Abstract
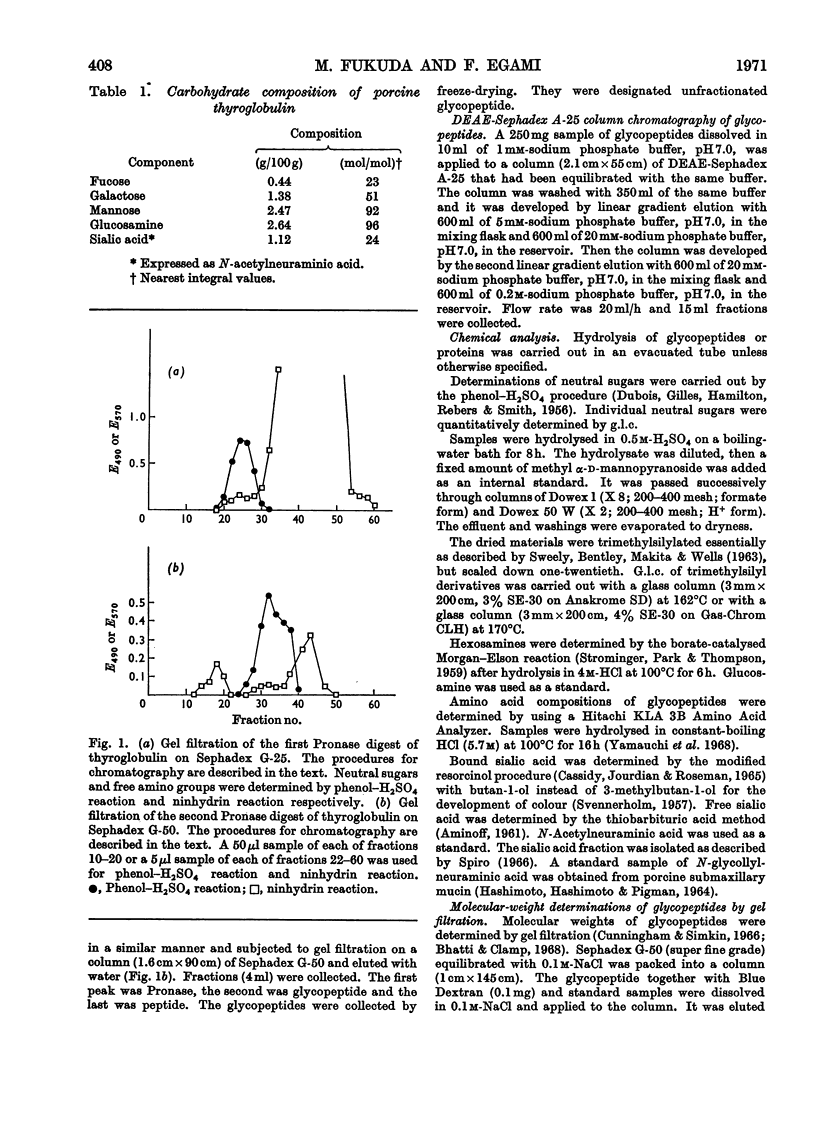
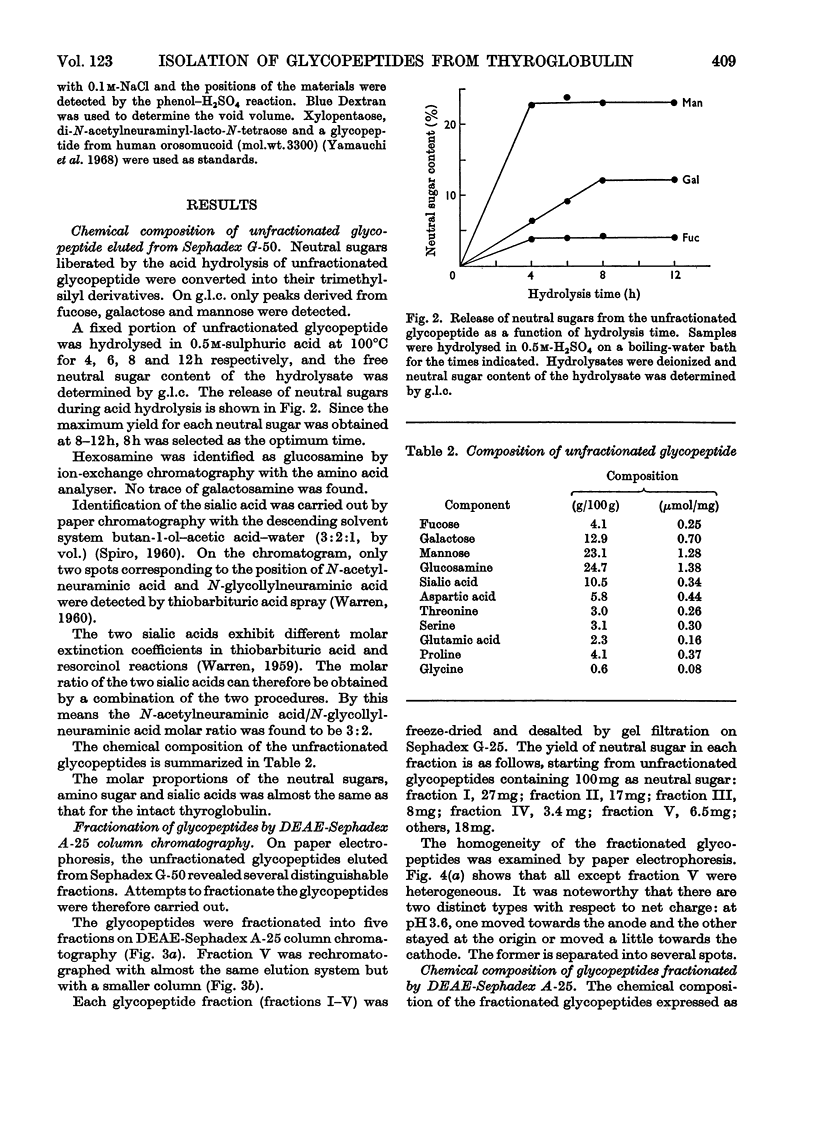
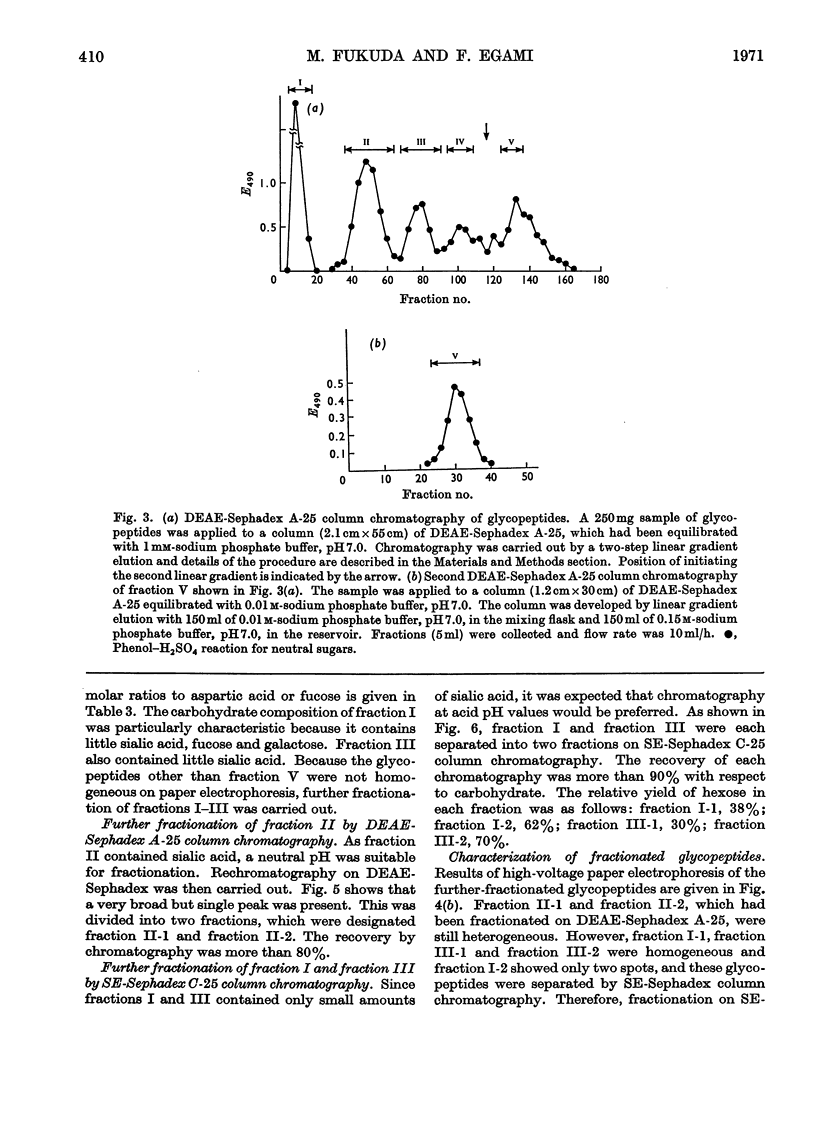
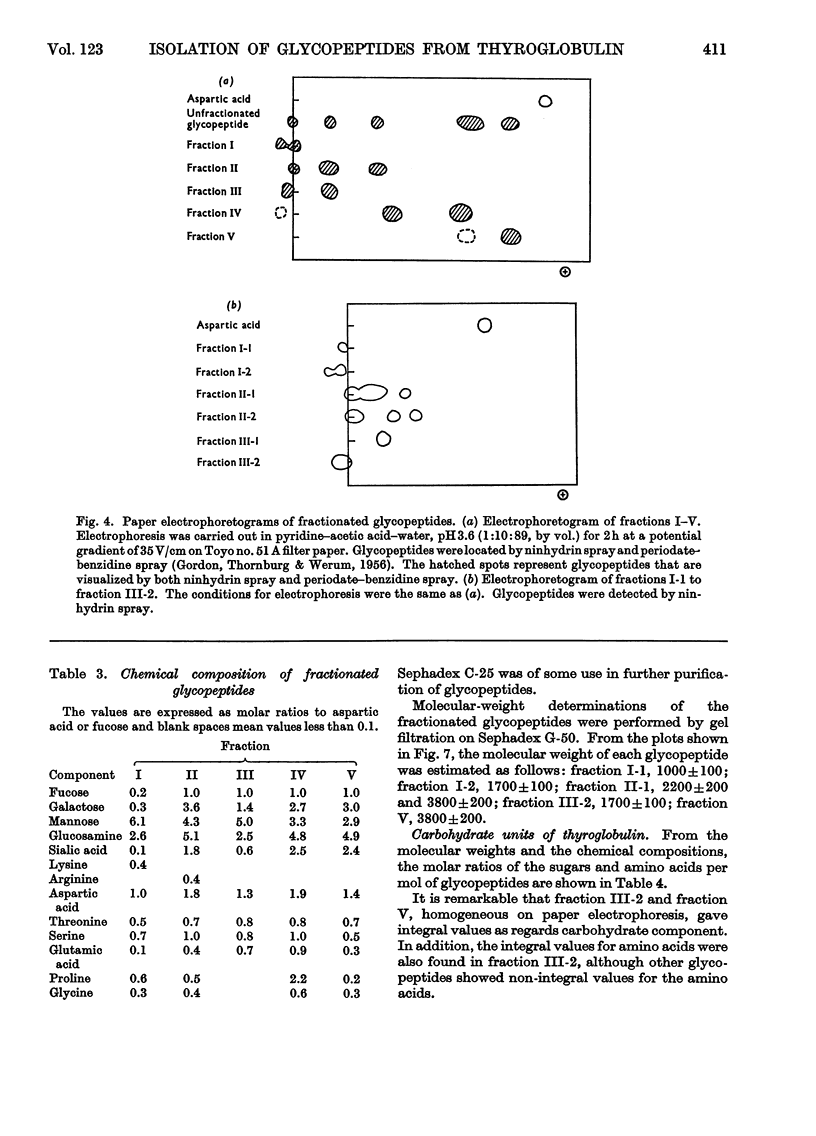
1. Glycopeptides were isolated by gel filtration on Sephadex G-25 and Sephadex G-50 from a Pronase digest of porcine thyroglobulin. 2. Isolated glycopeptides were separated into five main fractions on a column of DEAE-Sephadex A-25. Of these fractions I to III were further purified by SE-Sephadex C-25 or DEAE-Sephadex A-25 column chromatography. Several of the purified glycopeptides were homogeneous on paper electrophoresis. 3. Based on the chemical composition and molecular weight of the fractionated glycopeptides, two distinct types of heterosaccharide chain were demonstrated. 4. One type of the heterosaccharide unit consisted of four to eight residues of mannose and two residues of glucosamine and had a molecular weight of 1000–1700. The other type of unit contained sialic acid, fucose and galactose in addition to mannose and glucosamine and had a molecular weight of about 3600. 5. Mild alkaline treatment of the glycopeptide did not result in the destruction of threonine and serine. 2-Acetamido-1-N-(4-l-aspartyl)-2-deoxy-β-d-glucopyranosylamine was isolated from partial acid hydrolysates.