LVOT gradients and symptoms leading to a significant reduction in the need for septal reduction therapy at Week 56. 162 A secondary analysis of the EXPLORER‐HCM trial showed improvement in several parameters at the cardiopulmonary exercise testing including peak oxygen uptake with mavacamten compared with placebo. 163 In addition, subgroup analysis of the EXPLORER‐HCM and MAVA‐LTE studies showed that mavacamten benefits were reproduced and maintained regardless of beta‐blocker use. 164 Future studies might also illuminate how far myosin inhibitors affect intracellular signalling and myocardial remodelling in HCM hearts. 165 The real‐world candidacy to mavacamten in a contemporary hypertrophic obstructive cardiomyopathy population has been explored. 166
In symptomatic non‐obstructive HCM, the novel ninerafaxstat, a drug targeting myocardial energetics, was safe and well tolerated and associated with better exercise performance and health status among those with lower KCCQ at baseline. 167
Peripartum cardiomyopathy (PPCM)
PPCM has a major impact on maternal morbidity and mortality during pregnancy. 151 , 168 , 169 Novel epidemiological data were published from the large ESC EORP PPCM Registry. Among 535 women with PPCM, 1 year all‐cause death, first hospitalization and recurrent rehospitalizations occurred in 8.4%, 14% and 3.5%, respectively. 170 Thrombo‐embolism and stroke at the time of PPCM diagnosis were reported in 5.5% and 1.1%, respectively. 171
Cardiac amyloidosis
The real prevalence of cardiac amyloidosis (CA) in the general population is still unknown because of underdiagnosis. 172 A higher prevalence of CA was reported among patients with unexplained LV hypertrophy (LVH) or suspected HCM and HFpEF and in the elderly with AS. 173 , 174 The most common forms of CA are immunoglobulin light chain (AL‐) and wild‐type (wt) or hereditary (h) TTR (ATTR‐) CA, even if more rare forms exist as well. 175
In a small cohort of 300 patients affected by ATTR‐CA, the hereditary form was detected in 12% of the entire population and in 5.3% of patients aged ≥70 years. Hereditary ATTR‐CA (ATTRh‐CA) was more frequent in females. 13 Among 2029 patients aged ≥70 years with ATTR‐CA from the UK NAC, up to 20.7% had a pathogenic TTR variant whose presence was associated with increased risk of all‐cause mortality, especially when related to the V122I mutation. 176 These data support routine genetic sequencing in all patients with ATTR‐CA regardless of age.
Disproportionately elevated levels of natriuretic peptide and troponin are characteristics of patients with CA. Thus, cardiac biomarkers might refine the diagnostic algorithm. Vergaro et al., analysing 1149 patients with suspected CA, found NT‐proBNP 180 ng/L and hs‐TnT 14 ng/L as optimal cut‐off to rule out the diagnosis of CA. 177
Several independent prognostic factors have been identified in patients with CA. 178 , 179 RV–PA coupling predicted the risk of mortality or HFH. The TAPSE/PASP ratio was more effective than TAPSE or PASP alone in predicting prognosis. 180 Prevalence, aetiologies and prognostic impact of moderate‐to‐severe mitral regurgitation (MR) and tricuspid regurgitation (TR) in patients with CA have been reported. The most common aetiologies were atrial functional MR, followed by primary infiltrative MR and secondary TR due to RV overload followed by atrial functional TR. 181 Combined moderate‐to‐severe MR and TR and isolated moderate‐to‐severe TR but not isolated MR have been associated with an increased independent risk of all‐cause death or worsening HF events. 181 Also, worsening of MR and TR at 12 and 24 months was independently associated with a worse prognosis. 182 Patients with the V122I mutation showed a more rapid decline in structural and functional echocardiographic parameters compared with both wild‐type and T60A ATTR‐CA. 182 Atrial amyloidosis is an early manifestation of CA and could be found even in the absence of systemic disease and ventricular involvement, being able to cause AF and thrombo‐embolic events. 183 , 184
Great progress has been made in the treatment of CA. 185 Tafamidis, a stabilizer of the native TTR tetramer structure, is recommended for the treatment of patients with ATTR‐CA and NYHA Class I or II. 4 Also, in patients with severe HF symptoms (NYHA III), it was observed a reduction of all‐cause mortality with continuous tafamidis treatment compared with delayed tafamidis treatment (placebo then tafamidis) over a median follow‐up of 5 years. 186 , 187 The ATTRibute‐CM trial demonstrated that another stabilizer of TTR tetramer, acoramidis, reduced all‐cause mortality and CV hospitalizations in addition to improving functional capacity and QoL, compared with placebo. 188 An analysis of the APOLLO study showed that patisiran (RNA interference therapeutic that inhibits hepatic synthesis of TTR) may delay the progression of LV chamber dysfunction after 9 months of therapy. 189 The APOLLO‐B trial enrolled 360 patients with ATTR‐CA (variant or wild‐type) and a history of HF with the aim to investigate the effect of patisiran versus placebo on functional capacity and QoL at 1 year follow‐up. Patisiran reduced the decline in the 6 min walk test distance and improved the KCCQ Overall Summary score (KCCQ‐OSS); significant benefits were not observed for the secondary composite endpoint of all‐cause death, CV events and change from baseline in the 6 min walk test distance. 190 These new data, as well as data from the HELIOS‐B trial, might lead to changes in the indications for small interfering RNA (patisiran and vutrisiran) that are currently approved for hereditary TTR amyloidosis with polyneuropathy only. 185
Myocarditis
Acute myocarditis is a possible cause of ventricular dysfunction and a risk condition for malignant arrhythmias; viral infection is the most common aetiology, although viral identification is often not easy without an endomyocardial biopsy. 191 Some rare cases of myocarditis have been associated with COVID‐19 vaccination 192 ; a clinical consensus document of the HFA summarized incidence, diagnosis, pathophysiology and therapy for COVID‐19 vaccination‐related myocarditis. 193
Anakinra (a recombinant non‐glycosylated form of human interleukin‐1 receptor antagonist) showed a neutral effect on the risk of complications in patients with low‐risk acute myocarditis in the ARAMIS trial. 194
Treatment of HFrEF
Pharmacological therapies
Medical therapy has changed the prognosis of patients with HFrEF. 3 , 4 , 44 The four pillars for HFrEF treatment include beta‐blockers, angiotensin‐converting enzyme inhibitors (ACEis)/angiotensin receptor–neprilysin inhibitor (ARNI) sacubitril/valsartan or angiotensin receptor blockers (ARBs), MRAs and SGLT2 inhibitors. 4 Early benefits of GDMT support its early initiation. 195 , 196 Despite several strategies proposed to implement GDMT, 197 , 198 rates of prescription and titration remain suboptimal. 199 , 200 , 201 , 202 , 203 , 204
Several factors may influence the prescription of GDMT. First, significant differences were found in the management and implementation of therapy for HF between HF specialists and non‐specialists, supporting the idea that specific courses may improve physicians knowledge and ultimately benefit patients. 205 Also, pharmaco‐disparities must be addressed to improve HFrEF outcomes globally; indeed, despite higher prices in high‐income countries, GDMT was more accessible and affordable than in low‐ and middle‐income countries. 206
Obesity was independently associated with a higher prescription of each treatment and the achievement of the target dose. 207
CKD and hyperkalaemia are often advocated as reasons for under‐prescription of RAAS inhibitors. 204 , 208 , 209 , 210 , 211 Among the 31 668 patients with HFrEF, comorbid CKD was associated with lower rates of evidence‐based therapy prescription. However, low rates of prescription were observed even in categories of estimated glomerular filtration rate (eGFR) where these therapies are recommended and have demonstrated benefit. 212 Guidetti et al. showed the safety of MRAs in patients with severe CKD. 213 In a pre‐specified pooled analysis of PARADIGM‐HF and PARAGON‐HF trials, sacubitril/valsartan reduced the risk of serious adverse renal outcomes regardless of baseline renal function compared with valsartan or enalapril. 214 Post hoc analysis of EMPHASIS‐HF and TOPCAT Americas region trials showed an acute slight decline in eGFR (−2.4 and −2.0 mL/min/1.73 m2, respectively) after MRA initiation, and then stable eGFR values during follow‐up were described. 215 Moreover, a retrospective study using data from the Taiwan National Health Insurance Research Database (NHIRD) found a reduction of CV and all‐cause mortality with an MRA also in patients with HF and end‐stage renal disease starting maintenance dialysis. 216
Potassium binders represent a novel opportunity for enabling GDMT up‐titration. 217 , 218
SGLT2 inhibitors
SGLT2 inhibitors are now established as safe and effective drugs for the treatment of HF across the entire spectrum of LVEF. 4 , 44 , 219 , 220 A comprehensive meta‐analysis of five main trials with SGLT2 inhibitors in HF (DAPA‐HF, DELIVER, EMPEROR‐Reduced, EMPEROR‐Preserved and SOLOIST‐WHF) proved statistically significant reductions in HFH by 28%, CV death by 13% and all‐cause mortality by 8%. 220 Secondary analysis of SGLT2 inhibitors trials in HF showed a clinical benefit regardless of age, 221 aetiology of HF, body mass index (BMI), liver and renal function, 222 , 223 AF, 224 background therapy 225 and severity of HFH. 226 The DAPA‐VO2 trial enrolled 90 stable patients with HFrEF and showed a significant increase of peak VO2 at 3 months with dapagliflozin compared with placebo. 227
The use of SGLT2 inhibitors was not associated with a clinically relevant risk of hypotension, volume depletion or renal adverse events. 228 , 229 A mild decrease in eGFR rate may be expected in the first period after the initiation of SGLT2 inhibitors, without increasing the risk of short‐ or long‐term HF, mortality or kidney injury events. 223 , 230
Recent evidence in patients with acute HF or with a recent hospitalization due to worsening HF, 223 , 231 , 232 , 233 as well as the safety profile and tolerability, supports the early initiation of SGLT2 inhibitors in both ambulatory and in‐hospital settings as first‐line therapy. 196 However, a survey involving 615 cardiologists worldwide showed that the ‘historical sequential’ approach (ACEi, beta‐blockers, MRA and, lastly, SGLT2 inhibitors) remains more popular than the initiation of SGLT2 inhibitors as first‐line therapy. 234
An analysis of patients registered in the Swedish HF Registry with eligibility characteristics for SGLT2 inhibitors demonstrated a three‐fold increase in their use between 1 November 2020 and 5 August 2022. However, more than 4 in 10 eligible patients remained without therapy. Discontinuation rates at 6 and 12 months were 13.1% and 20.0%, respectively. 235
HF therapies after acute myocardial infarction
The superiority of sacubitril/valsartan compared with ramipril among high‐risk survivors of AMI is still debated. 236
One of the mechanisms of action for the benefits of MRA in HF patients is the positive remodelling through the antifibrotic effect. A reduction in serum procollagen type I C‐terminal propeptide (PICP, a biomarker of cardiac fibrosis) concentration was found following the administration of spironolactone in a population at risk of HF in the HOMAGE (Heart ‘Omics’ in AGEing) trial 237 ; furthermore, a decrease in PICP with spironolactone was correlated with improved diastolic dysfunction as assessed by E/e′. 238 In the REMI study that enrolled 119 patients with a fist acute ST‐elevation myocardial infarction (STEMI), Monzo et al. showed higher post‐STEMI aldosterone concentration correlating with more adverse LV remodelling even in the subgroup of patients with LVEF > 40%. 239
The impact of SGLT2 inhibitors on patients after AMI is still debated. In the DAPA‐MI trial, among 4017 patients with AMI (67% with LVEF between 30% and 49%) and without a history of T2DM or chronic HF, dapagliflozin showed better cardiometabolic outcomes (reduced onset of T2DM and weight loss) compared with placebo but did not impact major outcomes (CV death or HFH). 240
Similarly, among 6522 patients after AMI (78.3% with LVEF < 45% and 56.9% with acute signs or symptoms of congestion) at increased risk of HF enrolled in the EMPACT‐MI trial, treatment with empagliflozin did not lead to a significantly lower risk of a first HFH or death from any cause than placebo (HR 0.90, 95% CI 0.76–1.06, P = 0.21). Nevertheless, empagliflozin significantly reduced the risk of HFH. 241
Soluble guanylate cyclase stimulators
Vericiguat reduced the composite outcome of CV death or first HFH in HFrEF patients with a recent episode of worsening HF. 140 , 242 In a pre‐specified echocardiographic sub‐study of patients enrolled in the VICTORIA trial, significant improvements in LV structure and function occurred over 8 months in the vericiguat group but similarly in the placebo group. 243
Prior HFH within 6 months was the most common criterion limiting eligibility to vericiguat in a real‐world HF population. 244 Butler et al. summarized evidence supporting the rationale for investigating the use of soluble guanylate cyclase stimulators in stable low‐risk HF: first, the treatment effect of HFrEF medications is not always consistent across the risk spectrum; second, if soluble guanylate cyclase stimulators have cardioprotective effects, these effects may be highlighted when the medication is initiated earlier in the disease process; and third, a novel trial with a longer follow‐up may provide data on its effect on CV mortality. 245
The ongoing VICTOR trial (A Study of Vericiguat in Participants with Chronic Heart Failure With Reduced Ejection Fraction) will evaluate the effect of vericiguat in stable chronic HFrEF patients (NCT05093933).
Myosin activators
The selective cardiac myosin activator omecamtiv mecarbil might be more effective in patients with more severe HFrEF. 246 , 247 , 248 These data are also confirmed by a pre‐specified analysis of the GALACTIC‐HF (Global Approach to Lowering Adverse Cardiac outcomes Through Improving Contractility in HF) trial that showed a greater effect of omecamtiv mecarbil on the primary composite outcome of a first HF event or CV death in patients with a higher baseline NT‐proBNP. 249
Non‐pharmacological options
Exercise training
In a Cochrane systematic review and meta‐analysis of 60 trials (8728 participants with HF), exercise‐based cardiac rehabilitation reduced all‐cause hospitalization and improved HRQL. 250 In patients with advanced HF implanted with an LVAD, the Ex‐VAD trial demonstrated that 12 weeks of supervised exercise training versus usual care had positive effects on submaximal exercise capacity and physical QoL, although it did not improve peakVO2. 251
Implantable defibrillator therapy
A post hoc analysis of the PARADIGM‐HF trial showed a reduction of the risk of ventricular arrhythmia with ARNI versus enalapril; the effect was independent of baseline implantable cardiac defibrillator (ICD)/CRT—defibrillator (CRT‐D) use and greater in patients with a non‐ischaemic aetiology. 252 As medical therapy improves, it may be necessary to reconsider the indications and timing of ICD implantation for primary prevention of sudden death. 253 A careful analysis of predictors of recurrent major arrhythmic events could improve the selection of patients who could benefit from ICD implantation. 254 , 255 In an observational retrospective cohort study including 698 patients with non‐ischaemic cardiomyopathy, late gadolinium enhancement (LGE) on magnetic resonance imaging (MRI) was the only independent predictor of appropriate ICD therapies, sustained ventricular arrhythmias, resuscitated cardiac arrest and SCD. 256 Genetic testing is also useful in stratifying the risk of arrhythmic events. 151 , 255
CRT
Cleland et al. conducted a meta‐analysis of COMPANION (Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure) and CARE‐HF (Cardiac Resynchronization—Heart Failure) trials to identify patient characteristics that predicted the effect of CRT—pacemaker (CRT‐P) on clinical outcomes. Patients assigned to CRT‐P had lower rates for all‐cause mortality and the composite outcome of all‐cause mortality or HFH. No pre‐specified characteristic, including sex, aetiology of ventricular dysfunction, QRS duration (within the studied range) or morphology or PR interval, significantly influenced the effect of CRT‐P on all‐cause mortality or the composite outcome. However, CRT‐P had a greater effect on the composite outcome for patients with lower body surface area and those receiving beta‐blockers. 257 A CRT response among lamin A/C (LMNA) cardiomyopathy patients was associated with lower baseline LVEF or a high percentage of RV pacing prior to CRT in patients with pre‐existing cardiac implantable electronic device. In patients with ESC Class I guideline indication for CRT, response rates were 61%. Post‐CRT improvements in LVEF were associated with survival benefits. 258 The survival benefits of CRT were consistent also in patients with several comorbidities. 259
The BUDAPEST‐CRT Upgrade RCT randomly assigned in a 3:2 ratio 360 patients with symptomatic HF, LVEF ≤ 35% and intermittent or permanent RV pacing (≥20% of RV pacing burden) with wide‐paced QRS (>150 ms) to receive the CRT‐D upgrade or ICD. 260 The primary outcome was the composite of all‐cause mortality, HFH or <15% reduction of LV end‐systolic volume assessed at 12 months. The upgrade procedure was safe and showed an 11% reduction in the primary composite endpoint with consistent results in all patient subgroups (including patients with AF). 261
Excessive prolongation of the PR interval impairs the coupling of atrioventricular (AV) contraction. The HOPE‐HF (His Optimized Pacing Evaluated for Heart Failure) randomized, double‐blind, cross‐over trial evaluated whether AV‐optimized His pacing was preferable to no pacing. His bundle pacing did not improve peak oxygen uptake but improved QoL and symptoms without adverse effects. 262
Percutaneous treatment of mitral regurgitation
Moderate‐to‐severe secondary mitral regurgitation (SMR) has been associated with a poor prognosis in chronic HFrEF patients. 263 , 264 , 265
Prescription and up‐titration of GDMT are crucial before the correction of severe SMR. 4 Indeed, up to 40% of severe SMR improved after optimization of medical therapy in different cohorts. 266 , 267 Furthermore, triple GDMT prescription (beta‐blockers, renin–angiotensin system inhibitors and MRAs) at the time of transcatheter edge‐to‐edge mitral valve repair (M‐TEER) was associated with a better long‐term prognosis in large registries. 268 Using data from the EuroSMR registry, Adamo et al. showed that M‐TEER further allowed up‐titration of GDMT in 38% of patients. The degree of MR reduction between baseline and 6 month follow‐up was an independent predictor of GDMT up‐titration after M‐TEER. 269 Better tolerability of GDMT might be mediated by higher systolic blood pressure and improvement in renal function through improvement in haemodynamics and RV function. 138 Patients experiencing GDMT up‐titration after M‐TEER had a lower risk of all‐cause death or HFH compared with those without. 269
A 5 year follow‐up of the COAPT trial is now available. SMR correction with MitraClip device confirmed a significant reduction in HFH and all‐cause mortality compared with GDMT alone. 270 In the prospective, multicentre, international, single‐arm EXPAND study, third‐generation MitraClip system devices reduced MR to ≤1+ and MR ≤ 2+ in 93.0% and 98.5% of patients, respectively; this result was sustained at 1 year follow‐up. 271 The fourth‐generation MitraClip G4 System has further increased procedural success rates at 30 days in the EXPAND G4 study. 272
Several elements can influence the outcome of patients with severe MR undergoing M‐TEER, including LA and RV function. 138 , 273 , 274 , 275 , 276 , 277 , 278 Cardio‐hepatic syndrome was associated with a significant increase in 2 year mortality, 279 and low serum albumin levels were independently associated with reduced 4 year survival. 280 A risk score predicting all‐cause death or HFH using the COAPT trial data was developed. 281 However, the COAPT risk score showed a poor performance in the prognostic stratification of real‐world patients undergoing M‐TEER but a better performance in COAPT‐like patients. 282
Other options for transcatheter treatment of MR are emerging. Transcatheter mitral valve replacement (TMVR) is an alternative to M‐TEER. 283 , 284 Ludwig et al. analysed a propensity score‐matched comparison between the CHOICE‐MI registry (262 patients treated with TMVR) and the EuroSMR registry (1065 patients treated with M‐TEER) with 12 demographic, clinical and echocardiographic parameters; TMVR was associated with a greater reduction in MR severity and symptom improvement with no significant differences in mortality beyond 30 days (although post‐procedural mortality tended to be higher after TMVR). 285 Potential haemodynamic complications after TMVR, including LVOTO and afterload mismatch, and the peri‐procedural management of patients undergoing TMVR have been reviewed. 286
Percutaneous treatment of tricuspid regurgitation
TR is common in patients with HF and is associated with higher mortality rates. 181 , 287 , 288 , 289 , 290 Of the 11 298 patients included in the ESC‐HFA EORP Heart Failure Long‐Term Registry, 5.5% had isolated TR, and 11% had combined MR/TR; HFpEF was associated with an increased risk of isolated TR. TR, isolated or combined with MR, was associated with a worse prognosis. 291 In a different cohort of patients with severe combined MR/TR, an improvement in the degree of TR was observed after M‐TEER in about one third of cases. 274
Although tricuspid valve surgery should be the first therapeutic choice, mortality rates after isolated tricuspid surgery remain high, with up to 12% in‐hospital mortality. The TRI‐SCORE was proposed to predict in‐hospital mortality risk. 292
Transcatheter tricuspid valve repair might become a valuable alternative to surgery for severe TR. 287 , 293 , 294 The TRILUMINATE Pivotal, a prospective randomized trial of percutaneous tricuspid transcatheter edge‐to‐edge repair (T‐TEER) versus medical therapy for severe TR (93% with secondary TR), enrolled 350 symptomatic patients (NYHA II–IV) with LVEF > 20% and at least intermediate surgical risk. T‐TEER demonstrated a significant reduction in the severity of TR and an improvement in QoL (assessed by the KCCQ score). Supporting the absence of effective medical therapy for TR (as opposed to SMR), no improvement in the severity of TR was observed in the control group. 295
HFpEF
Epidemiology, clinical phenotypes and pathophysiology
HFpEF represents a heterogeneous clinical syndrome and accounts for more than half of HFH. 4 , 296 Cai et al. reported clinical characteristics and outcomes of 41 708 patients hospitalized with HFpEF between January 2017 and June 2021 in secondary and tertiary hospitals across 31 provinces of mainland China. The 1 year rate of clinical outcomes was 16.4%, the 1 year rate of HFH was 13.6% and CV death was 3.1%. 297
A scientific statement of the HFA outlined the most common HFpEF phenotypes and suggested an evidence‐based treatment strategy for each. 35
Obesity and T2DM are common comorbidities in HFpEF and might play a role in the pathogenesis of HFpEF. 36 , 298 Adverse myocardial remodelling might result from adipokine‐mediated inflammatory mechanisms and epicardial adipose tissue. 299 In the PROMIS‐HFpEF cohort, increased epicardial adipose tissue was associated with smaller indexed LV end‐diastolic and LA volumes, proteomic markers of adipose biology and inflammation, insulin resistance, endothelial dysfunction and dyslipidaemia but not with coronary flow reserve. 300 Other mechanisms potentially involved in the pathogenesis of HFpEF are endothelium‐independent microvascular dysfunction, subclinical inflammation, venous dysfunction and impaired myocardial energy homeostasis. 301 , 302 , 303 , 304
A post hoc analysis of the ATHENA trial showed that dronedarone was associated with reduced CV events in patients with paroxysmal or persistent AF or atrial flutter and HFmrEF or HFpEF. 305
Diagnosis and prognosis
The H2FPEF and HFA‐PEFF scores were proposed and validated to aid in the diagnosis of HFpEF, but their diagnostic performance varied in different populations. Tomasoni et al. showed that the HFA‐PEFF score had a higher diagnostic utility compared with the H2FPEF score and held an independent prognostic value for all‐cause mortality in patients with HFpEF caused by CA. 306
Exercise testing has a crucial role in the diagnosis and prognostic assessment of HFpEF. 307 , 308 , 309 Omote et al. performed invasive exercise testing in patients with exertional dyspnoea and LVEF ≥ 50% (n = 764). Among these patients, haemodynamic abnormalities currently used to confirm HFpEF diagnosis were also associated with an increased risk for adverse events. The greatest risk was observed in patients with elevated pulmonary arterial wedge pressure (PAWP) at rest, followed by patients with elevated exercise PAWP and normal resting PAWP. 310 In contrast to patients with HFrEF, between 10% and 25% of patients with HFpEF and without lung disease displayed arterial desaturation during exercise. Exertional hypoxaemia was associated with more severe haemodynamic abnormalities and increased mortality. 311 Among patients with HFpEF undergoing comprehensive echocardiography and invasive cardiopulmonary exercise testing, low compared with preserved biventricular cardiac power output reserve (< vs. ≥ median of 1.57 W) was associated with more advanced HFpEF, increased systemic vascular resistance and PVR, reduced exercise capacity and increased adverse events. 312