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein I., Wagh P. V., Winzler R. J. The structure of a glycopeptide from human orosomucoid (alpha-acid glycoprotein). J Biol Chem. 1969 Feb 25;244(4):658–665. [PubMed] [Google Scholar]
- Burge B. W., Strauss J. H., Jr Glycopeptides of the membrane glycoprotein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):449–466. doi: 10.1016/0022-2836(70)90314-1. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM L. W., CLOUSE R. W., FORD J. D. HETEROGENEITY OF THE CARBOHYDRATE MOIETY OF CRYSTALLINE OVALBUMIN. Biochim Biophys Acta. 1963 Oct 29;78:379–381. doi: 10.1016/0006-3002(63)91652-4. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Cunningham W., Simkin J. L. Studies on glycopeptides deried from acidic glycoproteins of guinea-pig serum. Biochem J. 1966 May;99(2):434–442. doi: 10.1042/bj0990434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELHOCH H. The properties of thyroglobulin. I. The effects of alkali. J Biol Chem. 1960 May;235:1326–1334. [PubMed] [Google Scholar]
- HASHIMOTO Y., HASHIMOTO S., PIGMAN W. PURIFICATION AND PROPERTIES OF PORCINE SUBMAXILLARY MUCIN. Arch Biochem Biophys. 1964 Feb;104:282–291. doi: 10.1016/s0003-9861(64)80015-1. [DOI] [PubMed] [Google Scholar]