Treatment
SGLT2 inhibitors
Based on the results of the EMPEROR‐Preserved and DELIVER trials, the 2023 Focus Update of 2021 HF guidelines introduced a CoR I, LoE A, for the use of SGLT2 inhibitors in patients with HFmrEF and HFpEF. 44 Global implementation of SGLT2 inhibitor use is warranted to prevent or postpone HFH and reduce HF‐related costs. 313
Among the 12 251 participants from DELIVER and EMPEROR‐Preserved, SGLT2 inhibitors reduced the composite endpoint of CV death or first HFH (HR 0.80, 95% CI 0.73–0.87) with a consistent reduction in both the components of CV death (HR 0.88, 95% CI 0.77–1.00) and first HFH (HR 0.74, 95% CI 0.67–0.83). 220
In a pre‐specified analysis of the DELIVER trial, dapagliflozin consistently reduced the risk of the primary endpoint compared with placebo, irrespective of baseline NYHA class, with an improvement in QoL more evident among NYHA III–IV patients. 314 A secondary analysis of EMPEROR‐Preserved assessed the effects of empagliflozin across the spectrum of kidney function. 315 Overall, 5988 patients were included and categorized according to concomitant CKD at baseline (n = 3198, 53.5% with CKD). The efficacy of empagliflozin on the primary outcome of HFH or CV death was consistent across a wide range of renal functions. Empagliflozin also reduced the progression to macroalbuminuria and the risk of acute kidney disease. In a further analysis of EMPEROR‐Preserved, empagliflozin, compared with placebo, led to a significant increase in albumin levels and was beneficial irrespective of baseline liver function. 316 The benefits of SGLT2 inhibitors were not influenced by background therapy or by the baseline history of AF. 317 , 318
Pooling data from the DAPA‐HF and DELIVER trials, Bhatt et al. analysed the benefits of dapagliflozin on health status, measured by the KCCQ, across the full spectrum of LVEF. A total of 11 007 participants were included. KCCQ was evaluated at 4 and 8 months. Dapagliflozin improved all key domains of health status irrespective of LVEF. 110 In a larger meta‐analysis, including 14 RCTs (21 737 participants), SGLT2 inhibitors demonstrated a significant improvement in QoL across the entire spectrum of LVEF as early as a 3 month follow‐up. Results were confirmed at 6 month follow‐up, and a wider effect was observed among patients with a recent episode of worsening HF. 319
ARNI
In a post hoc analysis of the PARAGON‐HF trial, a prior HFH (occurring pre‐randomization) was associated with an increased risk for renal events. HFpEF patients experiencing HFH could represent a distinct cohort at elevated risk for accelerated kidney disease progression. 320 Initiation of sacubitril/valsartan was associated with a modestly lower new loop diuretic requirement in follow‐up. 321
In the PARAGLIDE‐HF trial, enrolling 466 patients with LVEF > 40% and a recent stabilized episode of worsening HF (defined as HFH, emergency department visit or out‐of‐hospital urgent HF visit, all of them requiring IV diuretic agents within 30 days from randomization), sacubitril/valsartan reduced NT‐proBNP concentrations (benefit occurred early with biomarker values diverging at 1 week) and the risk of worsening renal function at the expense of more symptomatic hypotension compared with valsartan alone; however, secondary, the hierarchical outcome of CV death, HFH, urgent HF visits and change in NT‐proBNP was not significantly different. 322
In a pre‐specified participant‐level pooled analysis of PARAGLIDE‐HF and PARAGON‐HF, ARNI, compared with valsartan, significantly reduced total worsening HF events and CV death [rate ratio (RR) 0.86, 95% CI 0.75–0.98, P = 0.027] with statistical significance already reached by Day 9 after randomization; treatment benefits were larger in those with LVEF ≤ 60% (RR 0.78, 95% CI 0.66–0.91). 323
MRA
The TOPCAT trial was found to be neutral; however, the differences in patients and outcomes between the American and non‐American cohorts could explain the lack of benefit in the trial. 324 , 325
The STRUCTURE trial, including a subset of HFpEF patients with normal LV filling pressure at rest and increased LV filling pressure with exercise, showed an improvement in both exercise capacity and E/e′ with spironolactone, with a significant interaction between treatment with spironolactone and E/e′ on peak VO2. 326
An individual patient data meta‐analysis including 984 patients with HFpEF from three large trials (HOMAGE, Aldo‐DHF and TOPCAT) compared echocardiographic changes in patients on spironolactone versus placebo. The prescription of spironolactone was associated with a reduction in LA volume, LV mass and thickness and improved systolic and diastolic function. 327
Semaglutide
In the STEP‐HFpEF trial, the glucagon‐like peptide 1 (GLP‐1) agonist semaglutide administered once weekly at a dose of 2.4 mg for 1 year significantly decreased body weight (13.3% loss vs. 2.6% in the placebo group) and improved the KCCQ clinical summary score and 6 min walk distance among obese HFpEF patients. The main inclusion criteria were BMI above 30 kg/m2, NYHA Class II–IV, elevated natriuretic peptide levels (with thresholds stratified according to the BMI at baseline), LVEF > 45% and evidence of echocardiographic abnormalities. Most of the 529 participants (84%) had LVEF ≥ 50%. The decrease in NT‐proBNP levels was ~15% greater with semaglutide than with placebo. 328
The results of the STEP‐HFpEF DM trial are now published. Among patients with obesity‐related HFpEF and T2DM, semaglutide led to larger reductions in HF‐related symptoms and physical limitations and greater weight loss than placebo at 1 year. 329 , 330
Cardiac contractility modulation (CCM)
CCM may improve functional capacity and reduce HFHs. CCM‐HFpEF was a single‐arm, multicentre pilot study with the aim of assessing the potential benefits of CCM in 47 HFpEF patients. An increase in KCCQ (primary endpoint) by 18.0 (± 16.6) points (P < 0.001) was reported. The event‐free rate was 93.6%, and the safety profile was good. 331
Device‐based percutaneous treatments
Interatrial shunt devices might represent a new therapeutic strategy to decompress and reduce LA pressure. 332 Although initial results to reduce LA pressure seemed promising, the consecutive randomized, multicentre, blinded, sham‐controlled REDUCE‐LAP II trial reported no prognostic benefit, 333 and this might be attributed to latent pulmonary vascular disease (PVD). Schuster et al. hypothesized that non‐invasive characterization of cardiac and pulmonary physiology, through rest and exercise stress right heart catheterization, echocardiography and CV magnetic resonance, can more accurately select patients who would benefit most from an interatrial shunt device. Among the 75 patients with HFpEF enrolled, 24 had latent PVD, defined as increased PVR ≥ 1.74 Wood units during exercise stress. Patients with PVD had worse RV functional reserve. 334 In the RELIEVE‐HF open‐label roll‐in cohort, including symptomatic HF despite optimal GDMT with ≥1 HF hospitalization in the prior year or elevated natriuretic peptides, interatrial shunting with the Ventura device was safe and resulted in favourable clinical effects, namely, improvement in KCCQ‐OSS by 12–16 points at all follow‐up time points (all P < 0.004), with similar outcomes in patients with reduced and preserved LVEF. Also, improvements in LV and RV structure and function were consistent with reverse myocardial remodelling. 335
HF with supranormal EF
Some studies suggested that LVEF might have a U‐shaped relationship with outcomes, but results were inconsistent in different cohorts. 336 , 337 , 338 , 339
In RELAX‐AHF‐2, supranormal (sn) EF (HFsnEF), defined as LVEF ≥ 65%, was associated with a higher risk of non‐CV mortality but not all‐cause mortality. 340
Among the 11 573 patients hospitalized for HF and enrolled in the nationwide Japanese registry, 16.8% were classified as HFsnEF. Compared with HF with normal EF (50% ≤ LVEF ≤ 65%), HFsnEF patients were older, more likely to be women, and had lower natriuretic peptide values and smaller left ventricles. They had a similar risk of CV death or HFH and a lower adjusted HR for HFH. 341 In a merged dataset of 33 699 participants who had been enrolled in six randomized controlled HF trials, the incidence of most clinical outcomes (except non‐CV death) decreased as LVEF increased, with an LVEF inflection point of around 50% for all‐cause death and CV death, around 40% for pump failure death and around 35% for HFH. Higher than those thresholds, there was little further decline in the incidence rate. 339
Popovic et al. showed that HFsnEF patients had a smaller heart size, increased LV diastolic stiffness and leftward shift in the end‐diastolic pressure–volume relationship compared with HFpEF. 342
A reclassification of HF based on different LVEF categories was proposed (LVEF ≤ 35%, LVEF > 35% to <60%–65% and LVEF ≥ 60%–65%). 343
Advanced HF
Definition and prognosis
The 2018 HFA‐ESC definition of advanced HF required the presence of all the following criteria despite GDMT: persistence of severe symptoms (NYHA III–IV), severe cardiac dysfunction, episodes of congestion/arrhythmias/low output causing more than one unplanned hospitalization and severe impairment of exercise capacity. 4 The prognostic impact of this definition was shown in a contemporary, real‐world, multicentre high‐risk cohort of patients with HF and at least one ‘I NEED HELP’ criterion. 344 A further assessment of the ‘I NEED HELP’ criteria in this cohort was published. 345 , 346
Patients with advanced HF are burdened with very high mortality and present a challenging management. 248 , 344 , 347 , 348 A systematic review of observational studies including 862 046 patients reported a 1 year mortality rate that ranged from 8.47% for chronic HF to 29.74% for advanced HF patients. 349
Pharmacological therapies
Prescription and up‐titration of GDMT remained limited also in this high‐risk population of 699 patients with HFrEF and at least one ‘I NEED HELP’ marker for advanced HF enrolled in the HELP‐HF registry. Namely, beta‐blockers were administered to 574 (82%) patients, ACEi/ARB/ARNI was administered to 381 (55%) patients and 416 (60%) received MRA. Overall, ≥50% of target doses were reached in 41%, 22% and 56% of the patients on beta‐blockers, ACEi/ARB/ARNI and MRA, respectively. Reasons for under‐prescription were unknown in a significant proportion of patients, suggesting a potential role of clinical inertia. 204
Inotropes may represent a potentially useful strategy not only in the short term but also in the chronic treatment of advanced HF. 248 , 348 A recent clinical consensus statement of HFA‐ESC reviewed traditional and novel drugs with inotropic effects. 350
The LeoDOR multicentre, double‐blind, randomized trial evaluated the efficacy and safety of intermittent levosimendan therapy (infusion every 3 weeks for 12 weeks) in advanced HF following HFH. The infusion did not improve post‐hospitalization clinical stability, even if, due to the COVID‐19 pandemic, the statistical power of the study was reduced due to the impossibility of enrolling the planned number of patients. 351
Long‐term mechanical circulatory support (MCS)
In view of the shortage of heart donors and the difficulty in accessing transplantation due to possible contraindications, long‐term MCS devices represent a valid alternative for patients with advanced HF. 352 In an analysis depicting the evolving landscape of LVAD carriers in Europe over 13 years, improved 1 year survival was observed in patients implanted more recently with continuous‐flow LVAD, despite older recipients with more comorbidities. This was likely due to increased centre expertise and improved patient selection (less acutely ill) and pump technology. 353
Nevertheless, severe complications, including bleeding or thrombosis, might occur and require a careful selection of recipients. The ARIES HM3 trial demonstrated that a strategy with a vitamin K antagonist (VKA) alone was non‐inferior to combination treatment with VKA and aspirin in patients who underwent implantation with a HeartMate 3 (HM3) LVAD; in addition, survival free from bleeding and stroke seemed to favour the arm without aspirin. 354 Uriel et al. showed a reduction in the incidence of moderate‐to‐severe de novo AR with the fully magnetically levitated HM3 LVAD compared with the axial‐flow HeartMate II LVAD. 355
Palliative care
A comparison of 2021 ESC and 2022 American Heart Association (AHA)/American College of Cardiology (ACC)/Heart Failure Society of America (HFSA) HF guidelines regarding the provision of palliative care showed nuanced differences. 356 A clinical consensus statement from the HFA of ESC provided practical guidance promoting cultural competence in the management of patients with advanced HF needing palliative care. 357
Worsening HF
Worsening HF can be defined as worsening symptoms and signs of HF in patients with pre‐existing HF, requiring intensification of treatment, most often diuretic therapy. It requires a prior diagnosis of HF, excluding episodes of new‐onset HF. 140 Episodes of worsening HF characterize the clinical course of patients with chronic HF. Worsening HF must be kept distinct from acute HF, which is a much broader entity including also new‐onset HF as well as different clinical presentations such as acute pulmonary oedema, RV failure and cardiogenic shock (CS). Sites of care for worsening HF include hospitalization, emergency department visit with IV therapy, generally loop diuretics and ambulatory treatment, either as outpatients receiving IV therapy in an outpatient setting or as outpatients treated with an escalation of their oral diuretic therapy. 140 Episodes of worsening HF are associated with poorer QoL, increased risks of hospitalization and death and are a major burden on healthcare resources.
The results of the VICTORIA and PARAGLIDE‐HF trials have been discussed above.
The optimization of GDMT with a fifth drug (vericiguat) in patients who are symptomatic and with LVEF < 45% should be advised after a worsening HF event.
Acute HF
Hospitalization due to acute HF has a dramatic burden in terms of symptoms, morbidity and mortality. 140 , 358 , 359 , 360 , 361 In a study including 283 048 patients hospitalized for HF from 2008 to 2017 in Australia and New Zealand, HFH was associated with a loss of 7.3 years in life expectancy, compared with the general population. Survival rates were 48%, 34% and 17% at 3, 5 and 10 years, respectively. 360
Precipitating factors and prognostic markers
Several precipitating factors have been recognized. Gualandro et al. assessed the rate of acute HF after non‐cardiac surgery in a large series of 9164 consecutive high‐risk patients. The incidence of acute HF after non‐cardiac surgery was 2.5% in the general population and 10% in patients with a history of HF. Post‐operative acute HF was an independent predictor of all‐cause mortality and HF readmissions. 362
Valvular heart disease is frequently associated with acute HF. On one hand, a new significant valvular lesion can be the cause of acute decompensation; on the other hand, acute HF may worsen an already compromised haemodynamic status caused by a chronic valve disease. A scientific statement of the HFA, the Association for Acute CardioVascular Care and the European Association of Percutaneous Cardiovascular Interventions provided insights into the epidemiology and treatment options in patients with valvular heart disease (VHD) and acute HF. 363
Lee et al. investigated the relationship between patient‐reported symptoms, evaluated by the KCCQ total symptom score (KCCQ‐TSS), and pulmonary congestion, assessed by lung ultrasound, physical examination and chest X‐ray, in patients with acute HF. A lower KCCQ‐TSS was associated with worse NYHA class and peripheral oedema but not with pulmonary congestion. 364 Kapłon‐Cieślicka et al. assessed prevalence, hospital course and post‐discharge outcomes in patients with hyponatraemia in acute HF. Among 8298 patients enrolled in the ESC Heart Failure Long‐Term Registry, hyponatraemia at admission (possibly dilutional) was associated with worse in‐hospital and post‐discharge outcomes, especially if it did not resolve at discharge; conversely, hyponatraemia developing during hospitalization (possibly depletional) was associated with a lower risk. 365
Treatment
Diuretics and decongestion strategy
IV diuretics are the first option for the treatment of congestion in patients hospitalized due to acute HF. Evidence regarding the optimal strategy of diuretic administration is limited. Among the 15 078 patients included in the REPORT‐HF registry, the median time‐to‐diuretics was 67 min (range from 17 to 190 min). Time‐to‐diuretic administration did not have an impact on in‐hospital mortality but was associated with an increase in mortality risk at 30 days, especially in patients at higher risk. 366
The ADVOR trial examined the effect of acetazolamide on decongestion in patients with acute HF on top of standard loop diuretic therapy. Overall, 519 patients were enrolled (mean age 78 years, 63% male, mean LVEF 43% and median NT‐proBNP 6173 pg/mL). The addition of acetazolamide resulted in a greater incidence of the primary endpoint (i.e., successful decongestion within 3 days after randomization) with more decongestion also at discharge and a shorter length of hospital stay versus placebo. 367 , 368 In a pre‐specified sub‐analysis of the ADVOR trial, acetazolamide on top of standardized loop diuretic therapy did not lead to clinically important hypokalaemia or hyponatraemia and improved decongestion over the entire range of baseline serum potassium and sodium levels. 369 A greater efficacy was hypothesized in patients with baseline or loop diuretic‐induced elevated bicarbonate levels (a marker of proximal nephron NaHCO3 retention). 370
The CLOROTIC trial showed a greater decrease in body weight at 72 h and a greater diuretic response with the addition of hydrochlorothiazide (HCTZ), compared with placebo, on top of furosemide in patients hospitalized for acute HF. 371 Patients with eGFR ≥60 mL/min/1.73 m2 had greater weight loss compared with those with eGFR <60 mL/min/1.73 m2, but no significant differences were observed with the addition of HCTZ in terms of diuretic response, mortality or rehospitalizations, or safety endpoints across different eGFR values at baseline. 372
Torsemide compared with furosemide did not result in a significant difference in all‐cause mortality over 12 months among patients discharged after HFH. 373
Early assessment of urinary sodium (UNa) concentration is useful to assess intrinsic renal sodium avidity. 374 In the PUSH‐AHF trial, natriuresis‐guided diuretic therapy in patients with acute HF significantly improved natriuresis and diuresis up to 48 h without impacting all‐cause mortality and/or HF hospitalization at 180 days. 375
In patients hospitalized for acute HF, in‐hospital initiation of MRAs was associated with improved post‐discharge outcomes, independent of LVEF and other potential confounders. 376
SGLT2 inhibitors
The EMPULSE trial randomized 530 patients hospitalized for acute HF, when clinically stable, to receive empagliflozin 10 mg once daily or placebo for up to 90 days. Empagliflozin provided a clinical benefit, defined as a hierarchical composite of death from any cause, number of HF events and time‐to‐first HF event, or a 5‐point or greater difference in change from baseline in KCCQ‐TSS at 90 days, as assessed using a win ratio (stratified win ratio 1.36, 95% CI 1.09–1.68, P = 0.0054). 377 The initiation of empagliflozin resulted also in the improvement of all analysed indexes of congestion in the trial. 233
In the EMPAG‐HF trial, enrolling 60 patients within 12 h of hospitalization for acute HF, early addition of empagliflozin to standard diuretic therapy increased urine output without affecting markers of renal function. 378 Packer and Butler analysed similarities and distinctions in the diuretic effects of acetazolamide and SGLT2 inhibitors. 379
Other drugs
The phase 2a SEISMiC trial randomized 60 patients with acute HF with pre‐CS, defined as systolic blood pressure <90 mmHg without hypoperfusion, venous lactate ≥2 mmol/L and/or mechanical or inotropic support, to istaroxime 1.0–1.5 μg/kg/min or placebo for 24 h. Istaroxime improved systolic blood pressure without significant differences in serious adverse events. The most frequent adverse events were nausea, vomiting and infusion site pain in the istaroxime‐treated patients. 380
Morphine has been used for decades in patients developing acute cardiogenic pulmonary oedema because it reduces anxiety and dyspnoea and improves the vasoconstriction accompanying hypertensive crises but without evidence from RCTs. The MIMO (MIdazolam versus MOrphine) is a multicentre, open‐label RCT comparing midazolam with morphine in patients with pulmonary oedema. The trial was stopped early after a planned interim analysis by the safety monitoring committee. Overall, 111 patients were randomized at that time (55 to midazolam and 56 to morphine). No differences were found in the primary endpoint (in‐hospital mortality), but serious adverse events were less common with midazolam versus morphine. 381
Cardiogenic shock
The mortality rate in patients with CS remains extremely high. 382
The Society for Cardiovascular Angiography and Interventions (SCAI) shock stage classification, released in 2019, has been revised. 383 Medical therapy of CS has been recently reviewed. 384
Mechanical circulatory support
MCS devices represent an option for the treatment of CS. Schrage et al. assessed the association between MCS use and the primary endpoint of 30 day mortality in a 1:1 propensity‐matched cohort of patients with non‐ischaemic CS. In the matched cohort, MCS use was associated with a lower 30 day mortality. This finding was consistent through all tested subgroups except when CS severity was considered, indicating risk reduction especially in patients with deteriorating CS. However, complications occurred more frequently in patients with MCS (e.g., severe bleeding and access site‐related ischaemia). 385 The large European nationwide observational cohort study (InEK GmbH) included patients with AMI‐related CS treated with Impella (ABIOMED, Danvers, MA, USA) and/or veno‐arterial extracorporeal membrane oxygenation (VA‐ECMO) in 2020–2021. Impella patients were older and less frequently presented after an out‐of‐hospital cardiac arrest. In‐hospital mortality was lower in the Impella versus VA‐ECMO cohort. Adverse events including acute haemorrhagic anaemia (36% vs. 68%, P < 0.001), cerebrovascular accidents (4% vs. 11%, P < 0.001), thrombo‐embolisms of the extremities (5% vs. 8%, P < 0.001), systemic inflammatory response syndrome (21% vs. 25%, P = 0.004), acute kidney injury (44% vs. 53%, P < 0.001) and acute liver failure (7% vs. 12%, P < 0.001) occurred less frequently in Impella‐supported patients. Impella patients had shorter hospital stays and lower hospital costs. Notably, possible unmeasured and unadjusted confounders might have influenced the results. 386
The ECLS‐SHOCK trial tested whether routine early implementation of extracorporeal life support (ECLS) compared with usual medical treatment alone improved survival in patients with myocardial infarction and CS with planned early revascularization. Enrolled patients were at high risk for adverse outcomes (median lactate level was 6.9 mmol/L; median LVEF was 30%; and 77.7% received cardiopulmonary resuscitation before randomization). Early ECLS therapy did not reduce the risk of death from any cause at the 30 day follow‐up versus medical therapy alone, whereas the risk of major bleedings and vascular complications was increased. 387 The results were in line with an individual patient data meta‐analysis by Zeymer et al. 388 Park et al. evaluated the feasibility of an early LV unloading strategy compared with a conventional strategy in VA‐ECMO. A total of 60 patients were randomized in a 1:1 ratio to receive early (LV unloading performed at the time of VA‐ECMO insertion) or conventional LV unloading strategies. The early LV unloading strategy was performed using a percutaneous transseptal LA cannulation via the femoral vein incorporated into the extracorporeal membrane oxygenation (ECMO) venous circuit. Compared with the conventional approach, early LV unloading did not improve the VA‐ECMO weaning rate, despite a rapid improvement in pulmonary congestion. Also, there were no significant differences in survival at discharge. 389
Varshney et al. evaluated outcomes associated with bridging strategies to durable LVAD or heart transplantation in patients with acute decompensated CS from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Patients bridged with VA‐ECMO had the highest mortality (22%), followed by catheter‐based temporary MCS (10%), intra‐aortic balloon pump (9%) and medical therapy (7%). 390
Further trials testing the use of MCS in patients with severe CS are ongoing.
Before discharge, early discharge and after discharge
A scientific statement by the HFA summarized recent findings that have implications for clinical management in both the pre‐discharge and early post‐discharge phases after a hospitalization for acute HF. 141 First, the early detection and effective treatment of residual or recurrent congestion may reduce the risk of rehospitalization. Second, the initiation and up‐titration of GDMT are crucial to improve both the short‐ and long‐term clinical course. 141
Schrage et al. investigated the association of HFH with the initiation or discontinuation of GDMT and consequent outcomes. Among 6893 patients with LVEF < 50% who experienced an HFH from the Swedish HF Registry, hospitalization usually led to the implementation of GDMT, although it remained suboptimal. Early initiation of GDMT was associated with better survival. 391
In the STRONG‐HF trial, an intensive treatment strategy of rapid up‐titration of GDMT and close follow‐up after an acute HF admission reduced symptoms, improved QoL and reduced the risk of 180 day all‐cause death or HFH compared with usual care. 392 Achieving higher doses of GDMT 2 weeks after discharge was feasible and safe in most patients. 393 The high‐intensity care (HIC) strategy was safe and significantly reduced all‐cause mortality and HFH at 180 days compared with usual care, irrespective of age, sex, baseline systolic blood pressure, LVEF, NT‐proBNP, baseline self‐assessed health status and non‐cardiac comorbidities. 9 , 34 , 394 , 395 , 396 , 397 , 398
Importantly, early up‐titration of GDMT also significantly improved all dimensions of QoL. 398
In the HIC arm, the following pre‐specified safety indicators were used to guide up‐titration: eGFR < 30 mL/min/1.73 m2, serum potassium >5.0 mmol/L, systolic blood pressure <95 mmHg, heart rate <55 b.p.m. and NT‐proBNP > 10% higher than pre‐discharge values. 211 Three hundred thirteen of the 542 patients in the HIC arm (57.7%) met ≥1 safety indicator at any follow‐up visit 1–6 weeks after discharge. An increase in NT‐proBNP was the most frequent safety indicator. These patients achieved slightly lower GDMT doses, but higher than in the usual care group. Importantly, no significant increase in the primary outcome of 180 day HFH or death was reported when safety indicators were appropriately addressed according to the study protocol, highlighting the relevance of close follow‐up during rapid and early up‐titration of GDMT. 211 An early decrease in eGFR during rapid up‐titration of GDMT was associated with more evidence of congestion, yet lower doses of GDMT during follow‐up. 399
Based on the results of the STRONG‐HF, recommendations for an intensive strategy of initiation and rapid up‐titration of evidence‐based treatment before discharge and frequent and careful follow‐up visits in the first 6 weeks following an HFH were introduced. 44
Telemedicine and congestion remote monitoring
Remote monitoring in patients with HF includes telemedicine and implantable or wearable devices that can monitor impedance, pulmonary arterial pressure or arrhythmias. The benefits of non‐invasive remote patient management were confirmed across the entire spectrum of LVEF in a pre‐specified analysis of the TIM‐HF2 trial. 400
A systematic meta‐analysis, including 8 RCTs and 4347 HF patients, compared device‐based remote monitoring of congestion to standard therapy; a haemodynamic‐guided strategy with invasive devices was associated with a significant reduction in the composite endpoint of all‐cause death or HFH mainly driven by the reduction of HFH, while an impedance‐guided strategy did not show a significant reduction. 401 The CardioMEMS system is one of the most studied invasive pulmonary arterial pressure monitoring devices. A pre‐specified subgroup analysis of the MEMS‐HF study showed that the benefits of remote monitoring are confirmed regardless of the presence and subtypes of PH at baseline. 402 In the open‐label, randomized MONITOR‐HF trial, which enrolled 348 patients with symptomatic HF (NYHA III) and a recent episode of worsening HF, haemodynamic monitoring with CardioMEMS significantly improved QoL and reduced HFH irrespective of the LVEF. 403 Indications for implantation of haemodynamic PA pressure monitoring devices are likely to be strengthened in upcoming guidelines.
Novel devices for congestion monitoring of patients with HF, as an interatrial sensor able to transmit LA pressure or an IVC sensor able to measure IVC cross‐sectional area, are being studied (Figure 4). 404 , 405 , 406
Figure 4.

Invasive device for haemodynamic pressure monitoring and main findings from trials. HFH, heart failure hospitalization; IVC, inferior vena cava; LAP, left atrial pressure; PAP, pulmonary artery pressure; QoL, quality of life.
Conclusions
In recent years, there has been great progress in the management of HF. The 2021 ESC guidelines for the management of HF established the four pillars of HFrEF treatment with ACEi/ARNI, beta‐blockers, MRA and SGLT2 inhibitors. A fifth drug, vericiguat, is becoming available across several countries for patients experiencing an episode of worsening HF. SGLT2 inhibitors are the first class of drugs recommended for the treatment of HFmrEF and HFpEF in the 2023 focused update of HF guidelines.
Beghini, A. , Sammartino, A. M. , Papp, Z. , von Haehling, S. , Biegus, J. , Ponikowski, P. , Adamo, M. , Falco, L. , Lombardi, C. M. , Pagnesi, M. , Savarese, G. , Metra, M. , and Tomasoni, D. (2025) 2024 update in heart failure. ESC Heart Failure, 12: 8–42. 10.1002/ehf2.14857.
References
- 1. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352‐380. doi: 10.1002/ejhf.2115 [DOI] [PubMed] [Google Scholar]
- 2. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc Res 2023;118:3272‐3287. doi: 10.1093/cvr/cvac013 [DOI] [PubMed] [Google Scholar]
- 3. Riccardi M, Sammartino AM, Piepoli M, Adamo M, Pagnesi M, Rosano G, et al. Heart failure: An update from the last years and a look at the near future. ESC Heart Fail 2022;9:3667‐3693. doi: 10.1002/ehf2.14257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Authors/Task Force Members , McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2022;24:4‐131. doi: 10.1002/ejhf.2333 [DOI] [PubMed] [Google Scholar]
- 5. Seferovic PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinkovic I, et al. The Heart Failure Association Atlas: Heart failure epidemiology and management statistics 2019. Eur J Heart Fail 2021;23:906‐914. doi: 10.1002/ejhf.2143 [DOI] [PubMed] [Google Scholar]
- 6. Butt JH, Claggett BL, Miao ZM, Jering KS, Sim D, van der Meer P, et al. Geographic differences in patients with acute myocardial infarction in the PARADISE‐MI trial. Eur J Heart Fail 2023;25:1228‐1242. doi: 10.1002/ejhf.2851 [DOI] [PubMed] [Google Scholar]
- 7. Docherty KF, Jackson AM, Macartney M, Campbell RT, Petrie MC, Pfeffer MA, et al. Declining risk of heart failure hospitalization following first acute myocardial infarction in Scotland between 1991–2016. Eur J Heart Fail 2023;25:1213‐1224. doi: 10.1002/ejhf.2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stretti L, Zippo D, Coats AJS, Anker MS, von Haehling S, Metra M, et al. A year in heart failure: An update of recent findings. ESC Heart Fail. 2021;8:4370‐4393. doi: 10.1002/ehf2.13760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Čerlinskaitė‐Bajorė K, Lam CSP, Sliwa K, Adamo M, Ter Maaten JM, Léopold V, et al. Sex‐specific analysis of the rapid up‐titration of guideline‐directed medical therapies after a hospitalization for acute heart failure: Insights from the STRONG‐HF trial. Eur J Heart Fail 2023;25:1156‐1165. doi: 10.1002/EJHF.2882 [DOI] [PubMed] [Google Scholar]
- 10. Wussler D, Belkin M, Maeder MT, Walter J, Shrestha S, Kupska K, et al. Comprehensive vasodilatation in women with acute heart failure: Novel insights from the GALACTIC randomized controlled trial. Eur J Heart Fail 2023;25:2218‐2229. doi: 10.1002/ejhf.3065 [DOI] [PubMed] [Google Scholar]
- 11. Ravera A, Santema BT, de Boer RA, Anker SD, Samani NJ, Lang CC, et al. Distinct pathophysiological pathways in women and men with heart failure. Eur J Heart Fail 2022;24:1532‐1544. doi: 10.1002/ejhf.2534 [DOI] [PubMed] [Google Scholar]
- 12. Keshvani N, Shah S, Ayodele I, Chiswell K, Alhanti B, Allen LA, et al. Sex differences in long‐term outcomes following acute heart failure hospitalization: Findings from the Get With The Guidelines‐Heart Failure registry. Eur J Heart Fail 2023;25:1544‐1554. doi: 10.1002/ejhf.3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel RK, Ioannou A, Razvi Y, Chacko L, Venneri L, Bandera F, et al. Sex differences among patients with transthyretin amyloid cardiomyopathy—From diagnosis to prognosis. Eur J Heart Fail 2022;24:2355‐2363. doi: 10.1002/ejhf.2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aimo A, Tomasoni D, Porcari A, Vergaro G, Castiglione V, Passino C, et al. Left ventricular wall thickness and severity of cardiac disease in women and men with transthyretin amyloidosis. Eur J Heart Fail 2023;25:510‐514. doi: 10.1002/ejhf.2824 [DOI] [PubMed] [Google Scholar]
- 15. Schroeder M, Lim YMF, Savarese G, Suzart‐Woischnik K, Baudier C, Dyszynski T, et al. Sex differences in the generalizability of randomized clinical trials in heart failure with reduced ejection fraction. Eur J Heart Fail 2023;25:912‐921. doi: 10.1002/ejhf.2868 [DOI] [PubMed] [Google Scholar]
- 16. Gonzalez A, Richards AM, de Boer RA, Thum T, Arfsten H, Hulsmann M, et al. Cardiac remodelling—Part 1: From cells and tissues to circulating biomarkers. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:927‐943. doi: 10.1002/ejhf.2493 [DOI] [PubMed] [Google Scholar]
- 17. Packer M. Foetal recapitulation of nutrient surplus signalling by O‐GlcNAcylation and the failing heart. Eur J Heart Fail 2023;25:1199‐1212. doi: 10.1002/ejhf.2972 [DOI] [PubMed] [Google Scholar]
- 18. Shi C, Aboumsallem JP, Suthahar N, de Graaf AO, Jansen JH, van Zeventer IA, et al. Clonal haematopoiesis of indeterminate potential: Associations with heart failure incidence, clinical parameters and biomarkers. Eur J Heart Fail 2023;25:4‐13. doi: 10.1002/ejhf.2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van der Hoef CCS, Boorsma EM, Emmens JE, van Essen BJ, Metra M, Ng LL, et al. Biomarker signature and pathophysiological pathways in patients with chronic heart failure and metabolic syndrome. Eur J Heart Fail 2023;25:163‐173. doi: 10.1002/ejhf.2760 [DOI] [PubMed] [Google Scholar]
- 20. van Woerden G, van Veldhuisen DJ, Westenbrink BD, de Boer RA, Rienstra M, Gorter TM. Connecting epicardial adipose tissue and heart failure with preserved ejection fraction: Mechanisms, management and modern perspectives. Eur J Heart Fail 2022;24:2238‐2250. doi: 10.1002/ejhf.2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garofalo M, Corso R, Tomasoni D, Adamo M, Lombardi CM, Inciardi RM, et al. Inflammation in acute heart failure. Front Cardiovasc Med 2023;10:1235178. doi: 10.3389/fcvm.2023.1235178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ceelen D, Voors AA, Tromp J, van Veldhuisen DJ, Dickstein K, de Boer RA, et al. Pathophysiological pathways related to high plasma growth differentiation factor 15 concentrations in patients with heart failure. Eur J Heart Fail 2022;24:308‐320. doi: 10.1002/ejhf.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solberg OG, Aaberge L, Bosse G, Ueland T, Gullestad L, Aukrust P, et al. Microvascular function and inflammatory activation in Takotsubo cardiomyopathy. ESC Heart Fail. 2023;10:3216‐3222. doi: 10.1002/ehf2.14461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohebi R, Liu Y, van Kimmenade R, Gaggin HK, Murphy SP, Januzzi JL Jr. Inflammation across universal definition of heart failure stages: The CASABLANCA study. Eur J Heart Fail 2023;25:152‐160. doi: 10.1002/ejhf.2742 [DOI] [PubMed] [Google Scholar]
- 25. Michou E, Wussler D, Belkin M, Simmen C, Strebel I, Nowak A, et al. Quantifying inflammation using interleukin‐6 for improved phenotyping and risk stratification in acute heart failure. Eur J Heart Fail 2023;25:174‐184. doi: 10.1002/ejhf.2767 [DOI] [PubMed] [Google Scholar]
- 26. Buckley LF, Dorbala P, Claggett BL, Libby P, Tang W, Coresh J, et al. Circulating neutrophil‐related proteins associate with incident heart failure and cardiac dysfunction: The ARIC study. Eur J Heart Fail 2023;25:1923‐1932. doi: 10.1002/ejhf.3008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lund LH, Lam CSP, Pizzato PE, Gabrielsen A, Michaelsson E, Nelander K, et al. Rationale and design of ENDEAVOR: A sequential phase 2b–3 randomized clinical trial to evaluate the effect of myeloperoxidase inhibition on symptoms and exercise capacity in heart failure with preserved or mildly reduced ejection fraction. Eur J Heart Fail 2023;25:1696‐1707. doi: 10.1002/ejhf.2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Screever EM, Yousif LIE, Moslehi JJ, Salem JE, Voors AA, Silljé HHW, et al. Circulating immune checkpoints predict heart failure outcomes. ESC Heart Fail. 2023;10:2330‐2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Coats AJS, Abraham WT, Zile MR, Lindenfeld JA, Weaver FA, Fudim M, et al. Baroreflex activation therapy with the Barostim™ device in patients with heart failure with reduced ejection fraction: A patient level meta‐analysis of randomized controlled trials. Eur J Heart Fail 2022;24:1665‐1673. doi: 10.1002/ejhf.2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fudim M, Fail PS, Litwin SE, Shaburishvili T, Goyal P, Hummel SL, et al. Endovascular ablation of the right greater splanchnic nerve in heart failure with preserved ejection fraction: Early results of the REBALANCE‐HF trial roll‐in cohort. Eur J Heart Fail 2022;24:1410‐1414. doi: 10.1002/ejhf.2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xueyuan L, Yanping X, Jiaoqiong G, Yuehui Y. Autonomic nervous modulation: Early treatment for pulmonary artery hypertension. ESC Heart Fail.n/a doi: 10.1002/ehf2.14616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Badrov MB, Keir DA, Tomlinson G, Notarius CF, Millar PJ, Kimmerly DS, et al. Normal and excessive muscle sympathetic nerve activity in heart failure: Implications for future trials of therapeutic autonomic modulation. Eur J Heart Fail 2023;25:201‐210. doi: 10.1002/ejhf.2749 [DOI] [PubMed] [Google Scholar]
- 33. Yang M, Kondo T, Adamson C, Butt JH, Abraham WT, Desai AS, et al. Impact of comorbidities on health status measured using the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with reduced and preserved ejection fraction. Eur J Heart Fail 2023;25:1606‐1618. doi: 10.1002/ejhf.2962 [DOI] [PubMed] [Google Scholar]
- 34. Chioncel O, Davison B, Adamo M, Antohi LE, Arrigo M, Barros M, et al. Non‐cardiac comorbidities and intensive up‐titration of oral treatment in patients recently hospitalized for heart failure: Insights from the STRONG‐HF trial. Eur J Heart Fail 2023;25:1994‐2006. doi: 10.1002/ejhf.3039 [DOI] [PubMed] [Google Scholar]
- 35. Anker SD, Usman MS, Anker MS, Butler J, Böhm M, Abraham WT, et al. Patient phenotype profiling in heart failure with preserved ejection fraction to guide therapeutic decision making. A scientific statement of the Heart Failure Association, the European Heart Rhythm Association of the European Society of Cardiology, and the European Society of Hypertension. Eur J Heart Fail 2023;25:936‐955. doi: 10.1002/ejhf.2894 [DOI] [PubMed] [Google Scholar]
- 36. Tomasoni D, Vitale C, Guidetti F, Benson L, Braunschweig F, Dahlstrom U, et al. The role of multimorbidity in patients with heart failure across the left ventricular ejection fraction spectrum: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2023; doi: 10.1002/ejhf.3112 [DOI] [PubMed] [Google Scholar]
- 37. Dewan P, Ferreira JP, Butt JH, Petrie MC, Abraham WT, Desai AS, et al. Impact of multimorbidity on mortality in heart failure with reduced ejection fraction: Which comorbidities matter most? An analysis of PARADIGM‐HF and ATMOSPHERE. Eur J Heart Fail 2023;25:687‐697. doi: 10.1002/ejhf.2856 [DOI] [PubMed] [Google Scholar]
- 38. Pugliese NR, De Biase N, Del Punta L, Balletti A, Armenia S, Buralli S, et al. Deep phenotype characterization of hypertensive response to exercise: Implications on functional capacity and prognosis across the heart failure spectrum. Eur J Heart Fail 2023;25:497‐509. doi: 10.1002/ejhf.2827 [DOI] [PubMed] [Google Scholar]
- 39. Giannoni A, Borrelli C, Gentile F, Sciarrone P, Spiesshofer J, Piepoli M, et al. Autonomic and respiratory consequences of altered chemoreflex function: Clinical and therapeutic implications in cardiovascular diseases. Eur J Heart Fail 2023;25:642‐656. doi: 10.1002/ejhf.2819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Januzzi JL Jr, Butler J, Del Prato S, Ezekowitz JA, Ibrahim NE, Lam CSP, et al. Rationale and design of the Aldose Reductase Inhibition for Stabilization of Exercise Capacity in Heart Failure Trial (ARISE‐HF) in patients with high‐risk diabetic cardiomyopathy. Am Heart J 2023;256:25‐36. doi: 10.1016/j.ahj.2022.11.003 [DOI] [PubMed] [Google Scholar]
- 41. Sakaniwa R, Tromp J, Streng KW, Suthahar N, Kieneker LM, Postmus D, et al. Trajectories of renal biomarkers and new‐onset heart failure in the general population: Findings from the PREVEND study. Eur J Heart Fail 2023;25:1072‐1079. doi: 10.1002/ejhf.2925 [DOI] [PubMed] [Google Scholar]
- 42. Morales J, Handelsman Y. Cardiovascular outcomes in patients with diabetes and kidney disease: JACC review topic of the week. J Am Coll Cardiol 2023;82:161‐170. doi: 10.1016/j.jacc.2023.04.052 [DOI] [PubMed] [Google Scholar]
- 43. Sharma A, Inzucchi SE, Testani JM, Ofstad AP, Fitchett D, Mattheus M, et al. Kidney and heart failure events are bidirectionally associated in patients with type 2 diabetes and cardiovascular disease. ESC Heart Fail.n/a doi: 10.1002/ehf2.14601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2023;44:3627‐3639. doi: 10.1093/eurheartj/ehad195 [DOI] [PubMed] [Google Scholar]
- 45. The E‐KCG , Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med 2023;388:117‐127. doi: 10.1056/NEJMoa2204233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nuffield Department of Population Health Renal Studies Group, SGLT2 inhibitor Meta‐Analysis Cardio‐Renal Trialists' Consortium . Impact of diabetes on the effects of sodium glucose co‐transporter‐2 inhibitors on kidney outcomes: Collaborative meta‐analysis of large placebo‐controlled trials. Lancet 2022;400:1788‐1801. doi: 10.1016/S0140-6736(22)02074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Filippatos G, Pitt B, Agarwal R, Farmakis D, Ruilope LM, Rossing P, et al. Finerenone in patients with chronic kidney disease and type 2 diabetes with and without heart failure: A prespecified subgroup analysis of the FIDELIO‐DKD trial. Eur J Heart Fail 2022;24:996‐1005. doi: 10.1002/ejhf.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur Heart J 2022;43:474‐484. doi: 10.1093/eurheartj/ehab777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 2020;383:2219‐2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
- 50. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 2021;385:2252‐2263. doi: 10.1056/NEJMoa2110956 [DOI] [PubMed] [Google Scholar]
- 51. Chunawala ZS, Qamar A, Arora S, Pandey A, Fudim M, Vaduganathan M, et al. Prognostic significance of obstructive coronary artery disease in patients admitted with acute decompensated heart failure: The ARIC study community surveillance. Eur J Heart Fail 2022;24:2140‐2149. doi: 10.1002/ejhf.2617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med 2022;387:1351‐1360. doi: 10.1056/NEJMoa2206606 [DOI] [PubMed] [Google Scholar]
- 53. Iaconelli A, Pellicori P, Dolce P, Busti M, Ruggio A, Aspromonte N, et al. Coronary revascularization for heart failure with coronary artery disease: A systematic review and meta‐analysis of randomized trials. Eur J Heart Fail 2023;25:1094‐1104. doi: 10.1002/ejhf.2911 [DOI] [PubMed] [Google Scholar]
- 54. Shahim B, Shahim A, Adamo M, Chioncel O, Benson L, Crespo‐Leiro MG, et al. Prevalence, characteristics and prognostic impact of aortic valve disease in patients with heart failure and reduced, mildly reduced, and preserved ejection fraction: An analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2023;25:1049‐1060. doi: 10.1002/ejhf.2908 [DOI] [PubMed] [Google Scholar]
- 55. De Biase N, Mazzola M, Del Punta L, Di Fiore V, De Carlo M, Giannini C, et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: A specific phenotype of heart failure with preserved ejection fraction? Eur J Heart Fail 2023;25:1947‐1958. doi: 10.1002/ejhf.3018 [DOI] [PubMed] [Google Scholar]
- 56. Mack MJ, Leon MB, Thourani VH, Pibarot P, Hahn RT, Genereux P, et al. Transcatheter aortic‐valve replacement in low‐risk patients at five years. N Engl J Med 2023;389:1949‐1960. doi: 10.1056/NEJMoa2307447 [DOI] [PubMed] [Google Scholar]