- JOHANSEN P. G., MARSHALL R. D., NEUBERGER A. Carbohydrates in protein. 3 The preparation and some of the properties of a glycopeptide from hen's-egg albumin. Biochem J. 1961 Mar;78:518–527. doi: 10.1042/bj0780518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. T., Li S. C., Shetlar M. R. Isolation of glycopeptides from rat liver microsomes involved in the biosynthesis of plasma glycoprotein. J Biol Chem. 1968 Feb 10;243(3):656–665. [PubMed] [Google Scholar]
- NARASIMHAMURTHY P. V., RAGHUPATHY E., CHAIKOFF I. L. STUDIES ON THYROID PROTEINS. I. ISOLATION AND PROPERTIES OF A GLYCOPEPTIDE FROM SHEEP THYROGLOBULIN. Biochemistry. 1965 Apr;4:611–618. doi: 10.1021/bi00880a001. [DOI] [PubMed] [Google Scholar]
- Rawitch A. B., Liao T. H., Pierce J. C. The amino acid sequence of a tryptic glycopeptide from human thyroglobulin. Biochim Biophys Acta. 1968 Aug 13;160(3):360–367. doi: 10.1016/0005-2795(68)90208-0. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. Studies on fetuin, a glycoprotein of fetal serum. II. Nature of the carbohydrate units. J Biol Chem. 1962 Feb;237:382–388. [PubMed] [Google Scholar]
- SPIRO R. G. THE CARBOHYDRATE UNITS OF THYROGLOBULIN. J Biol Chem. 1965 Apr;240:1603–1610. [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid galactosyltransferase. J Biol Chem. 1968 Dec 25;243(24):6529–6537. [PubMed] [Google Scholar]