- 57. Basic C, Hansson P‐O, Sandström TZ, Johansson B, Fu M, Mandalenakis Z. Heart failure outcomes in low‐risk patients with atrial fibrillation: A case–control study of 680 523 Swedish individuals. ESC Heart Fail. 2023;10:2281‐2289. doi: 10.1002/ehf2.14375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hamatani Y, Iguchi M, Minami K, Ishigami K, Esato M, Tsuji H, et al. Utility of left ventricular ejection fraction in atrial fibrillation patients without pre‐existing heart failure. ESC Heart Failure 2023;10:3091‐3101. doi: 10.1002/ehf2.14500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Patel RB, Greene SJ, Xu H, Alhanti B, Peterson P, Yancy CW, et al. Intersection of atrial fibrillation and heart failure with mildly reduced and preserved ejection fraction in >400 000 participants in the Get With The Guidelines‐Heart Failure Registry. Eur J Heart Fail 2023;25:63‐73. doi: 10.1002/ejhf.2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. González‐Ferrero T, Bergonti M, López‐Canoa JN, Arias FG‐R, Eiras Penas S, Spera F, et al. Atrial fibrillation ablation in patients with arrhythmia‐induced cardiomyopathy: A prospective multicentre study. ESC Heart Fail 2023;10:3055‐3066. doi: 10.1002/ehf2.14448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. von Olshausen G, Benson L, Dahlstrom U, Lund LH, Savarese G, Braunschweig F. Catheter ablation for patients with atrial fibrillation and heart failure: Insights from the Swedish Heart Failure Registry. Eur J Heart Fail 2022;24:1636‐1646. doi: 10.1002/ejhf.2604 [DOI] [PubMed] [Google Scholar]
- 62. Zakeri R, Ahluwalia N, Tindale A, Omar F, Packer M, Khan H, et al. Long‐term outcomes following catheter ablation versus medical therapy in patients with persistent atrial fibrillation and heart failure with reduced ejection fraction. Eur J Heart Fail 2023;25:77‐86. doi: 10.1002/ejhf.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sohns C, Fox H, Marrouche NF, Crijns H, Costard‐Jaeckle A, Bergau L, et al. Catheter ablation in end‐stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380‐1389. doi: 10.1056/NEJMoa2306037 [DOI] [PubMed] [Google Scholar]
- 64. Aktaa S, Gale CP, Brida M, Giannakoulas G, Kovacs G, Adir Y, et al. European Society of Cardiology quality indicators for the care and outcomes of adults with pulmonary arterial hypertension. Developed in collaboration with the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023;25:469‐477. doi: 10.1002/ejhf.2830 [DOI] [PubMed] [Google Scholar]
- 65. Kramer T, Nattmann P, Gerhardt F, Stafiej P, Dumitrescu D, ten Freyhaus H, et al. Impact of rapid sequential combination therapy on distinct haemodynamic measures in newly diagnosed pulmonary arterial hypertension. ESC Heart Fail.n/a doi: 10.1002/ehf2.14611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rosenkranz S, Channick R, Chin KM, Jenner B, Gaine S, Galie N, et al. The impact of comorbidities on selexipag treatment effect in patients with pulmonary arterial hypertension: Insights from the GRIPHON study. Eur J Heart Fail 2022;24:205‐214. doi: 10.1002/ejhf.2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Greutmann M, Tobler D, Engel R, Heg D, Mueller C, Frenk A, et al. Effect of phosphodiesterase‐5 inhibition on SystEmic Right VEntricular size and function. A multicentre, double‐blind, randomized, placebo‐controlled trial: SERVE. Eur J Heart Fail 2023;25:1105‐1114. doi: 10.1002/ejhf.2924 [DOI] [PubMed] [Google Scholar]
- 68. Cooper TJ, Cleland JGF, Guazzi M, Pellicori P, Ben Gal T, Amir O, et al. Effects of sildenafil on symptoms and exercise capacity for heart failure with reduced ejection fraction and pulmonary hypertension (the SilHF study): A randomized placebo‐controlled multicentre trial. Eur J Heart Fail 2022;24:1239‐1248. doi: 10.1002/ejhf.2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garcia‐Alvarez A, Blanco I, Garcia‐Lunar I, Jorda P, Rodriguez‐Arias JJ, Fernandez‐Friera L, et al. β3 adrenergic agonist treatment in chronic pulmonary hypertension associated with heart failure (SPHERE‐HF): A double blind, placebo‐controlled, randomized clinical trial. Eur J Heart Fail 2023;25:373‐385. doi: 10.1002/ejhf.2745 [DOI] [PubMed] [Google Scholar]
- 70. Lyon AR, Lopez‐Fernandez T, Couch LS, Asteggiano R, Aznar MC, Bergler‐Klein J, et al. 2022 ESC guidelines on cardio‐oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio‐Oncology Society (IC‐OS). Eur Heart J 2022;43:4229‐4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 71. Nouhravesh N, Strange JE, Holt A, Tonnesen J, Andersen CF, Nielsen SK, et al. Patient mortality following new‐onset heart failure stratified by cancer type and status. Eur J Heart Fail 2023;25:1859‐1867. doi: 10.1002/ejhf.2984 [DOI] [PubMed] [Google Scholar]
- 72. Dobbin SJH, Shen L, Petrie MC, Packer M, Solomon SD, McMurray JJV, et al. Characteristics and outcomes of patients with a history of cancer recruited to heart failure trials. Eur J Heart Fail 2023;25:488‐496. doi: 10.1002/ejhf.2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Graham FJ, Masini G, Pellicori P, Cleland JGF, Greenlaw N, Friday J, et al. Natural history and prognostic significance of iron deficiency and anaemia in ambulatory patients with chronic heart failure. Eur J Heart Fail 2022;24:807‐817. doi: 10.1002/ejhf.2251 [DOI] [PubMed] [Google Scholar]
- 74. Filippatos G, Ponikowski P, Farmakis D, Anker SD, Butler J, Fabien V, et al. Association between hemoglobin levels and efficacy of intravenous ferric carboxymaltose in patients with acute heart failure and iron deficiency: An AFFIRM‐AHF subgroup analysis. Circulation 2023;147:1640‐1653. doi: 10.1161/CIRCULATIONAHA.122.060757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Metra M, Jankowska EA, Pagnesi M, Anker SD, Butler J, Dorigotti F, et al. Impact of ischaemic aetiology on the efficacy of intravenous ferric carboxymaltose in patients with iron deficiency and acute heart failure: Insights from the AFFIRM‐AHF trial. Eur J Heart Fail 2022;24:1928‐1939. doi: 10.1002/ejhf.2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Martens P, Dupont M, Dauw J, Nijst P, Bertrand PB, Tang WHW, et al. The effect of intravenous ferric carboxymaltose on right ventricular function—Insights from the IRON‐CRT trial. Eur J Heart Fail 2022;24:1106‐1113. doi: 10.1002/ejhf.2489 [DOI] [PubMed] [Google Scholar]
- 77. Caravita S, Faini A, Vignati C, Pelucchi S, Salvioni E, Cattadori G, et al. Intravenous iron therapy improves the hypercapnic ventilatory response and sleep disordered breathing in chronic heart failure. Eur J Heart Fail 2022;24:1940‐1949. doi: 10.1002/ejhf.2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mentz RJ, Garg J, Rockhold FW, Butler J, De Pasquale CG, Ezekowitz JA, et al. Ferric carboxymaltose in heart failure with iron deficiency. N Engl J Med 2023;389:975‐986. doi: 10.1056/NEJMoa2304968 [DOI] [PubMed] [Google Scholar]
- 79. Kalra PR, Cleland JGF, Petrie MC, Thomson EA, Kalra PA, Squire IB, et al. Intravenous ferric derisomaltose in patients with heart failure and iron deficiency in the UK (IRONMAN): An investigator‐initiated, prospective, randomised, open‐label, blinded‐endpoint trial. Lancet 2022;400:2199‐2209. doi: 10.1016/S0140-6736(22)02083-9 [DOI] [PubMed] [Google Scholar]
- 80. Anker SD, Khan MS, Butler J, von Haehling S, Jankowska EA, Ponikowski P, et al. Effect of intravenous iron replacement on recurrent heart failure hospitalizations and cardiovascular mortality in patients with heart failure and iron deficiency: A Bayesian meta‐analysis. Eur J Heart Fail 2023;25:1080‐1090. doi: 10.1002/ejhf.2860 [DOI] [PubMed] [Google Scholar]
- 81. Graham FJ, Pellicori P, Kalra PR, Ford I, Bruzzese D, Cleland JGF. Intravenous iron in patients with heart failure and iron deficiency: An updated meta‐analysis. Eur J Heart Fail 2023;25:528‐537. doi: 10.1002/ejhf.2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Salah HM, Savarese G, Rosano GMC, Ambrosy AP, Mentz RJ, Fudim M. Intravenous iron infusion in patients with heart failure: A systematic review and study‐level meta‐analysis. ESC Heart Fail. 2023;10:1473‐1480. doi: 10.1002/ehf2.14310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ponikowski P, Mentz RJ, Hernandez AF, Butler J, Khan MS, van Veldhuisen DJ, et al. Efficacy of ferric carboxymaltose in heart failure with iron deficiency: An individual patient data meta‐analysis. Eur Heart J 2023;44:5077‐5091. doi: 10.1093/eurheartj/ehad586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rosano GM, Kalantar‐Zadeh K, Jankowska EA. Hypophosphataemia risk associated with ferric carboxymaltose in heart failure: A pooled analysis of clinical trials. ESC Heart Fail. 2023;10:1294‐1304. doi: 10.1002/ehf2.14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. McEwan P, Harrison C, Binnie R, Lewis RD, Cohen‐Solal A, Lund LH, et al. Impact of ferric carboxymaltose for iron deficiency at discharge after heart failure hospitalization: A European multinational economic evaluation. Eur J Heart Fail 2023;25:389‐398. doi: 10.1002/EJHF.2788 [DOI] [PubMed] [Google Scholar]
- 86. Lindberg F, Lund LH, Benson L, Linde C, Orsini N, Carrero JJ, et al. Iron deficiency in heart failure: Screening, prevalence, incidence and outcome data from the Swedish Heart Failure Registry and the Stockholm CREAtinine Measurements collaborative project. Eur J Heart Fail 2023;25:1270‐1280. doi: 10.1002/EJHF.2879 [DOI] [PubMed] [Google Scholar]
- 87. Fuchs Andersen C, Omar M, Glenthoj A, El Fassi D, Moller HJ, Lindholm Kurtzhals JA, et al. Effects of empagliflozin on erythropoiesis in heart failure: Data from the Empire HF trial. Eur J Heart Fail 2023;25:226‐234. doi: 10.1002/ejhf.2735 [DOI] [PubMed] [Google Scholar]
- 88. Packer M. How can sodium‐glucose cotransporter 2 inhibitors stimulate erythrocytosis in patients who are iron‐deficient? Implications for understanding iron homeostasis in heart failure. Eur J Heart Fail 2022;24:2287‐2296. doi: 10.1002/ejhf.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Marques P, Matias P, Packer M, Vieira JT, Vasques‐Novoa F, Sharma A, et al. Erythropoietic response after intravenous iron in patients with heart failure and reduced ejection fraction with and without background treatment with sodium–glucose cotransporter 2 inhibitors. Eur J Heart Fail 2023;25:2191‐2198. doi: 10.1002/ejhf.2992 [DOI] [PubMed] [Google Scholar]
- 90. Tomasoni D, Adamo M, Italia L, Branca L, Chizzola G, Fiorina C, et al. Impact of COVID‐2019 outbreak on prevalence, clinical presentation and outcomes of ST‐elevation myocardial infarction. J Cardiovasc Med (Hagerstown) 2020;21:874‐881. doi: 10.2459/JCM.0000000000001098 [DOI] [PubMed] [Google Scholar]
- 91. Paris S, Inciardi RM, Lombardi CM, Tomasoni D, Ameri P, Carubelli V, et al. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID‐19 patients: Results of the Cardio‐COVID‐Italy multicentre study. Europace 2021;23:1603‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Zaccone G, Tomasoni D, Italia L, Lombardi CM, Metra M. Myocardial involvement in COVID‐19: An interaction between comorbidities and heart failure with preserved ejection fraction. A further indication of the role of inflammation. Curr Heart Fail Rep 2021;18:99‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Task Force for the management of C‐otESoC . European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID‐19 pandemic: Part 1—Epidemiology, pathophysiology, and diagnosis. Eur Heart J 2022;43:1033‐1058. doi: 10.1093/eurheartj/ehab696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Task Force for the management of C‐otESoC . ESC guidance for the diagnosis and management of cardiovascular disease during the COVID‐19 pandemic: Part 2—Care pathways, treatment, and follow‐up. Eur Heart J 2022;43:1059‐1103. doi: 10.1093/eurheartj/ehab697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Italia L, Tomasoni D, Bisegna S, Pancaldi E, Stretti L, Adamo M, et al. COVID‐19 and heart failure: From epidemiology during the pandemic to myocardial injury, myocarditis, and heart failure sequelae. Front Cardiovasc Med. 2021;8:713560. doi: 10.3389/fcvm.2021.713560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tomasoni D, Inciardi RM, Lombardi CM, Tedino C, Agostoni P, Ameri P, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID‐19. Results of the Cardio‐COVID‐Italy multicentre study. Eur J Heart Fail 2020;22:2238‐2247. doi: 10.1002/ejhf.2052 [DOI] [PubMed] [Google Scholar]
- 97. Bhatt AS, Dimond M, Fiuzat M, Vaduganathan M, Vardeny O, Divanji P, et al. Impact of COVID‐19 on heart failure clinical trials: Insights from the Heart Failure Collaboratory. JACC Heart failure 2023;11:254‐257. doi: 10.1016/J.JCHF.2022.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bhatt AS, Kosiborod MN, Claggett BL, Miao ZM, Vaduganathan M, Lam CSP, et al. Impact of COVID‐19 in patients with heart failure with mildly reduced or preserved ejection fraction enrolled in the DELIVER trial. Eur J Heart Fail 2023;25:2177‐2188. doi: 10.1002/EJHF.3043 [DOI] [PubMed] [Google Scholar]
- 99. Rosano G, Jankowska EA, Ray R, Metra M, Abdelhamid M, Adamopoulos S, et al. COVID‐19 vaccination in patients with heart failure: A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2021;23:1806‐1818. doi: 10.1002/EJHF.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hulscher N, Hodkinson R, Makis W, McCullough PA. Autopsy findings in cases of fatal COVID‐19 vaccine‐induced myocarditis. ESC Heart Fail.n/a doi: 10.1002/ehf2.14680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ammirati E, Lupi L, Palazzini M, Ciabatti M, Rossi VA, Gentile P, et al. Outcome and morphofunctional changes on cardiac magnetic resonance in patients with acute myocarditis following mRNA COVID‐19 vaccination. Circ Heart Fail 2023;16:e010315. doi: 10.1161/CIRCHEARTFAILURE.122.010315 [DOI] [PubMed] [Google Scholar]
- 102. Docherty KF, Lam CSP, Rakisheva A, Coats AJS, Greenhalgh T, Metra M, et al. Heart failure diagnosis in the general community—Who, how and when? A clinical consensus statement of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2023;25:1185‐1198. doi: 10.1002/EJHF.2946 [DOI] [PubMed] [Google Scholar]
- 103. Bayes‐Genis A, Rosano G. Unlocking the potential of natriuretic peptide testing in primary care: A roadmap for early heart failure diagnosis. Eur J Heart Fail 2023;25:1181‐1184. doi: 10.1002/EJHF.2950 [DOI] [PubMed] [Google Scholar]
- 104. Kozhuharov N, Martin J, Wussler D, Lopez‐Ayala P, Belkin M, Strebel I, et al. Clinical effect of obesity on N‐terminal pro‐B‐type natriuretic peptide cut‐off concentrations for the diagnosis of acute heart failure. Eur J Heart Fail 2022;24:1545‐1554. doi: 10.1002/ejhf.2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bayes‐Genis A, Docherty KF, Petrie MC, Januzzi JL, Mueller C, Andreson L, et al. Practical algorithms for early diagnosis of heart failure and heart stress using NT‐proBNP: A clinical consensus statement from the Heart Failure Association of the ESC. Eur J Heart Fail 2023;25:13‐1898. doi: 10.1002/EJHF.3036 [DOI] [PubMed] [Google Scholar]
- 106. Pagura L, Porcari A, Cameli M, Biagini E, Canepa M, Crotti L, et al. ECG/echo indexes in the diagnostic approach to amyloid cardiomyopathy: A head‐to‐head comparison from the AC‐TIVE study. Eur J Intern Med 2024;122:68‐77. doi: 10.1016/j.ejim.2023.09.026 [DOI] [PubMed] [Google Scholar]
- 107. Surendra K, Nürnberg S, Bremer JP, Knorr MS, Ückert F, Wenzel JP, et al. Pragmatic screening for heart failure in the general population using an electrocardiogram‐based neural network. ESC Heart Fail. 2023;10:975‐984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. de la Espriella R, Amiguet M, Miñana G, Rodríguez JC, Moyano P, Segarra D, et al. Bending oxygen saturation index and risk of worsening heart failure events in chronic heart failure. Eur J Heart Fail 2022;24:2108‐2117. doi: 10.1002/ejhf.2651 [DOI] [PubMed] [Google Scholar]
- 109. Anker MS, Potthoff SK, Lena A, Porthun J, Hadzibegovic S, Evertz R, et al. Cardiovascular health‐related quality of life in cancer: A prospective study comparing the ESC HeartQoL and EORTC QLQ‐C30 questionnaire. Eur J Heart Fail 2023;25:1635‐1647. doi: 10.1002/ejhf.2951 [DOI] [PubMed] [Google Scholar]
- 110. Bhatt AS, Kosiborod MN, Vaduganathan M, Claggett BL, Miao ZM, Kulac IJ, et al. Effect of dapagliflozin on health status and quality of life across the spectrum of ejection fraction: Participant‐level pooled analysis from the DAPA‐HF and DELIVER trials. Eur J Heart Fail 2023;25:981‐988. doi: 10.1002/ejhf.2909 [DOI] [PubMed] [Google Scholar]
- 111. Butler J, Spertus JA, Bamber L, Khan MS, Roessig L, Vlajnic V, et al. Defining changes in physical limitation from the patient perspective: Insights from the VITALITY‐HFpEF randomized trial. Eur J Heart Fail 2022;24:843‐850. doi: 10.1002/ejhf.2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Johansson I, Balasubramanian K, Bangdiwala S, Mielniczuk L, Hage C, Sharma SK, et al. Factors associated with health‐related quality of life in heart failure in 23 000 patients from 40 countries: Results of the G‐CHF research programme. Eur J Heart Fail 2022;24:1478‐1490. doi: 10.1002/ejhf.2535 [DOI] [PubMed] [Google Scholar]
- 113. Lindberg F, Lund LH, Benson L, Dahlström U, Karlström P, Linde C, et al. Trajectories in New York Heart Association functional class in heart failure across the ejection fraction spectrum: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2022;24:2093‐2104. doi: 10.1002/ejhf.2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Savarese G, Lindenfeld J, Stolfo D, Adams K, Ahmad T, Desai NR, et al. Use of patient‐reported outcomes in heart failure: From clinical trials to routine practice. Eur J Heart Fail 2023;25:139‐151. doi: 10.1002/ejhf.2778 [DOI] [PubMed] [Google Scholar]
- 115. Zannad F, Alikhaani J, Alikhaani S, Butler J, Gordon J, Jensen K, et al. Patient‐reported outcome measures and patient engagement in heart failure clinical trials: Multi‐stakeholder perspectives. Eur J Heart Fail 2023;25:478‐487. doi: 10.1002/ejhf.2828 [DOI] [PubMed] [Google Scholar]
- 116. Bayes‐Genis A, Aimo A, Jhund P, Richards M, de Boer RA, Arfsten H, et al. Biomarkers in heart failure clinical trials. A review from the Biomarkers Working Group of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:1767‐1777. doi: 10.1002/ejhf.2675 [DOI] [PubMed] [Google Scholar]
- 117. Klingenberg R, Holtkamp F, Grün D, Frey A, Jahns V, Jahns R, et al. Use of serial changes in biomarkers vs. baseline levels to predict left ventricular remodelling after STEMI. ESC Heart Fail 2023;10:432‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Aleshcheva G, Baumeier C, Harms D, Bock C‐T, Escher F, Schultheiss H‐P. MicroRNAs as novel biomarkers and potential therapeutic options for inflammatory cardiomyopathy. ESC Heart Fail. 2023;10:3410‐3418. doi: 10.1002/ehf2.14523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Oyama K, Giugliano RP, Ruff CT, Berg DD, Jarolim P, Tang M, et al. Serial assessment of biomarkers and heart failure outcomes in patients with atrial fibrillation. Eur J Heart Fail 2023;25:832‐841. doi: 10.1002/ejhf.2844 [DOI] [PubMed] [Google Scholar]
- 120. Pocock SJ, Ferreira JP, Packer M, Zannad F, Filippatos G, Kondo T, et al. Biomarker‐driven prognostic models in chronic heart failure with preserved ejection fraction: The EMPEROR–Preserved trial. Eur J Heart Fail 2022;24:1869‐1878. doi: 10.1002/EJHF.2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. McDowell K, Campbell R, Simpson J, Cunningham JW, Desai AS, Jhund PS, et al. Incremental prognostic value of biomarkers in PARADIGM‐HF. Eur J Heart Fail 2023;25:1406‐1414. doi: 10.1002/EJHF.2887 [DOI] [PubMed] [Google Scholar]
- 122. Bayes‐Genis A, Lupón J, Revuelta‐Lopez E, Llibre C, Gastelurrutia P, Domingo M, et al. Evolocumab has no effects on heart failure with reduced ejection fraction injury biomarkers: The EVO‐HF trial. Eur J Heart Fail 2023;25:1439‐1443. doi: 10.1002/ejhf.2932 [DOI] [PubMed] [Google Scholar]
- 123. Tsutsui H, Albert NM, Coats AJS, Anker SD, Bayes‐Genis A, Butler J, et al. Natriuretic peptides: Role in the diagnosis and management of heart failure: A scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. Eur J Heart Fail 2023;25:616‐631. doi: 10.1002/EJHF.2848 [DOI] [PubMed] [Google Scholar]
- 124. Myhre PL, Liu Y, Kulac IJ, Claggett BL, Prescott MF, Felker GM, et al. Changes in mid‐regional pro‐adrenomedullin during treatment with sacubitril/valsartan. Eur J Heart Fail 2023;25:1396‐1405. doi: 10.1002/ejhf.2957 [DOI] [PubMed] [Google Scholar]
- 125. Parvan R, Hosseinpour M, Moradi Y, Devaux Y, Cataliotti A, da Silva GJJ. Diagnostic performance of microRNAs in the detection of heart failure with reduced or preserved ejection fraction: A systematic review and meta‐analysis. Eur J Heart Fail 2022;24:2212‐2225. doi: 10.1002/ejhf.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wagh V, Nguemo F, Kiseleva Z, Mader RM, Hescheler J, Mohl W. Circulating microRNAs and cardiomyocyte proliferation in heart failure patients related to 10 years survival. ESC Heart Fail. 2023;10:3559‐3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Soussi S, Ahmadiankalati M, Jentzer JC, Marshall JC, Lawler PR, Herridge M, et al. Clinical phenotypes of cardiogenic shock survivors: Insights into late host responses and long‐term outcomes. ESC Heart Fail. 2023;11:1242‐1248. doi: 10.1002/ehf2.14596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Eidizadeh A, Schnelle M, Leha A, Edelmann F, Nolte K, Werhahn SM, et al. Biomarker profiles in heart failure with preserved vs. reduced ejection fraction: Results from the DIAST‐CHF study. ESC Heart Fail. 2023;10:200‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Smiseth OA, Morris DA, Cardim N, Cikes M, Delgado V, Donal E, et al. Multimodality imaging in patients with heart failure and preserved ejection fraction: An expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2022;23:2‐2, 13. [DOI] [PubMed] [Google Scholar]
- 130. Moura B, Aimo A, Al‐Mohammad A, Keramida K, Ben Gal T, Dorbala S, et al. Diagnosis and management of patients with left ventricular hypertrophy: Role of multimodality cardiac imaging. A scientific statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023;25:1493‐1506. doi: 10.1002/ejhf.2997 [DOI] [PubMed] [Google Scholar]
- 131. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: A systematic review and meta‐analysis of global longitudinal strain and ejection fraction. Heart 2014;100:1673‐1680. doi: 10.1136/heartjnl-2014-305538 [DOI] [PubMed] [Google Scholar]
- 132. Brann A, Miller J, Eshraghian E, Park JJ, Greenberg B. Global longitudinal strain predicts clinical outcomes in patients with heart failure with preserved ejection fraction. Eur J Heart Fail 2023;25:1755‐1765. doi: 10.1002/ejhf.2947 [DOI] [PubMed] [Google Scholar]
- 133. Harada T, Kagami K, Shina T, Sorimachi H, Yuasa N, Saito Y, et al. Diagnostic value of reduced left atrial compliance during ergometry exercise in heart failure with preserved ejection fraction. Eur J Heart Fail 2023;25:1293‐1303. doi: 10.1002/ejhf.2862 [DOI] [PubMed] [Google Scholar]
- 134. Gard EK, Beale AL, Telles F, Silvestry FE, Hanff T, Hummel SL, et al. Left atrial enlargement is associated with pulmonary vascular disease in heart failure with preserved ejection fraction. Eur J Heart Fail 2023;25:806‐814. doi: 10.1002/ejhf.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Aimo A, Vergaro G, González A, Barison A, Lupón J, Delgado V, et al. Cardiac remodelling—Part 2: Clinical, imaging and laboratory findings. A review from the Study Group on Biomarkers of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:944‐958. doi: 10.1002/EJHF.2522 [DOI] [PubMed] [Google Scholar]
- 136. Pascual‐Figal DA, Zamorano JL, Domingo M, Morillas H, Nuñez J, Cobo Marcos M, et al. Impact of dapagliflozin on cardiac remodelling in patients with chronic heart failure: The DAPA‐MODA study. Eur J Heart Fail 2023;25:1352‐1360. doi: 10.1002/ejhf.2884 [DOI] [PubMed] [Google Scholar]
- 137. Ansari Ramandi MM, van Melle JP, Gorter TM, Hoendermis ES, van Veldhuisen DJ, Nauta JF, et al. Right ventricular dysfunction in patients with new‐onset heart failure: Longitudinal follow‐up during guideline‐directed medical therapy. Eur J Heart Fail 2022;24:2226‐2234. doi: 10.1002/ejhf.2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Caiffa T, De Luca A, Biagini E, Lupi L, Bedogni F, Castrichini M, et al. Impact on clinical outcomes of right ventricular response to percutaneous correction of secondary mitral regurgitation. Eur J Heart Fail 2021;23:1765‐1774. [DOI] [PubMed] [Google Scholar]
- 139. Pellicori P, Platz E, Dauw J, Ter Maaten JM, Martens P, Pivetta E, et al. Ultrasound imaging of congestion in heart failure: Examinations beyond the heart. Eur J Heart Fail 2021;23:703‐712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Metra M, Tomasoni D, Adamo M, Bayes‐Genis A, Filippatos G, Abdelhamid M, et al. Worsening of chronic heart failure: Definition, epidemiology, management and prevention. A clinical consensus statement by the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023;25:776‐791. doi: 10.1002/ejhf.2874 [DOI] [PubMed] [Google Scholar]
- 141. Metra M, Adamo M, Tomasoni D, Mebazaa A, Bayes‐Genis A, Abdelhamid M, et al. Pre‐discharge and early post‐discharge management of patients hospitalized for acute heart failure: A scientific statement by the Heart Failure Association of the ESC. Eur J Heart Fail 2023;25:1115‐1131. doi: 10.1002/EJHF.2888 [DOI] [PubMed] [Google Scholar]
- 142. Segar MW, Khan MS, Patel KV, Vaduganathan M, Kannan V, Willett D, et al. Incorporation of natriuretic peptides with clinical risk scores to predict heart failure among individuals with dysglycaemia. Eur J Heart Fail 2022;24:169‐180. doi: 10.1002/EJHF.2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Jering KS, Campagnari C, Claggett B, Adler E, Klein L, Ahmad FS, et al. Improving clinical trial efficiency using a machine learning‐based risk score to enrich study populations. Eur J Heart Fail 2022;24:1418‐1426. doi: 10.1002/EJHF.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nakano K, Nochioka K, Yasuda S, Tamori D, Shiroto T, Sato Y, et al. Machine learning approach to stratify complex heterogeneity of chronic heart failure: A report from the CHART‐2 study. ESC Heart Fail. 2023;10:1597‐1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Khan MS, Arshad MS, Greene SJ, Van Spall HGC, Pandey A, Vemulapalli S, et al. Artificial intelligence and heart failure: A state‐of‐the‐art review. Eur J Heart Fail 2023;25:1507‐1525. doi: 10.1002/EJHF.2994 [DOI] [PubMed] [Google Scholar]
- 146. De Filippo O, Cammann VL, Pancotti C, Di Vece D, Silverio A, Schweiger V, et al. Machine learning‐based prediction of in‐hospital death for patients with takotsubo syndrome: The InterTAK‐ML model. Eur J Heart Fail 2023;25:2299‐2311. doi: 10.1002/ejhf.2983 [DOI] [PubMed] [Google Scholar]
- 147. Park JJ, Jang SY, Adler E, Ahmad F, Campagnari C, Yagil A, et al. A machine learning‐derived risk score predicts mortality in East Asian patients with acute heart failure. Eur J Heart Fail 2023;25:2331‐2333. doi: 10.1002/ejhf.3059 [DOI] [PubMed] [Google Scholar]
- 148. Goldsmith AJ, Jin M, Lucassen R, Duggan NM, Harrison NE, Wells W, et al. Comparison of pulmonary congestion severity using artificial intelligence‐assisted scoring versus clinical experts: A secondary analysis of BLUSHED‐AHF. Eur J Heart Fail 2023;25:1166‐1169. doi: 10.1002/ejhf.2881 [DOI] [PubMed] [Google Scholar]
- 149. Eda Y, Nabeta T, Iikura S, Takigami Y, Fujita T, Iida Y, et al. Non‐dilated left ventricular cardiomyopathy vs. dilated cardiomyopathy: Clinical background and outcomes. ESC Heart Fail.n/a doi: 10.1002/ehf2.14711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Keil L, Berisha F, Ritter S, Skibowski J, Subramanian H, Nikolaev VO, et al. Multimodal characterization of dilated cardiomyopathy: Geno‐ And Phenotyping of PrImary Cardiomyopathy (GrAPHIC). ESC Heart Fail. 2024;11:541‐549. doi: 10.1002/ehf2.14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Arbelo E, Protonotarios A, Gimeno JR, Arbustini E, Barriales‐Villa R, Basso C, et al. 2023 ESC guidelines for the management of cardiomyopathies: Developed by the Task Force on the Management of Cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J 2023;44:3503‐3626. doi: 10.1093/EURHEARTJ/EHAD194 [DOI] [PubMed] [Google Scholar]
- 152. Seferović PM, Polovina M, Rosano G, Bozkurt B, Metra M, Heymans S, et al. State‐of‐the‐art document on optimal contemporary management of cardiomyopathies. Eur J Heart Fail 2023;25:1899‐1922. doi: 10.1002/EJHF.2979 [DOI] [PubMed] [Google Scholar]
- 153. de Boer RA, Heymans S, Backs J, Carrier L, Coats AJS, Dimmeler S, et al. Targeted therapies in genetic dilated and hypertrophic cardiomyopathies: From molecular mechanisms to therapeutic targets. A position paper from the Heart Failure Association (HFA) and the Working Group on Myocardial Function of the European Society of Cardiology (ESC). Eur J Heart Fail 2022;24:406‐420. doi: 10.1002/ejhf.2414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Polovina M, Tschöpe C, Rosano G, Metra M, Crea F, Mullens W, et al. Incidence, risk assessment and prevention of sudden cardiac death in cardiomyopathies. Eur J Heart Fail 2023;25:2144‐2163. doi: 10.1002/EJHF.3076 [DOI] [PubMed] [Google Scholar]
- 155. Hammersley DJ, Jones RE, Owen R, Mach L, Lota AS, Khalique Z, et al. Phenotype, outcomes and natural history of early‐stage non‐ischaemic cardiomyopathy. Eur J Heart Fail 2023;25:2050‐2059. doi: 10.1002/EJHF.3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wilde AAM, Semsarian C, Márquez MF, Shamloo AS, Ackerman MJ, Ashley EA, et al. European Heart Rhythm Association (EHRA)/Heart Rhythm Society (HRS)/Asia Pacific Heart Rhythm Society (APHRS)/Latin American Heart Rhythm Society (LAHRS) Expert Consensus Statement on the state of genetic testing for cardiac diseases. EP Europace 2022;24:1307‐1367. doi: 10.1093/EUROPACE/EUAC030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Barat A, Chen CW, Patel‐Murray N, McMurray JJV, Packer M, Solomon SD, et al. Clinical characteristics of heart failure with reduced ejection fraction patients with rare pathogenic variants in dilated cardiomyopathy‐associated genes: A subgroup analysis of the PARADIGM‐HF trial. Eur J Heart Fail 2023;25:1256‐1266. doi: 10.1002/EJHF.2886 [DOI] [PubMed] [Google Scholar]
- 158. Jones RE, Hammersley DJ, Zheng S, McGurk KA, de Marvao A, Theotokis PI, et al. Assessing the association between genetic and phenotypic features of dilated cardiomyopathy and outcome in patients with coronary artery disease. Eur J Heart Fail 2023;26:46‐55. doi: 10.1002/EJHF.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Desai MY, Owens A, Geske JB, Wolski K, Saberi S, Wang A, et al. Dose‐blinded myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: Outcomes through 32 weeks. Circulation 2023;147:850‐863. doi: 10.1161/CIRCULATIONAHA.122.062534 [DOI] [PubMed] [Google Scholar]
- 160. Desai MY, Owens A, Geske JB, Wolski K, Naidu SS, Smedira NG, et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J Am Coll Cardiol 2022;80:95‐108. doi: 10.1016/J.JACC.2022.04.048 [DOI] [PubMed] [Google Scholar]
- 161. Zhang Y, Adamo M, Zou C, Porcari A, Tomasoni D, Rossi M, et al. Management of hypertrophic cardiomyopathy. J Cardiovasc Med (Hagerstown) 2024; doi: 10.2459/JCM.0000000000001616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Desai MY, Owens A, Wolski K, Geske JB, Saberi S, Wang A, et al. Mavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: Week 56 results from the VALOR‐HCM randomized clinical trial. JAMA Cardiol 2023;8:8. doi: 10.1001/JAMACARDIO.2023.3342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Wheeler MT, Olivotto I, Elliott PM, Saberi S, Owens AT, Maurer MS, et al. Effects of mavacamten on measures of cardiopulmonary exercise testing beyond peak oxygen consumption: A secondary analysis of the EXPLORER‐HCM randomized trial. JAMA Cardiol 2023;8:240‐247. doi: 10.1001/jamacardio.2022.5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Wheeler MT, Jacoby D, Elliott PM, Saberi S, Hegde SM, Lakdawala NK, et al. Effect of beta‐blocker therapy on the response to mavacamten in patients with symptomatic obstructive hypertrophic cardiomyopathy. Eur J Heart Fail 2023;25:260‐270. doi: 10.1002/ejhf.2737 [DOI] [PubMed] [Google Scholar]
- 165. Rixon C, Andreassen K, Shen X, Erusappan PM, Almaas VM, Palmero S, et al. Lumican accumulates with fibrillar collagen in fibrosis in hypertrophic cardiomyopathy. ESC Heart Fail. 2023;10:858‐871. doi: 10.1002/ehf2.14234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bertero E, Chiti C, Schiavo MA, Tini G, Costa P, Todiere G, et al. Real‐world candidacy to mavacamten in a contemporary hypertrophic obstructive cardiomyopathy population. Eur J Heart Fail 2024;26:59‐64. doi: 10.1002/ejhf.3120 [DOI] [PubMed] [Google Scholar]
- 167. Maron MS, Mahmod M, Abd Samat AH, Choudhury L, Massera D, Phelan DMJ, et al. Safety and efficacy of metabolic modulation with ninerafaxstat in patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol 2024; doi: 10.1016/j.jacc.2024.03.387 [DOI] [PubMed] [Google Scholar]
- 168. Hoevelmann J, Engel ME, Muller E, Hohlfeld A, Böhm M, Sliwa K, et al. A global perspective on the management and outcomes of peripartum cardiomyopathy: A systematic review and meta‐analysis. Eur J Heart Fail 2022;24:1719‐1736. doi: 10.1002/EJHF.2603 [DOI] [PubMed] [Google Scholar]
- 169. Imran TF, Ataklte F, Khalid M, Lopez D, Mohebali D, Bello NA, et al. Clinical predictors of right ventricular dysfunction and association with adverse outcomes in peripartum cardiomyopathy. ESC Heart Fail. 2024;11:422‐432. doi: 10.1002/ehf2.14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jackson AM, Bauersachs J, Petrie MC, van der Meer P, Laroche C, Farhan HA, et al. Outcomes at one year in women with peripartum cardiomyopathy: Findings from the ESC EORP PPCM Registry. Eur J Heart Fail 2023;26:34‐42. doi: 10.1002/EJHF.3055 [DOI] [PubMed] [Google Scholar]
- 171. Tromp J, Jackson AM, Abdelhamid M, Fouad D, Youssef G, Petrie MC, et al. Thromboembolic events in peripartum cardiomyopathy: Results from the ESC EORP PPCM registry. Eur J Heart Fail 2023;25:1464‐1466. doi: 10.1002/EJHF.2871 [DOI] [PubMed] [Google Scholar]
- 172. Merlo M, Pagura L, Porcari A, Cameli M, Vergaro G, Musumeci B, et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: Results from Phase 2 of the AC‐TIVE study, an Italian nationwide survey. Eur J Heart Fail 2022;24:1377‐1386. [DOI] [PubMed] [Google Scholar]
- 173. Antonopoulos AS, Panagiotopoulos I, Kouroutzoglou A, Koutsis G, Toskas P, Lazaros G, et al. Prevalence and clinical outcomes of transthyretin amyloidosis: A systematic review and meta‐analysis. Eur J Heart Fail 2022;24:1677‐1696. doi: 10.1002/EJHF.2589 [DOI] [PubMed] [Google Scholar]
- 174. Beuthner BE, Elkenani M, Evert K, Mustroph J, Jacob CF, Paul NB, et al. Histological assessment of cardiac amyloidosis in patients undergoing transcatheter aortic valve replacement. ESC Heart Fail.n/a doi: 10.1002/ehf2.14709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tomasoni D, Aimo A, Adamo M, Nardi M, Lombardi CM, Regazzoni V, et al. Echocardiographic findings in subjects with an amyloidogenic apolipoprotein A1 pathogenic variant. Amyloid 2023;30:335‐345. doi: 10.1080/13506129.2023.2190003 [DOI] [PubMed] [Google Scholar]
- 176. Porcari A, Razvi Y, Masi A, Patel R, Ioannou A, Rauf MU, et al. Prevalence, characteristics and outcomes of older patients with hereditary versus wild‐type transthyretin amyloid cardiomyopathy. Eur J Heart Fail 2023;25:515‐524. doi: 10.1002/EJHF.2776 [DOI] [PubMed] [Google Scholar]
- 177. Vergaro G, Castiglione V, Aimo A, Prontera C, Masotti S, Musetti V, et al. N‐terminal pro‐B‐type natriuretic peptide and high‐sensitivity troponin T hold diagnostic value in cardiac amyloidosis. Eur J Heart Fail 2023;25:335‐346. doi: 10.1002/EJHF.2769 [DOI] [PubMed] [Google Scholar]
- 178. Zaroui A, Kharoubi M, Gounot R, Oghina S, Degoutte C, Bezard M, et al. Prognostic mortality factors in advanced light chain cardiac amyloidosis: A prospective cohort study. ESC Heart Fail.n/a doi: 10.1002/ehf2.14671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Rosengren S, Skibsted Clemmensen T, Hvitfeldt Poulsen S, Tolbod L, Harms HJ, Wikström G, et al. Outcome prediction by myocardial external efficiency from 11C‐acetate positron emission tomography in cardiac amyloidosis. ESC Heart Fail. 2024;11:44‐53. doi: 10.1002/ehf2.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tomasoni D, Adamo M, Porcari A, Aimo A, Bonfioli GB, Castiglione V, et al. Right ventricular to pulmonary artery coupling and outcome in patients with cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2023;24:1405‐1414. doi: 10.1093/ehjci/jead145 [DOI] [PubMed] [Google Scholar]
- 181. Tomasoni D, Aimo A, Porcari A, Bonfioli GB, Castiglione V, Saro R, et al. Prevalence and clinical outcomes of isolated or combined moderate to severe mitral and tricuspid regurgitation in patients with cardiac amyloidosis. Eur Heart J Cardiovasc Imaging 2024; doi: 10.1093/ehjci/jeae060 [DOI] [PubMed] [Google Scholar]
- 182. Chacko L, Karia N, Venneri L, Bandera F, Passo BD, Buonamici L, et al. Progression of echocardiographic parameters and prognosis in transthyretin cardiac amyloidosis. Eur J Heart Fail 2022;24:1700‐1712. doi: 10.1002/EJHF.2606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Vergaro G, Aimo A, Rapezzi C, Castiglione V, Fabiani I, Pucci A, et al. Atrial amyloidosis: Mechanisms and clinical manifestations. Eur J Heart Fail 2022;24:2019‐2019, 2028. doi: 10.1002/EJHF.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Akintoye E, Majid M, Klein AL, Hanna M. Prognostic utility of left atrial strain to predict thrombotic events and mortality in amyloid cardiomyopathy. JACC Cardiovasc Imaging 2023;16:1371‐1383. doi: 10.1016/j.jcmg.2023.01.015 [DOI] [PubMed] [Google Scholar]
- 185. Tomasoni D, Bonfioli GB, Aimo A, Adamo M, Canepa M, Inciardi RM, et al. Treating amyloid transthyretin cardiomyopathy: Lessons learned from clinical trials. Front Cardiovasc Med 2023;10:10. doi: 10.3389/fcvm.2023.1154594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Elliott P, Gundapaneni B, Sultan MB, Ines M, Garcia‐Pavia P. Improved long‐term survival with tafamidis treatment in patients with transthyretin amyloid cardiomyopathy and severe heart failure symptoms. Eur J Heart Fail 2023;25:2060‐2064. doi: 10.1002/ejhf.2974 [DOI] [PubMed] [Google Scholar]
- 187. Tubben A, Nienhuis HLA, van der Meer P. Tafamidis in patients with severe heart failure due to transthyretin amyloidosis cardiomyopathy: Improved long‐term survival. Eur J Heart Fail 2023;25:2065‐2066. doi: 10.1002/ejhf.3053 [DOI] [PubMed] [Google Scholar]
- 188. Gillmore JD, Judge DP, Cappelli F, Fontana M, Garcia‐Pavia P, Gibbs S, et al. Efficacy and safety of acoramidis in transthyretin amyloid cardiomyopathy. N Engl J Med 2024;390:132‐142. doi: 10.1056/NEJMoa2305434 [DOI] [PubMed] [Google Scholar]
- 189. Rosenblum HR, Griffin JM, Minamisawa M, Prasad N, Vest J, White MT, et al. Effect of patisiran on stroke volume in hereditary transthyretin‐mediated amyloidosis: Insights from pressure–volume analysis of the APOLLO study. Eur J Heart Fail 2023;25:727‐736. doi: 10.1002/EJHF.2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Maurer MS, Kale P, Fontana M, Berk JL, Grogan M, Gustafsson F, et al. Patisiran treatment in patients with transthyretin cardiac amyloidosis. New England Journal of Medicine. 2023;389:1553‐1565. doi: 10.1056/NEJMoa2300757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Lei Z, Cao J, Wu J, Lu Y, Ni L, Hu X. Identification of the communal pathogenesis and immune landscape between viral myocarditis and dilated cardiomyopathy. ESC Heart Fail. 2024;11:282‐292. doi: 10.1002/ehf2.14585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Yamamoto M, Tajiri K, Ayuzawa S, Ieda M. Pathological findings of clinically suspected myocarditis temporally associated with COVID‐19 vaccination. Eur J Heart Fail 2022;24:1132‐1138. doi: 10.1002/ejhf.2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Heidecker B, Dagan N, Balicer R, Eriksson U, Rosano G, Coats A, et al. Myocarditis following COVID‐19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail 2022;24:2000‐2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Morrow DA, Verbrugge FH. In‐perspective: The ARAMIS double‐blind randomized placebo‐controlled trial of anakinra for the treatment of acute myocarditis. Eur Heart J Acute Cardiovasc Care 2023;12:627‐628. doi: 10.1093/EHJACC/ZUAD102 [DOI] [PubMed] [Google Scholar]
- 195. Monzo L, Girerd N, Duarte K, Ferreira JP, McMurray JJV, van Veldhuisen DJ, et al. Time to clinical benefit of eplerenone among patients with heart failure and reduced ejection fraction: A subgroups analysis from the EMPHASIS‐HF trial. Eur J Heart Fail 2023;25:1444‐1449. doi: 10.1002/ejhf.2952 [DOI] [PubMed] [Google Scholar]
- 196. Tomasoni D, Fonarow GC, Adamo M, Anker SD, Butler J, Coats AJS, et al. Sodium–glucose co‐transporter 2 inhibitors as an early, first‐line therapy in patients with heart failure and reduced ejection fraction. Eur J Heart Fail 2022;24:431‐441. doi: 10.1002/EJHF.2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Musella F, Rosano GMC, Hage C, Benson L, Guidetti F, Moura B, et al. Patient profiles in heart failure with reduced ejection fraction: Prevalence, characteristics, treatments and outcomes in a real‐world heart failure population. Eur J Heart Fail 2023;25:1246‐1253. doi: 10.1002/EJHF.2892 [DOI] [PubMed] [Google Scholar]
- 198. Jalloh MB, Granger CB, Fonarow GC, Van Spall HGC. Multi‐level implementation strategies to improve uptake of evidence‐based therapies in heart failure. Eur Heart J 2023;44:2055‐2058. doi: 10.1093/eurheartj/ehad150 [DOI] [PubMed] [Google Scholar]