- Spiro M. J., Spiro R. G. Glycoprotein biosynthesis: studies on thyroglobulin. Thyroid sialyltransferase. J Biol Chem. 1968 Dec 25;243(24):6520–6528. [PubMed] [Google Scholar]
- Spiro R. G., Spiro M. J. Glycoprotein biosynthesis: studies on thyroglobulin. Characterization of a particulate precursor and radioisotope incorporation by thyroid slices and particle systems. J Biol Chem. 1966 Mar 25;241(6):1271–1282. [PubMed] [Google Scholar]
- Spiro R. G. Studies on fetuin, a glycoprotein of fetal serum. I. Isolation, chemical composition, and physiochemical properties. J Biol Chem. 1960 Oct;235(10):2860–2869. [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WARREN L. Thiobarbituric acid spray reaction for deoxy sugars and sialic acids. Nature. 1960 Apr 16;186:237–237. doi: 10.1038/186237a0. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Makino M., Yamashina I. The amino acid sequence, the peptide-carbohydrate linkages, and the composition and size of the polysaccharide units of the glycopeptides from alpha-1-acid glycoprotein of human plasma. J Biochem. 1968 Nov;64(5):683–698. doi: 10.1093/oxfordjournals.jbchem.a128946. [DOI] [PubMed] [Google Scholar]