- 199. Giovinazzo S, Carmisciano L, Toma M, Benenati S, Tomasoni D, Sormani MP, et al. Sacubitril/valsartan in real‐life European patients with heart failure and reduced ejection fraction: A systematic review and meta‐analysis. ESC Heart Fail. 2021;8:3547‐3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Savarese G, Kishi T, Vardeny O, Adamsson Eryd S, Bodegard J, Lund LH, et al. Heart failure drug treatment—Inertia, titration, and discontinuation: A multinational observational study (EVOLUTION HF). JACC Heart Fail 2022; [DOI] [PubMed] [Google Scholar]
- 201. Carubelli V, Lombardi C, Specchia C, Peveri G, Oriecuia C, Tomasoni D, et al. Adherence and optimization of angiotensin converting enzyme inhibitor/angiotensin II receptors blockers and beta‐blockers in patients hospitalized for acute heart failure. ESC Heart Fail. 2021;8:1944‐1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bhatt AS, Vaduganathan M, Solomon SD, Schneeweiss S, Lauffenburger JC, Rj D. Sacubitril/valsartan use patterns among older adults with heart failure in clinical practice: A population‐based cohort study of >25 000 Medicare beneficiaries. Eur J Heart Fail 2022;24:1506‐1515. [DOI] [PubMed] [Google Scholar]
- 203. Fatima K, Butler J, Fonarow GC. Residual risk in heart failure and the need for simultaneous implementation and innovation. Eur J Heart Fail 2023;25:1477‐1480. doi: 10.1002/ejhf.3005 [DOI] [PubMed] [Google Scholar]
- 204. Tomasoni D, Pagnesi M, Colombo G, Chiarito M, Stolfo D, Baldetti L, et al. Guideline‐directed medical therapy in severe heart failure with reduced ejection fraction: An analysis from the HELP‐HF registry. Eur J Heart Fail 2023;26:327‐337. doi: 10.1002/ejhf.3081 [DOI] [PubMed] [Google Scholar]
- 205. Fauvel C, Saldarriaga Giraldo CI, Barassa A, Shchendrygina A, Mapelli M, Jakus N, et al. Differences between heart failure specialists and non‐specialists regarding heart failure drug implementation and up‐titration. Eur J Heart Fail 2023;25:1884‐1886. doi: 10.1002/ejhf.3010 [DOI] [PubMed] [Google Scholar]
- 206. Averbuch T, Esfahani M, Khatib R, Kayima J, Miranda JJ, Wadhera RK, et al. Pharmaco‐disparities in heart failure: A survey of the affordability of guideline recommended therapy in 10 countries. ESC Heart Failure. 2023;10:3152‐3163. doi: 10.1002/ehf2.14468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Cappelletto C, Stolfo D, Orsini N, Benson L, Rodolico D, Rosano GMC, et al. Use of and association between heart failure pharmacological treatments and outcomes in obese versus non‐obese patients with heart failure with reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2023;25:698‐710. doi: 10.1002/ejhf.2795 [DOI] [PubMed] [Google Scholar]
- 208. Mullens W, Martens P, Testani JM, Tang WHW, Skouri H, Verbrugge FH, et al. Renal effects of guideline‐directed medical therapies in heart failure: A consensus document from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2022;24:603‐619. doi: 10.1002/EJHF.2471 [DOI] [PubMed] [Google Scholar]
- 209. Zahir D, Bonde A, Madelaire C, Malmborg M, Butt JH, Fosbol E, et al. Temporal trends in initiation of mineralocorticoid receptor antagonists and risk of subsequent withdrawal in patients with heart failure: A nationwide study in Denmark from 2003–2017. Eur J Heart Fail 2022;24:539‐547. doi: 10.1002/EJHF.2418 [DOI] [PubMed] [Google Scholar]
- 210. Lombardi CM, Carubelli V, Peveri G, Inciardi RM, Pagnesi M, Ravera A, et al. Prognostic significance of serum potassium in patients hospitalized for acute heart failure. ESC Heart Fail 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Tomasoni D, Davison B, Adamo M, Pagnesi M, Mebazaa A, Edwards C, et al. Safety indicators in patients receiving high‐intensity care after hospital admission for acute heart failure: The STRONG‐HF trial. J Card Fail 2023;30:525‐537. doi: 10.1016/j.cardfail.2023.09.002 [DOI] [PubMed] [Google Scholar]
- 212. Janse RJ, Fu EL, Dahlström U, Benson L, Lindholm B, van Diepen M, et al. Use of guideline‐recommended medical therapy in patients with heart failure and chronic kidney disease: From physician's prescriptions to patient's dispensations, medication adherence and persistence. Eur J Heart Fail 2022;24:2185‐2195. doi: 10.1002/ejhf.2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Guidetti F, Lund LH, Benson L, Hage C, Musella F, Stolfo D, et al. Safety of continuing mineralocorticoid receptor antagonist treatment in patients with heart failure with reduced ejection fraction and severe kidney disease: Data from Swedish Heart Failure Registry. Eur J Heart Fail 2023;25:2164‐2173. doi: 10.1002/ejhf.3049 [DOI] [PubMed] [Google Scholar]
- 214. Mc Causland FR, Lefkowitz MP, Claggett B, Packer M, Senni M, Gori M, et al. Angiotensin–neprilysin inhibition and renal outcomes across the spectrum of ejection fraction in heart failure. Eur J Heart Fail 2022;24:1591‐1598. doi: 10.1002/ejhf.2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Vaduganathan M, Ferreira JP, Rossignol P, Neuen BL, Claggett BL, Pfeffer MA, et al. Effects of steroidal mineralocorticoid receptor antagonists on acute and chronic estimated glomerular filtration rate slopes in patients with chronic heart failure. Eur J Heart Fail 2022;24:1586‐1590. doi: 10.1002/EJHF.2635 [DOI] [PubMed] [Google Scholar]
- 216. Lin DSH, Lin FJ, Lin YS, Lee JK, Lin YH. The effects of mineralocorticoid receptor antagonists on cardiovascular outcomes in patients with end‐stage renal disease and heart failure. Eur J Heart Fail 2023;25:98‐107. doi: 10.1002/EJHF.2740 [DOI] [PubMed] [Google Scholar]
- 217. Butler J, Anker SD, Siddiqi TJ, Coats AJS, Dorigotti F, Filippatos G, et al. Patiromer for the management of hyperkalaemia in patients receiving renin–angiotensin–aldosterone system inhibitors for heart failure: Design and rationale of the DIAMOND trial. Eur J Heart Fail 2022;24:230‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Butler J, Anker SD, Lund LH, Coats AJS, Filippatos G, Siddiqi TJ, et al. Patiromer for the management of hyperkalemia in heart failure with reduced ejection fraction: The DIAMOND trial. Eur Heart J 2022;43:4362‐4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Pagnesi M, Baldetti L, Aimo A, Inciardi RM, Tomasoni D, Vizzardi E, et al. Prognostic benefit of new drugs for HFrEF: A systematic review and network meta‐analysis. J Clin Med 2022;11:2‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta‐analysis of five randomised controlled trials. The Lancet 2022;400:757‐767. doi: 10.1016/S0140-6736(22)01429-5 [DOI] [PubMed] [Google Scholar]
- 221. Filippatos G, Anker SD, Butler J, Farmakis D, Ferreira JP, Gollop ND, et al. Effects of empagliflozin on cardiovascular and renal outcomes in heart failure with reduced ejection fraction according to age: A secondary analysis of EMPEROR‐Reduced. Eur J Heart Fail 2022;24:2297‐2304. doi: 10.1002/EJHF.2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Adamson C, Cowan LM, de Boer RA, Diez M, Drożdż J, Dukát A, et al. Liver tests and outcomes in heart failure with reduced ejection fraction: Findings from DAPA‐HF. Eur J Heart Fail 2022;24:1856‐1868. doi: 10.1002/EJHF.2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Voors AA, Damman K, Teerlink JR, Angermann CE, Collins SP, Kosiborod M, et al. Renal effects of empagliflozin in patients hospitalized for acute heart failure: From the EMPULSE trial. Eur J Heart Fail 2022;24:1844‐1852. doi: 10.1002/EJHF.2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Butt JH, Docherty KF, Jhund PS, de Boer RA, Böhm M, Desai AS, et al. Dapagliflozin and atrial fibrillation in heart failure with reduced ejection fraction: Insights from DAPA‐HF. Eur J Heart Fail 2022;24:513‐525. doi: 10.1002/EJHF.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Ferreira JP, Blatchford JP, Teerlink JR, Kosiborod MN, Angermann CE, Biegus J, et al. Mineralocorticoid receptor antagonist use and the effects of empagliflozin on clinical outcomes in patients admitted for acute heart failure: Findings from EMPULSE. Eur J Heart Fail 2023;25:1797‐1805. doi: 10.1002/EJHF.2982 [DOI] [PubMed] [Google Scholar]
- 226. Chatur S, Kondo T, Claggett BL, Docherty K, Miao ZM, Desai AS, et al. Effects of dapagliflozin on heart failure hospitalizations according to severity of inpatient course: Insights from DELIVER and DAPA‐HF. Eur J Heart Fail 2023;25:1364‐1371. doi: 10.1002/EJHF.2912 [DOI] [PubMed] [Google Scholar]
- 227. Palau P, Amiguet M, Domínguez E, Sastre C, Mollar A, Seller J, et al. Short‐term effects of dapagliflozin on maximal functional capacity in heart failure with reduced ejection fraction (DAPA‐VO2): A randomized clinical trial. Eur J Heart Fail 2022;24:1816‐1826. doi: 10.1002/EJHF.2560 [DOI] [PubMed] [Google Scholar]
- 228. Vukadinović D, Abdin A, Anker SD, Rosano GMC, Mahfoud F, Packer M, et al. Side effects and treatment initiation barriers of sodium–glucose cotransporter 2 inhibitors in heart failure: A systematic review and meta‐analysis. Eur J Heart Fail 2022;24:1625‐1632. doi: 10.1002/ejhf.2584 [DOI] [PubMed] [Google Scholar]
- 229. Chatur S, Cunningham JW, Vaduganathan M, Mc Causland FR, Claggett BL, Desai AS, et al. Renal and blood pressure effects of dapagliflozin in recently hospitalized patients with heart failure with mildly reduced or preserved ejection fraction: Insights from the DELIVER trial. Eur J Heart Fail 2023;25:1170‐1175. doi: 10.1002/ejhf.2915 [DOI] [PubMed] [Google Scholar]
- 230. Zannad F, Ferreira JP, Gregson J, Kraus BJ, Mattheus M, Hauske SJ, et al. Early changes in estimated glomerular filtration rate post‐initiation of empagliflozin in EMPEROR‐Reduced. Eur J Heart Fail 2022;24:1829‐1839. doi: 10.1002/EJHF.2578 [DOI] [PubMed] [Google Scholar]
- 231. Kosiborod MN, Angermann CE, Collins SP, Teerlink JR, Ponikowski P, Biegus J, et al. Effects of empagliflozin on symptoms, physical limitations, and quality of life in patients hospitalized for acute heart failure: Results from the EMPULSE trial. Circulation 2022;146:279‐288. doi: 10.1161/CIRCULATIONAHA.122.059725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Biegus J, Fudim M, Salah HM, Heerspink HJL, Voors AA, Ponikowski P. Sodium–glucose cotransporter‐2 inhibitors in heart failure: Potential decongestive mechanisms and current clinical studies. Eur J Heart Fail 2023;25:1526‐1536. doi: 10.1002/ejhf.2967 [DOI] [PubMed] [Google Scholar]
- 233. Biegus J, Voors AA, Collins SP, Kosiborod MN, Teerlink JR, Angermann CE, et al. Impact of empagliflozin on decongestion in acute heart failure: The EMPULSE trial. Eur Heart J 2023;44:41‐50. doi: 10.1093/eurheartj/ehac530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Fauvel C, Bonnet G, Mullens W, Giraldo CIS, Mežnar AZ, Barasa A, et al. Sequencing and titrating approach of therapy in heart failure with reduced ejection fraction following the 2021 European Society of Cardiology guidelines: An international cardiology survey. Eur J Heart Fail 2023;25:213‐222. doi: 10.1002/EJHF.2743 [DOI] [PubMed] [Google Scholar]
- 235. Stolfo D, Lund LH, Benson L, Lindberg F, Ferrannini G, Dahlström U, et al. Real‐world use of sodium–glucose cotransporter 2 inhibitors in patients with heart failure and reduced ejection fraction: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2023;25:1648‐1658. doi: 10.1002/ejhf.2971 [DOI] [PubMed] [Google Scholar]
- 236. Berwanger O, Pfeffer M, Claggett B, Jering KS, Maggioni AP, Steg PG, et al. Sacubitril/valsartan versus ramipril for patients with acute myocardial infarction: Win‐ratio analysis of the PARADISE‐MI trial. Eur J Heart Fail 2022;24:1918‐1927. [DOI] [PubMed] [Google Scholar]
- 237. Ravassa S, López B, Ferreira JP, Girerd N, Bozec E, Pellicori P, et al. Biomarker‐based assessment of collagen cross‐linking identifies patients at risk of heart failure more likely to benefit from spironolactone effects on left atrial remodelling. Insights from the HOMAGE clinical trial. Eur J Heart Fail 2022;24:321‐331. doi: 10.1002/EJHF.2394 [DOI] [PubMed] [Google Scholar]
- 238. Kobayashi M, Girerd N, Ferreira JP, Kevin D, Huttin O, González A, et al. The association between markers of type I collagen synthesis and echocardiographic response to spironolactone in patients at risk of heart failure: Findings from the HOMAGE trial. Eur J Heart Fail 2022;24:1559‐1568. doi: 10.1002/EJHF.2579 [DOI] [PubMed] [Google Scholar]
- 239. Monzo L, Huttin O, Ferreira JP, Lamiral Z, Bozec E, Beaumont M, et al. Role of aldosterone in mid‐ and long‐term left ventricular remodelling after acute myocardial infarction: The REMI study. Eur J Heart Fail 2023;25:1742‐1752. doi: 10.1002/EJHF.2986 [DOI] [PubMed] [Google Scholar]
- 240. James S, Erlinge D, Storey RF, McGuire DK, de Belder M, Eriksson N, et al. Dapagliflozin in myocardial infarction without diabetes or heart failure. NEJM Evidence 2023;3: doi: 10.1056/EVIDoa2300286 [DOI] [PubMed] [Google Scholar]
- 241. Butler J, Jones WS, Udell JA, Anker SD, Petrie MC, Harrington J, et al. Empagliflozin after acute myocardial infarction. N Engl J Med 2024;390:1455‐1466. doi: 10.1056/NEJMoa2314051 [DOI] [PubMed] [Google Scholar]
- 242. Olivella A, Almenar‐Bonet L, Moliner P, Coloma E, Martínez‐Rubio A, Paz Bermejo M, et al. Role of vericiguat in management of patients with heart failure with reduced ejection fraction after worsening episode. ESC Heart Fail.n/a doi: 10.1002/ehf2.14647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Pieske B, Pieske‐Kraigher E, Lam CSP, Melenovský V, Sliwa K, Lopatin Y, et al. Effect of vericiguat on left ventricular structure and function in patients with heart failure with reduced ejection fraction: The VICTORIA echocardiographic substudy. Eur J Heart Fail 2023;25:1012‐1021. doi: 10.1002/EJHF.2836 [DOI] [PubMed] [Google Scholar]
- 244. Nguyen NV, Lindberg F, Benson L, Ferrannini G, Imbalzano E, Mol PGM, et al. Eligibility for vericiguat in a real‐world heart failure population according to trial, guideline and label criteria: Data from the Swedish Heart Failure Registry. Eur J Heart Fail 2023;25:1418‐1428. doi: 10.1002/EJHF.2939 [DOI] [PubMed] [Google Scholar]
- 245. Butler J, Usman MS, Anstrom KJ, Blaustein RO, Bonaca MP, Ezekowitz JA, et al. Soluble guanylate cyclase stimulators in patients with heart failure with reduced ejection fraction across the risk spectrum. Eur J Heart Fail 2022;24:2029‐2036. doi: 10.1002/EJHF.2720 [DOI] [PubMed] [Google Scholar]
- 246. Felker GM, Solomon SD, Claggett B, Diaz R, Jjv M, Metra M, et al. Assessment of omecamtiv mecarbil for the treatment of patients with severe heart failure: A post hoc analysis of data from the GALACTIC‐HF randomized clinical trial. JAMA Cardiol 2022;7:26‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Lewis GD, Voors AA, Cohen‐Solal A, Metra M, Whellan DJ, Ezekowitz JA, et al. Effect of omecamtiv mecarbil on exercise capacity in chronic heart failure with reduced ejection fraction: The METEORIC‐HF randomized clinical trial. JAMA 2022;328:259‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Tomasoni D, Jkk V‐N, Pagnesi M, Adamo M, Lombardi CM, Gustafsson F, et al. Advanced heart failure: Guideline‐directed medical therapy, diuretics, inotropes, and palliative care. ESC Heart Fail. 2022;9:1507‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Docherty KF, McMurray JJV, Claggett BL, Miao ZM, Adams KF, Arias‐Mendoza A, et al. Efficacy of omecamtiv mecarbil in heart failure with reduced ejection fraction according to N‐terminal pro‐B‐type natriuretic peptide level: Insights from the GALACTIC‐HF trial. Eur J Heart Fail 2023;25:248‐259. doi: 10.1002/EJHF.2763 [DOI] [PubMed] [Google Scholar]
- 250. Molloy CD, Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, et al. Exercise‐based cardiac rehabilitation for adults with heart failure—2023 Cochrane systematic review and meta‐analysis. Eur J Heart Fail 2023:2263‐2273. doi: 10.1002/EJHF.3046 [DOI] [PubMed] [Google Scholar]
- 251. Feuerstein A, Schoenrath F, Belyavskiy E, Knierim J, Friede T, Placzek M, et al. Supervised exercise training in patients with advanced heart failure and left ventricular assist device: A multicentre randomized controlled trial (Ex‐VAD trial). Eur J Heart Fail 2023;25:2252‐2262. doi: 10.1002/EJHF.3032 [DOI] [PubMed] [Google Scholar]
- 252. Curtain JP, Jackson AM, Shen L, Jhund PS, Docherty KF, Petrie MC, et al. Effect of sacubitril/valsartan on investigator‐reported ventricular arrhythmias in PARADIGM‐HF. Eur J Heart Fail 2022;24:551‐561. doi: 10.1002/EJHF.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Abdelhamid M, Rosano G, Metra M, Adamopoulos S, Böhm M, Chioncel O, et al. Prevention of sudden death in heart failure with reduced ejection fraction: Do we still need an implantable cardioverter‐defibrillator for primary prevention? Eur J Heart Fail 2022;24:1460‐1466. [DOI] [PubMed] [Google Scholar]
- 254. Schrage B, Lund LH, Benson L, Dahlström U, Shadman R, Linde C, et al. Predictors of primary prevention implantable cardioverter‐defibrillator use in heart failure with reduced ejection fraction: Impact of the predicted risk of sudden cardiac death and all‐cause mortality. Eur J Heart Fail 2022;24:1212‐1222. doi: 10.1002/EJHF.2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Mirelis JG, Escobar‐Lopez L, Ochoa JP, Espinosa MÁ, Villacorta E, Navarro M, et al. Combination of late gadolinium enhancement and genotype improves prediction of prognosis in non‐ischaemic dilated cardiomyopathy. Eur J Heart Fail 2022;24:1183‐1196. doi: 10.1002/EJHF.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. di Marco A, Brown P, Mateus G, Faga V, Nucifora G, Claver E, et al. Late gadolinium enhancement and the risk of ventricular arrhythmias and sudden death in NYHA class I patients with non‐ischaemic cardiomyopathy. Eur J Heart Fail 2023;25:740‐750. doi: 10.1002/EJHF.2793 [DOI] [PubMed] [Google Scholar]
- 257. Cleland JGF, Bristow MR, Freemantle N, Olshansky B, Gras D, Saxon L, et al. The effect of cardiac resynchronization without a defibrillator on morbidity and mortality: An individual patient data meta‐analysis of COMPANION and CARE‐HF. Eur J Heart Fail 2022;24:1080‐1090. doi: 10.1002/ejhf.2524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Sidhu K, Castrini AI, Parikh V, Reza N, Owens A, Tremblay‐Gravel M, et al. The response to cardiac resynchronization therapy in LMNA cardiomyopathy. Eur J Heart Fail 2022;24:685‐693. doi: 10.1002/ejhf.2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Fudim M, Dalgaard F, Friedman DJ, Abraham WT, Cleland JGF, Curtis AB, et al. Comorbidities and clinical response to cardiac resynchronization therapy: Patient‐level meta‐analysis from eight clinical trials. Eur J Heart Fail 2023; doi: 10.1002/ejhf.3029 [DOI] [PubMed] [Google Scholar]
- 260. Merkely B, Gellér L, Zima E, Osztheimer I, Molnár L, Földesi C, et al. Baseline clinical characteristics of heart failure patients with reduced ejection fraction enrolled in the BUDAPEST‐CRT Upgrade trial. Eur J Heart Fail 2022;24:1652‐1661. doi: 10.1002/EJHF.2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Merkely B, Hatala R, Wranicz JK, Duray G, Földesi C, Som Z, et al. Upgrade of right ventricular pacing to cardiac resynchronization therapy in heart failure: A randomized trial. Eur Heart J 2023;44:4259‐4269. doi: 10.1093/EURHEARTJ/EHAD591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Whinnett ZI, Shun‐Shin MJ, Tanner M, Foley P, Chandrasekaran B, Moore P, et al. Effects of haemodynamically atrio‐ventricular optimized His bundle pacing on heart failure symptoms and exercise capacity: The His Optimized Pacing Evaluated for Heart Failure (HOPE‐HF) randomized, double‐blind, cross‐over trial. Eur J Heart Fail 2023;25:274‐283. doi: 10.1002/EJHF.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Malagoli A, Rossi L, Zanni A, Sticozzi C, Piepoli MF, Benfari G. Quantified mitral regurgitation and left atrial function in heart failure with reduced ejection fraction: Interplay and outcome implications. Eur J Heart Fail 2022;24:694‐702. doi: 10.1002/EJHF.2429 [DOI] [PubMed] [Google Scholar]
- 264. Pagnesi M, Calì F, Chiarito M, Stolfo D, Baldetti L, Lombardi CM, et al. Prognostic role of mitral regurgitation in patients with advanced heart failure. Eur J Intern Med 2023;122:102‐108. doi: 10.1016/j.ejim.2023.11.002 [DOI] [PubMed] [Google Scholar]
- 265. Bruno RR, Uzel R, Spieker M, Datz C, Oehler D, Bönner F, et al. The impact of gender and frailty on the outcome of older patients with functional mitral regurgitation. ESC Heart Fail. 2023;10:2948‐2954. doi: 10.1002/ehf2.14478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Januzzi JL, Omar AMS, Liu Y, Murphy S, Butler J, Felker GM, et al. Association between sacubitril/valsartan initiation and mitral regurgitation severity in heart failure with reduced ejection fraction: The PROVE‐HF study. Circulation 2022;146:1638‐1640. doi: 10.1161/CIRCULATIONAHA.122.061693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Spinka G, Bartko PE, Heitzinger G, Prausmüller S, Winter MP, Arfsten H, et al. Guideline directed medical therapy and reduction of secondary mitral regurgitation. Eur Heart J Cardiovasc Imaging 2022;23:755‐764. doi: 10.1093/EHJCI/JEAC068 [DOI] [PubMed] [Google Scholar]
- 268. Varshney AS, Shah M, Vemulapalli S, Kosinski A, Bhatt AS, Sandhu AT, et al. Heart failure medical therapy prior to mitral transcatheter edge‐to‐edge repair: The STS/ACC Transcatheter Valve Therapy Registry. Eur Heart J 2023;44:4650‐4661. doi: 10.1093/eurheartj/ehad584 [DOI] [PubMed] [Google Scholar]
- 269. Adamo M, Tomasoni D, Stolz L, Stocker TJ, Pancaldi E, Koell B, et al. Impact of transcatheter edge‐to‐edge mitral valve repair on guideline‐directed medical therapy uptitration. JACC Cardiovasc Interv 2023;16:896‐905. doi: 10.1016/j.jcin.2023.01.362 [DOI] [PubMed] [Google Scholar]
- 270. Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, et al. Five‐year follow‐up after transcatheter repair of secondary mitral regurgitation. N Engl J Med 2023;388:2037‐2048. doi: 10.1056/NEJMOA2300213/SUPPL_FILE/NEJMOA2300213_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 271. Orban M, Rottbauer W, Williams M, Mahoney P, von Bardeleben RS, Price MJ, et al. Transcatheter edge‐to‐edge repair for secondary mitral regurgitation with third‐generation devices in heart failure patients—Results from the Global EXPAND Post‐Market study. Eur J Heart Fail 2023;25:411‐421. doi: 10.1002/EJHF.2770 [DOI] [PubMed] [Google Scholar]
- 272. von Bardeleben RS, Rogers JH, Mahoney P, Price MJ, Denti P, Maisano F, et al. Real‐world outcomes of fourth‐generation mitral transcatheter repair: 30‐day results from EXPAND G4. J Am Coll Cardiol Intv 2023;16:1463‐1473. doi: 10.1016/j.jcin.2023.05.013 [DOI] [PubMed] [Google Scholar]
- 273. Doldi PM, Stolz L, Kalbacher D, Köll B, Geyer M, Ludwig S, et al. Right ventricular dysfunction predicts outcome after transcatheter mitral valve repair for primary mitral valve regurgitation. Eur J Heart Fail 2022;24:2162‐2171. doi: 10.1002/EJHF.2661 [DOI] [PubMed] [Google Scholar]
- 274. Adamo M, Pagnesi M, Ghizzoni G, Estévez‐Loureiro R, Raposeiras‐Roubin S, Tomasoni D, et al. Evolution of tricuspid regurgitation after transcatheter edge‐to‐edge mitral valve repair for secondary mitral regurgitation and its impact on mortality. Eur J Heart Fail 2022;24:2175‐2184. doi: 10.1002/EJHF.2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Adamo M, Fiorelli F, Melica B, D'Ortona R, Lupi L, Giannini C, et al. COAPT‐like profile predicts long‐term outcomes in patients with secondary mitral regurgitation undergoing MitraClip implantation. JACC Cardiovasc Interv 2021;14:15‐25. doi: 10.1016/J.JCIN.2020.09.050 [DOI] [PubMed] [Google Scholar]
- 276. Adamo M, Cani DS, Gavazzoni M, Taramasso M, Lupi L, Fiorelli F, et al. Impact of disproportionate secondary mitral regurgitation in patients undergoing edge‐to‐edge percutaneous mitral valve repair. EuroIntervention 2020;16:413‐420. doi: 10.4244/EIJ-D-19-01114 [DOI] [PubMed] [Google Scholar]
- 277. Lupi L, Italia L, Pagnesi M, Pancaldi E, Ancona F, Stella S, et al. Prognostic value of right ventricular longitudinal strain in patients with secondary mitral regurgitation undergoing transcatheter edge‐to‐edge mitral valve repair. Eur Heart J Cardiovasc Imaging 2023;24:1509‐1517. doi: 10.1093/ehjci/jead103 [DOI] [PubMed] [Google Scholar]
- 278. Adamo M, Inciardi RM, Tomasoni D, Dallapellegrina L, Estévez‐Loureiro R, Stolfo D, et al. Changes in right ventricular‐to‐pulmonary artery coupling after transcatheter edge‐to‐edge repair in secondary mitral regurgitation. JACC Cardiovasc Imaging 2022;15:2038‐2047. doi: 10.1016/j.jcmg.2022.08.012 [DOI] [PubMed] [Google Scholar]
- 279. Stolz L, Orban M, Karam N, Lubos E, Wild M, Weckbach L, et al. Cardio‐hepatic syndrome in patients undergoing mitral valve transcatheter edge‐to‐edge repair. Eur J Heart Fail 2023;25:872‐884. doi: 10.1002/EJHF.2842 [DOI] [PubMed] [Google Scholar]
- 280. Feng KY, Ambrosy AP, Zhou Z, Li D, Kong J, Zaroff JG, et al. Association between serum albumin and outcomes in heart failure and secondary mitral regurgitation: The COAPT trial. Eur J Heart Fail 2023;25:553‐561. doi: 10.1002/EJHF.2809 [DOI] [PubMed] [Google Scholar]
- 281. Shah N, Madhavan MV, Gray WA, Brener SJ, Ahmad Y, Lindenfeld J, et al. Prediction of death or HF hospitalization in patients with severe FMR: The COAPT risk score. J Am Coll Cardiol Intv 2022;15:1893‐1905. doi: 10.1016/j.jcin.2022.08.005 [DOI] [PubMed] [Google Scholar]
- 282. Adamo M, Rubbio AP, Zaccone G, Pighi M, Massussi M, Tomasoni D, et al. Prediction of mortality and heart failure hospitalisations in patients undergoing M‐TEER: External validation of the COAPT risk score. EuroIntervention 2023;18:1408‐1417. doi: 10.4244/EIJ-D-22-00992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Ben Ali W, Ludwig S, Duncan A, Weimann J, Nickenig G, Tanaka T, et al. Characteristics and outcomes of patients screened for transcatheter mitral valve implantation: 1‐year results from the CHOICE‐MI registry. Eur J Heart Fail 2022;24:887‐898. doi: 10.1002/ejhf.2492 [DOI] [PubMed] [Google Scholar]
- 284. Wild MG, Kreidel F, Hell MM, Praz F, Mach M, Adam M, et al. Transapical mitral valve implantation for treatment of symptomatic mitral valve disease: A real‐world multicentre experience. Eur J Heart Fail 2022;24:899‐907. doi: 10.1002/ejhf.2434 [DOI] [PubMed] [Google Scholar]
- 285. Ludwig S, Kalbacher D, Ali WB, Weimann J, Adam M, Duncan A, et al. Transcatheter mitral valve replacement or repair for secondary mitral regurgitation: A propensity score‐matched analysis. Eur J Heart Fail 2023;25:399‐410. doi: 10.1002/EJHF.2797 [DOI] [PubMed] [Google Scholar]
- 286. Hungerford SL, Dahle G, Duncan A, Hayward CS, Muller DWM. Peri‐procedural management of transcatheter mitral valve replacement in patients with heart failure. Eur J Heart Fail 2023;25:890‐901. doi: 10.1002/EJHF.2758 [DOI] [PubMed] [Google Scholar]
- 287. Hahn RT. Tricuspid regurgitation. New England Journal of Medicine 2023;388:1876‐1891. doi: 10.1056/NEJMra2216709 [DOI] [PubMed] [Google Scholar]
- 288. Heitzinger G, Pavo N, Koschatko S, Jantsch C, Winter MP, Spinka G, et al. Contemporary insights into the epidemiology, impact and treatment of secondary tricuspid regurgitation across the heart failure spectrum. Eur J Heart Fail 2023;25:857‐867. doi: 10.1002/ejhf.2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Adamo M, Metra M, Claggett BL, Miao ZM, Diaz R, Felker GM, et al. Tricuspid regurgitation and clinical outcomes in heart failure with reduced ejection fraction. JACC Heart Fail 2024;12:552‐563. doi: 10.1016/j.jchf.2023.11.018 [DOI] [PubMed] [Google Scholar]
- 290. Pagnesi M, Riccardi M, Chiarito M, Stolfo D, Baldetti L, Lombardi CM, et al. Characteristics and outcomes of patients with tricuspid regurgitation and advanced heart failure. J Cardiovasc Med (Hagerstown) 2024;25:200‐209. doi: 10.2459/JCM.0000000000001582 [DOI] [PubMed] [Google Scholar]
- 291. Adamo M, Chioncel O, Benson L, Shahim B, Crespo‐Leiro MG, Anker SD, et al. Prevalence, clinical characteristics and outcomes of heart failure patients with or without isolated or combined mitral and tricuspid regurgitation: An analysis from the ESC‐HFA Heart Failure Long‐Term Registry. Eur J Heart Fail 2023;25:1061‐1071. doi: 10.1002/ejhf.2929 [DOI] [PubMed] [Google Scholar]
- 292. Dreyfus J, Audureau E, Bohbot Y, Coisne A, Lavie‐Badie Y, Bouchery M, et al. TRI‐SCORE: A new risk score for in‐hospital mortality prediction after isolated tricuspid valve surgery. Eur Heart J 2022;43:654‐662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Russo G, Taramasso M, Pedicino D, Gennari M, Gavazzoni M, Pozzoli A, et al. Challenges and future perspectives of transcatheter tricuspid valve interventions: Adopt old strategies or adapt to new opportunities? Eur J Heart Fail 2022;24:442‐454. [DOI] [PubMed] [Google Scholar]
- 294. Stocker TJ, Cohen DJ, Arnold SV, Sommer S, Braun D, Stolz L, et al. Durability of benefit after transcatheter tricuspid valve intervention: Insights from actigraphy. Eur J Heart Fail 2022;24:1293‐1301. doi: 10.1002/ejhf.2467 [DOI] [PubMed] [Google Scholar]
- 295. Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med 2023;388:1833‐1842. doi: 10.1056/NEJMoa2300525 [DOI] [PubMed] [Google Scholar]
- 296. Iacovoni A, Palmieri V, Abete R, Vecchi AL, Mortara A, Gori M, et al. Right and left ventricular structures and functions in acute HFpEF: Comparing the hypertensive pulmonary edema and worsening heart failure phenotypes. J Cardiovasc Med (Hagerstown) 2022;23:663‐671. doi: 10.2459/JCM.0000000000001366 [DOI] [PubMed] [Google Scholar]
- 297. Cai A, Qiu W, Zhou Y, Feng Y, Chen J, Xia S, et al. Clinical characteristics and 1‐year outcomes in hospitalized patients with heart failure with preserved ejection fraction: Results from the China Cardiovascular Association Database‐Heart Failure Center Registry. Eur J Heart Fail 2022;24:2048‐2062. doi: 10.1002/EJHF.2654 [DOI] [PubMed] [Google Scholar]
- 298. Jackson AM, Rørth R, Liu J, Kristensen SL, Anand IS, Claggett BL, et al. Diabetes and pre‐diabetes in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2022;24:497‐509. doi: 10.1002/EJHF.2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Crum Y, Hoendermis ES, van Veldhuisen DJ, van Woerden G, Lobeek M, Dickinson MG, et al. Epicardial adipose tissue and pericardial constraint in heart failure with preserved ejection fraction. ESC Heart Fail.n/a doi: 10.1002/ehf2.14739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Venkateshvaran A, Faxen UL, Hage C, Michaëlsson E, Svedlund S, Saraste A, et al. Association of epicardial adipose tissue with proteomics, coronary flow reserve, cardiac structure and function, and quality of life in heart failure with preserved ejection fraction: Insights from the PROMIS‐HFpEF study. Eur J Heart Fail 2022;24:2251‐2260. doi: 10.1002/EJHF.2709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. van de Bovenkamp AA, Geurkink KTJ, Oosterveer FTP, de Man FS, Kok WEM, Bronzwaer PNA, et al. Trimetazidine in heart failure with preserved ejection fraction: A randomized controlled cross‐over trial. ESC Heart Fail. 2023;10:2998‐3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Erhardsson M, Ljung Faxén U, Venkateshvaran A, Svedlund S, Saraste A, Lagerström Fermer M, et al. Regional differences and coronary microvascular dysfunction in heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10:3729‐3734. doi: 10.1002/ehf2.14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Nishihara T, Miyoshi T, Nakashima M, Ichikawa K, Takaya Y, Nakayama R, et al. Association of perivascular fat attenuation on computed tomography and heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10:2447‐2457. doi: 10.1002/ehf2.14419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Vernooij RWM, van Ommen A‐MLN, Valstar GB, Cramer MJ, Teske AJ, Menken R, et al. Association of mild kidney dysfunction with diastolic dysfunction and heart failure with preserved ejection fraction. ESC Heart Fail. 2024;11:315‐326. doi: 10.1002/ehf2.14511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Vaduganathan M, Piccini JP, Camm AJ, Crijns HJGM, Anker SD, Butler J, et al. Dronedarone for the treatment of atrial fibrillation with concomitant heart failure with preserved and mildly reduced ejection fraction: A post‐hoc analysis of the ATHENA trial. Eur J Heart Fail 2022;24:1094‐1094, 1101. doi: 10.1002/EJHF.2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Tomasoni D, Aimo A, Merlo M, Nardi M, Adamo M, Bellicini MG, et al. Value of the HFA‐PEFF and H2FPEF scores in patients with heart failure and preserved ejection fraction caused by cardiac amyloidosis. Eur J Heart Fail 2022;24:2374‐2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Guazzi M, Wilhelm M, Halle M, van Craenenbroeck E, Kemps H, de Boer RA, et al. Exercise testing in heart failure with preserved ejection fraction: An appraisal through diagnosis, pathophysiology and therapy—A clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur J Heart Fail 2022;24:1327‐1345. doi: 10.1002/ejhf.2601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Saito Y, Obokata M, Harada T, Kagami K, Sorimachi H, Yuasa N, et al. Disproportionate exercise‐induced pulmonary hypertension in relation to cardiac output in heart failure with preserved ejection fraction: A non‐invasive echocardiographic study. Eur J Heart Fail 2023;25:792‐802. doi: 10.1002/ejhf.2821 [DOI] [PubMed] [Google Scholar]
- 309. Lanzarone E, Baratto C, Vicenzi M, Villella F, Rota I, Dewachter C, et al. Haemodynamic validation of the three‐step HFA‐PEFF algorithm to diagnose heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10:2588‐2595. doi: 10.1002/ehf2.14436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Omote K, Verbrugge FH, Sorimachi H, Omar M, Popovic D, Obokata M, et al. Central haemodynamic abnormalities and outcome in patients with unexplained dyspnoea. Eur J Heart Fail 2023;25:185‐196. doi: 10.1002/EJHF.2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Omar M, Omote K, Sorimachi H, Popovic D, Kanwar A, Alogna A, et al. Hypoxaemia in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2023;25:1593‐1603. doi: 10.1002/EJHF.2930 [DOI] [PubMed] [Google Scholar]
- 312. Alogna A, Omar M, Popovic D, Sorimachi H, Omote K, Reddy YNV, et al. Biventricular cardiac power reserve in heart failure with preserved ejection fraction. Eur J Heart Fail 2023;25:956‐966. doi: 10.1002/ejhf.2867 [DOI] [PubMed] [Google Scholar]
- 313. Talha KM, Butler J, Greene SJ, Aggarwal R, Anker SD, Claggett BL, et al. Potential global impact of sodium‐glucose cotransporter‐2 inhibitors in heart failure. Eur J Heart Fail 2023;25:999‐1009. doi: 10.1002/EJHF.2864 [DOI] [PubMed] [Google Scholar]
- 314. Ostrominski JW, Vaduganathan M, Claggett BL, de Boer RA, Desai AS, Dobreanu D, et al. Dapagliflozin and New York Heart Association functional class in heart failure with mildly reduced or preserved ejection fraction: The DELIVER trial. Eur J Heart Fail 2022;24:1892‐1901. doi: 10.1002/EJHF.2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Sharma A, Ferreira JP, Zannad F, Pocock SJ, Filippatos G, Pfarr E, et al. Cardiac and kidney benefits of empagliflozin in heart failure across the spectrum of kidney function: Insights from the EMPEROR‐Preserved trial. Eur J Heart Fail 2023;25:1337‐1348. doi: 10.1002/ejhf.2857 [DOI] [PubMed] [Google Scholar]
- 316. Böhm M, Butler J, Krawczyk M, Mahfoud F, Haring B, Filippatos G, et al. Liver tests, cardiovascular outcomes and effects of empagliflozin in patients with heart failure and preserved ejection fraction: The EMPEROR‐Preserved trial. Eur J Heart Fail 2023;25:1375‐1383. doi: 10.1002/ejhf.2922 [DOI] [PubMed] [Google Scholar]
- 317. Filippatos G, Farmakis D, Butler J, Zannad F, Ferreira JP, Ofstad AP, et al. Empagliflozin in heart failure with preserved ejection fraction with and without atrial fibrillation. Eur J Heart Fail 2023;25:970‐977. doi: 10.1002/ejhf.2861 [DOI] [PubMed] [Google Scholar]
- 318. Yang M, Butt JH, Kondo T, Jering KS, Docherty KF, Jhund PS, et al. Dapagliflozin in patients with heart failure with mildly reduced and preserved ejection fraction treated with a mineralocorticoid receptor antagonist or sacubitril/valsartan. Eur J Heart Fail 2022;24:2307‐2319. doi: 10.1002/EJHF.2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Oriecuia C, Tomasoni D, Sala I, Bonfioli GB, Adamo M, Gussago C, et al. Sodium glucose co‐transporter 2 inhibitors and quality of life in patients with heart failure: A comprehensive systematic review and meta‐analysis of randomized controlled trials. Eur Heart J Cardiovasc Pharmacother 2023;10:147‐157. doi: 10.1093/ehjcvp/pvad088 [DOI] [PubMed] [Google Scholar]
- 320. Chatur S, Vaduganathan M, Peikert A, Claggett BL, McCausland FR, Skali H, et al. Longitudinal trajectories in renal function before and after heart failure hospitalization among patients with heart failure with preserved ejection fraction in the PARAGON‐HF trial. Eur J Heart Fail 2022;24:1906‐1914. doi: 10.1002/ejhf.2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Chatur S, Claggett BL, Vardeny O, Jering K, Desai AS, Pfeffer MA, et al. Sacubitril/valsartan and loop diuretic requirement in heart failure with preserved ejection fraction in the PARAGON‐HF trial. Eur J Heart Fail 2023;25:87‐94. doi: 10.1002/EJHF.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Mentz RJ, Ward JH, Hernandez AF, Lepage S, Morrow DA, Sarwat S, et al. Angiotensin‐neprilysin inhibition in patients with mildly reduced or preserved ejection fraction and worsening heart failure. J Am Coll Cardiol 2023;82:1‐12. doi: 10.1016/j.jacc.2023.04.019 [DOI] [PubMed] [Google Scholar]
- 323. Vaduganathan M, Mentz RJ, Claggett BL, Miao ZM, Kulac IJ, Ward JH, et al. Sacubitril/valsartan in heart failure with mildly reduced or preserved ejection fraction: A pre‐specified participant‐level pooled analysis of PARAGLIDE‐HF and PARAGON‐HF. Eur Heart J 2023;44:2982‐2993. doi: 10.1093/eurheartj/ehad344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34‐42. doi: 10.1161/CIRCULATIONAHA.114.013255 [DOI] [PubMed] [Google Scholar]
- 325. Pfeffer MA, Claggett B. Behind the scenes of TOPCAT—Bending to inform. NEJM Evid 2022;1:EVIDctcs2100007. doi: 10.1056/EVIDctcs2100007 [DOI] [PubMed] [Google Scholar]
- 326. Kosmala W, Rojek A, Przewlocka‐Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of aldosterone antagonism on exercise tolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol 2016;68:1823‐1834. doi: 10.1016/j.jacc.2016.07.763 [DOI] [PubMed] [Google Scholar]
- 327. Ferreira JP, Cleland JG, Girerd N, Bozec E, Rossignol P, Pellicori P, et al. Spironolactone effect on cardiac structure and function of patients with heart failure and preserved ejection fraction: A pooled analysis of three randomized trials. Eur J Heart Fail 2023;25:108‐113. doi: 10.1002/EJHF.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. New England Journal of Medicine. 2023;389:1069‐1084. doi: 10.1056/NEJMOA2306963/SUPPL_FILE/NEJMOA2306963_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 329. Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Christensen L, Davies M, et al. Design and baseline characteristics of STEP‐HFpEF program evaluating semaglutide in patients with obesity HFpEF phenotype. JACC Heart Fail 2023;11:11‐1010. doi: 10.1016/J.JCHF.2023.05.010 [DOI] [PubMed] [Google Scholar]
- 330. Kosiborod MN, Petrie MC, Borlaug BA, Butler J, Davies MJ, Hovingh GK, et al. Semaglutide in patients with obesity‐related heart failure and type 2 diabetes. N Engl J Med 2024;390:1394‐1407. doi: 10.1056/NEJMoa2313917 [DOI] [PubMed] [Google Scholar]
- 331. Linde C, Grabowski M, Ponikowski P, Rao I, Stagg A, Tschöpe C. Cardiac contractility modulation therapy improves health status in patients with heart failure with preserved ejection fraction: A pilot study (CCM‐HFpEF). Eur J Heart Fail 2022;24:2275‐2284. doi: 10.1002/EJHF.2619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Riccardi M, Tomasoni D, Vizzardi E, Metra M, Adamo M. Device‐based percutaneous treatments to decompress the left atrium in heart failure with preserved ejection fraction. Heart Fail Rev 2023;28:315‐330. doi: 10.1007/S10741-022-10280-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Shah SJ, Borlaug BA, Chung ES, Cutlip DE, Debonnaire P, Fail PS, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP‐HF II): A randomised, multicentre, blinded, sham‐controlled trial. Lancet 2022;399:1130‐1140. [DOI] [PubMed] [Google Scholar]
- 334. Schuster A, Schulz A, Lange T, Evertz R, Hartmann F, Kowallick JT, et al. Concomitant latent pulmonary vascular disease leads to impaired global cardiac performance in heart failure with preserved ejection fraction. Eur J Heart Fail 2023;25:322‐331. doi: 10.1002/ejhf.2781 [DOI] [PubMed] [Google Scholar]
- 335. Rodés‐Cabau J, Lindenfeld J, Abraham WT, Zile MR, Kar S, Bayés‐Genís A, et al. Interatrial shunt therapy in advanced heart failure: Outcomes from the open‐label cohort of the RELIEVE‐HF trial. Eur J Heart Fail 2024; doi: 10.1002/ejhf.3215 [DOI] [PubMed] [Google Scholar]
- 336. Shah S, Segar MW, Kondamudi N, Ayers C, Chandra A, Matulevicius S, et al. Supranormal left ventricular ejection fraction, stroke volume, and cardiovascular risk: Findings from population‐based cohort studies. JACC Heart Fail. 2022;10:583‐594. [DOI] [PubMed] [Google Scholar]
- 337. Forrest IS, Rocheleau G, Bafna S, Argulian E, Narula J, Natarajan P, et al. Genetic and phenotypic profiling of supranormal ejection fraction reveals decreased survival and underdiagnosed heart failure. Eur J Heart Fail 2022;24:2118‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Ferreira JP, Verdonschot JAJ, Girerd N, Bozec E, Pellicori P, Collier T, et al. Influence of ejection fraction on biomarker expression and response to spironolactone in people at risk of heart failure: Findings from the HOMAGE trial. Eur J Heart Fail 2022;24:771‐778. doi: 10.1002/EJHF.2455 [DOI] [PubMed] [Google Scholar]
- 339. Kondo T, Dewan P, Anand IS, Desai AS, Packer M, Zile MR, et al. Clinical characteristics and outcomes in patients with heart failure: Are there thresholds and inflection points in left ventricular ejection fraction and thresholds justifying a clinical classification? Circulation 2023;148:732‐749. doi: 10.1161/CIRCULATIONAHA.122.063642 [DOI] [PubMed] [Google Scholar]
- 340. Van Essen BJ, Tromp J, Ter Maaten JM, Greenberg BH, Gimpelewicz C, Felker GM, et al. Characteristics and clinical outcomes of patients with acute heart failure with a supranormal left ventricular ejection fraction. Eur J Heart Fail 2022;25:35‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Horiuchi Y, Asami M, Ide T, Yahagi K, Komiyama K, Yuzawa H, et al. Prevalence, characteristics and cardiovascular and non‐cardiovascular outcomes in patients with heart failure with supra‐normal ejection fraction: Insight from the JROADHF study. Eur J Heart Fail 2023;25:989‐998. doi: 10.1002/EJHF.2895 [DOI] [PubMed] [Google Scholar]
- 342. Popovic D, Alogna A, Omar M, Sorimachi H, Omote K, Reddy YNV, et al. Ventricular stiffening and chamber contracture in heart failure with higher ejection fraction. Eur J Heart Fail 2023;25:657‐668. doi: 10.1002/EJHF.2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Packer M. A reclassification of heart failure based on recognition of heart failure with normal to supernormal ejection fraction, a clinically common form of cardiac contracture, with distinctive pathophysiological and therapeutic features. Eur J Heart Fail 2023;25:669‐672. doi: 10.1002/EJHF.2849 [DOI] [PubMed] [Google Scholar]
- 344. Pagnesi M, Lombardi CM, Chiarito M, Stolfo D, Baldetti L, Loiacono F, et al. Prognostic impact of the updated 2018 HFA‐ESC definition of advanced heart failure: Results from the HELP‐HF registry. Eur J Heart Fail 2022;24:1493‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Pagnesi M, Ghiraldin D, Vizzardi E, Chiarito M, Stolfo D, Baldetti L, et al. Detailed assessment of the “I need help” criteria in patients with heart failure: Insights from the HELP‐HF registry. Circ Heart Fail 2023;16:e011003. doi: 10.1161/CIRCHEARTFAILURE.123.011003 [DOI] [PubMed] [Google Scholar]
- 346. Pagnesi M, Sammartino AM, Chiarito M, Stolfo D, Baldetti L, Adamo M, et al. Clinical and prognostic implications of heart failure hospitalization in patients with advanced heart failure. J Cardiovasc Med (Hagerstown) 2024;25:149‐157. doi: 10.2459/JCM.0000000000001581 [DOI] [PubMed] [Google Scholar]
- 347. Lombardi CM, Cimino G, Pellicori P, Bonelli A, Inciardi RM, Pagnesi M, et al. Congestion in patients with advanced heart failure: Assessment and treatment. Heart Fail Clin 2021;17:575‐586. [DOI] [PubMed] [Google Scholar]
- 348. Jkk V‐N, Tomasoni D, Gustafsson F, Metra M. Contemporary drug treatment of advanced heart failure with reduced ejection fraction. Drugs 2022;82:375‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Shakoor A, Abou Kamar S, Malgie J, Kardys I, Schaap J, De Boer RA, et al. The different risk of new‐onset, chronic, worsening, and advanced heart failure: A systematic review and meta‐regression analysis. Eur J Heart Fail 2023;26:216‐229. doi: 10.1002/EJHF.3048 [DOI] [PubMed] [Google Scholar]
- 350. Gustafsson F, Damman K, Nalbantgil S, van Laake L, Tops LF, Thum T, et al. Inotropic therapy in patients with advanced heart failure. A clinical consensus statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2023;25:457‐468. doi: 10.1002/EJHF.2814 [DOI] [PubMed] [Google Scholar]
- 351. Pölzl G, Altenberger J, Comín‐Colet J, Delgado JF, Fedele F, García‐González MJ, et al. Repetitive levosimendan infusions for patients with advanced chronic heart failure in the vulnerable post‐discharge period: The multinational randomized LeoDOR trial. Eur J Heart Fail 2023;25:2007‐2017. doi: 10.1002/EJHF.3006 [DOI] [PubMed] [Google Scholar]
- 352. Crespo‐Leiro MG, Costanzo MR, Gustafsson F, Khush KK, Macdonald PS, Potena L, et al. Heart transplantation: Focus on donor recovery strategies, left ventricular assist devices, and novel therapies. Eur Heart J 2022;43:2237‐2246. [DOI] [PubMed] [Google Scholar]
- 353. Jakus N, Brugts JJ, Claggett B, Timmermans P, Pouleur AC, Rubiś P, et al. Improved survival of left ventricular assist device carriers in Europe according to implantation eras: Results from the PCHF‐VAD registry. Eur J Heart Fail 2022;24:1305‐1315. doi: 10.1002/EJHF.2526 [DOI] [PubMed] [Google Scholar]
- 354. Mehra MR, Netuka I, Uriel N, Katz JN, Pagani FD, Jorde UP, et al. Aspirin and hemocompatibility events with a left ventricular assist device in advanced heart failure: The ARIES‐HM3 randomized clinical trial. JAMA 2023;330:2171‐2181. doi: 10.1001/JAMA.2023.23204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Uriel N, Milano C, Agarwal R, Lee S, Cleveland J, Goldstein D, et al. Incidence and clinical correlates of de‐novo aortic regurgitation with a fully magnetically levitated left ventricular assist device: A MOMENTUM 3 trial portfolio analysis. Eur J Heart Fail 2023;25:286‐294. doi: 10.1002/EJHF.2746 [DOI] [PubMed] [Google Scholar]
- 356. Blum M, Goldstein NE, Jaarsma T, Allen LA, Gelfman LP. Palliative care in heart failure guidelines: A comparison of the 2021 ESC and the 2022 AHA/ACC/HFSA guidelines on heart failure. Eur J Heart Fail 2023:25‐1855. doi: 10.1002/EJHF.2981 [DOI] [PubMed] [Google Scholar]
- 357. Hill L, Baruah R, Beattie JM, Bistola V, Castiello T, Celutkienė J, et al. Culture, ethnicity, and socio‐economic status as determinants of the management of patients with advanced heart failure who need palliative care: A clinical consensus statement from the Heart Failure Association (HFA) of the ESC, the ESC Patient Forum, and the European Association of Palliative Care. Eur J Heart Fail 2023;25:25‐1492. doi: 10.1002/EJHF.2973 [DOI] [PubMed] [Google Scholar]
- 358. Tomasoni D, Lombardi CM, Sbolli M, Cotter G, Metra M. Acute heart failure: More questions than answers. Prog Cardiovasc Dis 2020;63:599‐606. doi: 10.1016/j.pcad.2020.04.007 [DOI] [PubMed] [Google Scholar]
- 359. Gupta AK, Tomasoni D, Sidhu K, Metra M, Ja E. Evidence‐based management of acute heart failure. Can J Cardiol 2021;37:621‐631. [DOI] [PubMed] [Google Scholar]
- 360. Hariharaputhiran S, Peng Y, Ngo L, Ali A, Hossain S, Visvanathan R, et al. Long‐term survival and life expectancy following an acute heart failure hospitalization in Australia and New Zealand. Eur J Heart Fail 2022;24:1519‐1528. doi: 10.1002/EJHF.2595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Lombardi C, Peveri G, Cani D, Latta F, Bonelli A, Tomasoni D, et al. In‐hospital and long‐term mortality for acute heart failure: Analysis at the time of admission to the emergency department. ESC Heart Fail. 2020;7:2650‐2661. doi: 10.1002/ehf2.12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Gualandro DM, Puelacher C, Chew MS, Andersson H, Lurati Buse G, Glarner N, et al. Acute heart failure after non‐cardiac surgery: Incidence, phenotypes, determinants and outcomes. Eur J Heart Fail 2023;25:347‐357. doi: 10.1002/EJHF.2773 [DOI] [PubMed] [Google Scholar]
- 363. Chioncel O, Adamo M, Nikolaou M, Parissis J, Mebazaa A, Yilmaz MB, et al. Acute heart failure and valvular heart disease: A scientific statement of the Heart Failure Association, the Association for Acute CardioVascular Care and the European Association of Percutaneous Cardiovascular Interventions of the European Society of Cardiology. Eur J Heart Fail 2023;25:1025‐1048. doi: 10.1002/ejhf.2918 [DOI] [PubMed] [Google Scholar]
- 364. Lee MMY, Campbell RT, Claggett BL, Lewis EF, Docherty KF, Lindner M, et al. Health‐related quality of life in acute heart failure: Association between patient‐reported symptoms and markers of congestion. Eur J Heart Fail 2023;25:54‐60. doi: 10.1002/EJHF.2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Kapłon‐Cieślicka A, Benson L, Chioncel O, Crespo‐Leiro MG, Coats AJS, Anker SD, et al. Hyponatraemia and changes in natraemia during hospitalization for acute heart failure and associations with in‐hospital and long‐term outcomes—From the ESC‐HFA EORP Heart Failure Long‐Term Registry. Eur J Heart Fail 2023;25:1571‐1583. doi: 10.1002/ejhf.2873 [DOI] [PubMed] [Google Scholar]
- 366. Ouwerkerk W, Tromp J, Cleland JGF, Angermann CE, Dahlstrom U, Ertl G, et al. Association of time‐to‐intravenous furosemide with mortality in acute heart failure: Data from REPORT‐HF. Eur J Heart Fail 2023;25:43‐51. doi: 10.1002/EJHF.2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Mullens W, Dauw J, Martens P, Meekers E, Nijst P, Verbrugge FH, et al. Acetazolamide in Decompensated Heart Failure with Volume Overload trial (ADVOR): Baseline characteristics. Eur J Heart Fail 2022;24:1601‐1610. doi: 10.1002/EJHF.2587 [DOI] [PubMed] [Google Scholar]
- 368. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide in acute decompensated heart failure with volume overload. N Engl J Med 2022;387:1185‐1195. [DOI] [PubMed] [Google Scholar]
- 369. Dhont S, Martens P, Meekers E, Dauw J, Verbrugge FH, Nijst P, et al. Sodium and potassium changes during decongestion with acetazolamide—A pre‐specified analysis from the ADVOR trial. Eur J Heart Fail 2023;25:1310‐1319. doi: 10.1002/ejhf.2863 [DOI] [PubMed] [Google Scholar]
- 370. Martens P, Verbrugge FH, Dauw J, Nijst P, Meekers E, Augusto SN, et al. Pre‐treatment bicarbonate levels and decongestion by acetazolamide: The ADVOR trial. Eur Heart J 2023;44:1995‐2005. doi: 10.1093/eurheartj/ehad236 [DOI] [PubMed] [Google Scholar]
- 371. Trullàs JC, Morales‐Rull JL, Casado J, Carrera‐Izquierdo M, Sánchez‐Marteles M, Conde‐Martel A, et al. Combining loop with thiazide diuretics for decompensated heart failure: The CLOROTIC trial. Eur Heart J 2023;44:411‐421. doi: 10.1093/EURHEARTJ/EHAC689 [DOI] [PubMed] [Google Scholar]
- 372. Trullàs JC, Morales‐Rull JL, Casado J, Carrera‐Izquierdo M, Sánchez‐Marteles M, Conde‐Martel A, et al. Combining loop and thiazide diuretics for acute heart failure across the estimated glomerular filtration rate spectrum: A post‐hoc analysis of the CLOROTIC trial. Eur J Heart Fail 2023;25:1784‐1793. doi: 10.1002/EJHF.2988 [DOI] [PubMed] [Google Scholar]
- 373. Mentz RJ, Anstrom KJ, Eisenstein EL, Sapp S, Greene SJ, Morgan S, et al. Effect of torsemide vs furosemide after discharge on all‐cause mortality in patients hospitalized with heart failure: The TRANSFORM‐HF randomized clinical trial. JAMA 2023;329:214‐223. doi: 10.1001/JAMA.2022.23924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Biegus J, Zymliński R, Testani J, Marciniak D, Zdanowicz A, Jankowska EA, et al. Renal profiling based on estimated glomerular filtration rate and spot urine sodium identifies high‐risk acute heart failure patients. Eur J Heart Fail 2021;23:729‐739. [DOI] [PubMed] [Google Scholar]
- 375. ter Maaten JM, Beldhuis IE, van der Meer P, Krikken JA, Postmus D, Coster JE, et al. Natriuresis‐guided diuretic therapy in acute heart failure: A pragmatic randomized trial. Nat Med 2023;29:2625‐2632. doi: 10.1038/s41591-023-02532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Beldhuis IE, Damman K, Pang PS, Greenberg B, Davison BA, Cotter G, et al. Mineralocorticoid receptor antagonist initiation during admission is associated with improved outcomes irrespective of ejection fraction in patients with acute heart failure. Eur J Heart Fail 2023;25:25‐1592. doi: 10.1002/EJHF.2975 [DOI] [PubMed] [Google Scholar]
- 377. Voors AA, Angermann CE, Teerlink JR, Collins SP, Kosiborod M, Biegus J, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat Med 2022;28:568‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Schulze PC, Bogoviku J, Westphal J, Aftanski P, Haertel F, Grund S, et al. Effects of early empagliflozin initiation on diuresis and kidney function in patients with acute decompensated heart failure (EMPAG‐HF). Circulation 2022;146:289‐298. [DOI] [PubMed] [Google Scholar]
- 379. Packer M, Butler J. Similarities and distinctions between acetazolamide and sodium–glucose cotransporter 2 inhibitors in patients with acute heart failure: Key insights into ADVOR and EMPULSE. Eur J Heart Fail 2023;25:1537‐1543. doi: 10.1002/ejhf.2968 [DOI] [PubMed] [Google Scholar]
- 380. Metra M, Chioncel O, Cotter G, Davison B, Filippatos G, Mebazaa A, et al. Safety and efficacy of istaroxime in patients with acute heart failure‐related pre‐cardiogenic shock—A multicentre, randomized, double‐blind, placebo‐controlled, parallel group study (SEISMiC). Eur J Heart Fail 2022;24:1967‐1977. doi: 10.1002/EJHF.2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Domínguez‐Rodríguez A, Suero‐Mendez C, Burillo‐Putze G, Gil V, Calvo‐Rodriguez R, Piñera‐Salmeron P, et al. Midazolam versus morphine in acute cardiogenic pulmonary oedema: Results of a multicentre, open‐label, randomized controlled trial. Eur J Heart Fail 2022;24:1953‐1962. doi: 10.1002/EJHF.2602 [DOI] [PubMed] [Google Scholar]
- 382. Beer BN, Jentzer JC, Weimann J, Dabboura S, Yan I, Sundermeyer J, et al. Early risk stratification in patients with cardiogenic shock irrespective of the underlying cause—The Cardiogenic Shock Score. Eur J Heart Fail 2022;24:657‐667. doi: 10.1002/EJHF.2449 [DOI] [PubMed] [Google Scholar]
- 383. Naidu SS, Baran DA, Jentzer JC, Hollenberg SM, van Diepen S, Basir MB, et al. SCAI SHOCK stage classification expert consensus update: A review and incorporation of validation studies: This statement was endorsed by the American College of Cardiology (ACC), American College of Emergency Physicians (ACEP), American Heart Association (AHA), European Society of Cardiology (ESC) Association for Acute Cardiovascular Care (ACVC), International Society for Heart and Lung Transplantation (ISHLT), Society of Critical Care Medicine (SCCM), and Society of Thoracic Surgeons (STS) in December 2021. J Am Coll Cardiol 2022;79:933‐946. doi: 10.1016/J.JACC.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 384. Riccardi M, Pagnesi M, Chioncel O, Mebazaa A, Cotter G, Gustafsson F, et al. Medical therapy of cardiogenic shock: Contemporary use of inotropes and vasopressors. Eur J Heart Fail 2024;26:411‐431. doi: 10.1002/ejhf.3162 [DOI] [PubMed] [Google Scholar]
- 385. Schrage B, Sundermeyer J, Beer BN, Bertoldi L, Bernhardt A, Blankenberg S, et al. Use of mechanical circulatory support in patients with non‐ischaemic cardiogenic shock. Eur J Heart Fail 2023;25:562‐572. doi: 10.1002/EJHF.2796 [DOI] [PubMed] [Google Scholar]
- 386. Bogerd M, ten Berg S, Peters EJ, Vlaar APJ, Engström AE, Otterspoor LC, et al. Impella and venoarterial extracorporeal membrane oxygenation in cardiogenic shock complicating acute myocardial infarction. Eur J Heart Fail 2023;25:2021‐2031. doi: 10.1002/ejhf.3025 [DOI] [PubMed] [Google Scholar]
- 387. Thiele H, Zeymer U, Akin I, Behnes M, Rassaf T, Mahabadi AA, et al. Extracorporeal life support in infarct‐related cardiogenic shock. N Engl J Med. 2023;389:1286‐1297. doi: 10.1056/NEJMOA2307227/SUPPL_FILE/NEJMOA2307227_DATA-SHARING.PDF [DOI] [PubMed] [Google Scholar]
- 388. Zeymer U, Freund A, Hochadel M, Ostadal P, Belohlavek J, Rokyta R, et al. Venoarterial extracorporeal membrane oxygenation in patients with infarct‐related cardiogenic shock: An individual patient data meta‐analysis of randomised trials. The Lancet. 2023;402:1338‐1346. doi: 10.1016/S0140-6736(23)01607-0 [DOI] [PubMed] [Google Scholar]
- 389. Park H, Yang JH, Ahn JM, Kang DY, Lee PH, Kim TO, et al. Early left atrial venting versus conventional treatment for left ventricular decompression during venoarterial extracorporeal membrane oxygenation support: The EVOLVE‐ECMO randomized clinical trial. Eur J Heart Fail 2023;25:2037‐2046. doi: 10.1002/ejhf.3014 [DOI] [PubMed] [Google Scholar]
- 390. Varshney AS, Berg DD, Zhou G, Sinnenberg L, Hirji S, DeFilippis EM, et al. Bridging strategies and cardiac replacement outcomes in patients with acute decompensated heart failure‐related cardiogenic shock. Eur J Heart Fail 2023;25:425‐435. doi: 10.1002/EJHF.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391. Schrage B, Lund LH, Benson L, Braunschweig F, Ferreira JP, Dahlström U, et al. Association between a hospitalization for heart failure and the initiation/discontinuation of guideline‐recommended treatments: An analysis from the Swedish Heart Failure Registry. Eur J Heart Fail 2023;25:25‐1144. doi: 10.1002/EJHF.2928 [DOI] [PubMed] [Google Scholar]
- 392. Mebazaa A, Davison B, Chioncel O, Cohen‐Solal A, Diaz R, Filippatos G, et al. Safety, tolerability and efficacy of up‐titration of guideline‐directed medical therapies for acute heart failure (STRONG‐HF): A multinational, open‐label, randomised, trial. Lancet (London, England) 2022;400:1938‐1952. doi: 10.1016/S0140-6736(22)02076-1 [DOI] [PubMed] [Google Scholar]
- 393. Cotter G, Deniau B, Davison B, Edwards C, Adamo M, Arrigo M, et al. Optimization of evidence‐based heart failure medications after an acute heart failure admission: A secondary analysis of the STRONG‐HF randomized clinical trial. JAMA Cardiol 2024;9:114‐124. doi: 10.1001/jamacardio.2023.4553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394. Arrigo M, Biegus J, Asakage A, Mebazaa A, Davison B, Edwards C, et al. Safety, tolerability and efficacy of up‐titration of guideline‐directed medical therapies for acute heart failure in elderly patients: A sub‐analysis of the STRONG‐HF randomized clinical trial. Eur J Heart Fail 2023;25:1145‐1155. doi: 10.1002/EJHF.2920 [DOI] [PubMed] [Google Scholar]
- 395. Pagnesi M, Metra M, Cohen‐Solal A, Edwards C, Adamo M, Tomasoni D, et al. Uptitrating treatment after heart failure hospitalization across the spectrum of left ventricular ejection fraction. J Am Coll Cardiol 2023;81:2131‐2144. doi: 10.1016/j.jacc.2023.03.426 [DOI] [PubMed] [Google Scholar]
- 396. Adamo M, Pagnesi M, Mebazaa A, Davison B, Edwards C, Tomasoni D, et al. NT‐proBNP and high intensity care for acute heart failure: The STRONG‐HF trial. Eur Heart J 2023;44:2947‐2962. doi: 10.1093/eurheartj/ehad335 [DOI] [PubMed] [Google Scholar]
- 397. Pagnesi M, Vilamajo OAG, Meirino A, Dumont CA, Mebazaa A, Davison B, et al. Blood pressure and intensive treatment up‐titration after acute heart failure hospitalization: Insights from the STRONG‐HF trial. Eur J Heart Fail 2024; doi: 10.1002/ejhf.3174 [DOI] [PubMed] [Google Scholar]
- 398. Celutkiene J, Cerlinskaite‐Bajore K, Cotter G, Edwards C, Adamo M, Arrigo M, et al. Impact of rapid up‐titration of guideline‐directed medical therapies on quality of life: Insights from the STRONG‐HF trial. Circ Heart Fail 2024;17:e011221. doi: 10.1161/CIRCHEARTFAILURE.123.011221 [DOI] [PubMed] [Google Scholar]
- 399. Ter Maaten JM, Mebazaa A, Davison B, Edwards C, Adamo M, Arrigo M, et al. Early changes in renal function during rapid up‐titration of guideline‐directed medical therapy following an admission for acute heart failure. Eur J Heart Fail 2023;25:2230‐2242. doi: 10.1002/ejhf.3074 [DOI] [PubMed] [Google Scholar]
- 400. Kerwagen F, Koehler K, Vettorazzi E, Stangl V, Koehler M, Halle M, et al. Remote patient management of heart failure across the ejection fraction spectrum: A pre‐specified analysis of the TIM‐HF2 trial. Eur J Heart Fail 2023;25:1671‐1681. doi: 10.1002/ejhf.2948 [DOI] [PubMed] [Google Scholar]
- 401. Zito A, Princi G, Romiti GF, Galli M, Basili S, Liuzzo G, et al. Device‐based remote monitoring strategies for congestion‐guided management of patients with heart failure: A systematic review and meta‐analysis. Eur J Heart Fail 2022;24:2333‐2341. doi: 10.1002/ejhf.2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Assmus B, Angermann CE, Alkhlout B, Asselbergs FW, Schnupp S, Brugts JJ, et al. Effects of remote haemodynamic‐guided heart failure management in patients with different subtypes of pulmonary hypertension: Insights from the MEMS‐HF study. Eur J Heart Fail 2022;24:2320‐2330. doi: 10.1002/EJHF.2656 [DOI] [PubMed] [Google Scholar]
- 403. Brugts JJ, Radhoe SP, Clephas PRD, Aydin D, van Gent MWF, Szymanski MK, et al. Remote haemodynamic monitoring of pulmonary artery pressures in patients with chronic heart failure (MONITOR‐HF): A randomised clinical trial. The Lancet. 2023;401:2113‐2123. doi: 10.1016/S0140-6736(23)00923-6 [DOI] [PubMed] [Google Scholar]
- 404. D'Amario D, Meerkin D, Restivo A, Ince H, Sievert H, Wiese A, et al. Safety, usability, and performance of a wireless left atrial pressure monitoring system in patients with heart failure: The VECTOR‐HF trial. Eur J Heart Fail 2023;25:902‐911. doi: 10.1002/EJHF.2869 [DOI] [PubMed] [Google Scholar]
- 405. Ivey‐Miranda JB, Wetterling F, Gaul R, Sheridan S, Asher JL, Rao VS, et al. Changes in inferior vena cava area represent a more sensitive metric than changes in filling pressures during experimental manipulation of intravascular volume and tone. Eur J Heart Fail 2022;24:455‐462. doi: 10.1002/EJHF.2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Sheridan WS, Wetterling F, Testani JM, Borlaug BA, Fudim M, Damman K, et al. Safety and performance of a novel implantable sensor in the inferior vena cava under acute and chronic intravascular volume modulation. Eur J Heart Fail 2023;25:754‐763. doi: 10.1002/EJHF.2822 [DOI] [PubMed] [Google Scholar]


