Abstract
A 58-year-old woman with a body mass index of 26.4 kg/m2 was referred because of high glycated hemoglobin (HbA1c) at a medical checkup. Her anti-glutamic acid decarboxylase antibody (GADA) titer was positive (16.0 U/mL; normal < 5.0 U/mL). Her HbA1c was controlled at 6.4%–7.5% using metformin, ipragliflozin, and sitagliptin. Two-and-a-half years later, she was diagnosed with endometrial cancer with pelvic lymph node metastasis and underwent surgery followed by chemotherapy with carboplatin and paclitaxel, then carboplatin and docetaxel. However, owing to enlargement of the metastatic nodules, combination therapy with pembrolizumab and lenvatinib (pem + len) was initiated (DAY 1). On DAY 36, her plasma glucose (PG) concentration was high; therefore, insulin degludec was administered once daily and self-monitoring of blood glucose commenced. On DAY 50, her PG and HbA1c were 509 mg/dL and 10.2%, respectively, and her insulin therapy was changed to a basal–bolus. Urinary ketones were negative. Treatment with pem + len was continued without interruption. Her GADA was negative 3 months before starting pem + len (DAY − 119), but was high (234 U/mL) on DAY 50, and then negative on DAYs 345 and 670. Her serum C-peptide concentration gradually decreased, but it did not disappear (DAYs − 119, 50, 156, 345, 607 and 670: 2.49, 1.80, 0.90, 0.21, 0.85 and 0.65 ng/mL, respectively). Human leukocyte antigen analysis revealed two susceptibility haplotypes (DRB1*04:05-DQB1*04:01-DPB1*02:01 and DRB1*04:05-DQB1*04:01-DPB1*05:01) for type 1 diabetes (T1D). This case is notable in that pembrolizumab-related T1D progressed more slowly than previously reported, and lenvatinib may have contributed to this delay.
Keywords: Pembrolizumab, Lenvatinib, Immune checkpoint inhibitor, Tyrosine kinase inhibitor, Type 1 diabetes, Slow progression
Introduction
Immune checkpoint inhibitor (ICI)-related type 1 diabetes mellitus (ICI–T1D) is a rare, but serious immune-related adverse event of ICI use, occurring in 0.2%–1.4% of patients who undergo ICI therapy for the treatment of cancer [1]. Most patients with ICI–T1D undergo anti-programmed cell death-1 (PD-1) or anti-programmed cell death ligand-1 (PD-L1) antibody therapy, and anti-PD-1 antibody administration has been most commonly reported [1]. Owing to the expansion of the indications for ICI therapy, the number of patients with ICI–T1D is expected to increase [1].
At the time of diagnosis of ICI–T1D, the serum C-peptide concentrations of patients are low in 84%–92% of instances [1, 2], and diabetic ketoacidosis (DKA) is present in 69.7% of patients [1]. It has been reported that the serum C-peptide concentrations of patients 1 week to 10 months after the onset of ICI–T1D are 0.02 ± 0.04 (mean ± SD) nmoL/L [1], and that the serum C-peptide concentrations of most Japanese patients decrease rapidly over a period of 2–3 weeks [3]. There have been no reported cases of recovery from diabetes when ICI–T1D develops and the serum C-peptide concentrations of patients decrease below 0.4 nmol/L [1].
We encountered a patient with type 1 diabetes mellitus (T1D) associated with combination therapy with pembrolizumab, an anti-PD-1 antibody, and lenvatinib, a multi-targeting tyrosine kinase inhibitor (TKI), for metastatic endometrial cancer during the course of probable slowly progressive type 1 diabetes mellitus (SPIDDM). Despite the continued use of pembrolizumab and lenvatinib, this patient experienced slower progression of T1D than previously reported cases of ICI–T1D.
Case presentation
A 58-year-old woman, who was 158 cm tall, weighed 66 kg, and had a body mass index (BMI) of 26.4 kg/m2, visited our hospital because of a high HbA1c level (7.7%) (Day 1). She had no family history of diabetes, but a high HbA1c (6.0%) had been identified 2 years previously. Routine laboratory measurements revealed several abnormalities, including a high fasting plasma glucose (PG) concentration, a high HbA1c, high circulating alanine aminotransferase and aspartate aminotransferase activities, a high low-density lipoprotein-cholesterol concentration, a high thyroid-stimulating hormone concentration, and a low free thyroxine concentration (Table 1). Mild fatty liver was identified on ultrasonography. Her blood pressure was normal. Immunologic assays revealed positivity for circulating anti-thyroglobulin antibody, anti-thyroid peroxidase antibody, and anti-glutamic acid decarboxylase antibody (GADA) (16.0 U/mL).
Table 1.
Laboratory data obtained for the patient at her first visit
| Hematology | (Normal range) | (Normal range) | |||
|---|---|---|---|---|---|
| White blood cell count | 5.9 × 103/μL | 3.3–8.6 × 103/μL | Glucose | 143 mg/dL | 73–109 mg/dL |
| Red blood cell count | 4.65 × 106/μL | 3.86–4.92 × 106/μL | Hemoglobin A1c | 8.2% | 4.9%–6.0% |
| Hemoglobin | 14.5 g/dL | 11.6–14.8 g/dL | Thyroid-stimulating hormone | 9.22 μIU/mL | 0.5–5.0 μIU/mL |
| Hematocrit | 42.4% | 35.1%–44.4% | Free thyroxine | 0.73 ng/dL | 0.9–1.7 ng/dL |
| Platelet count | 203 × 103/μL | 158–348 × 103/μL | Serological data | ||
| Blood chemistry | Hepatitis B surface antigen | (–) | |||
| Total protein | 7.7 g/dL | 6.6–8.1 g/dL | Anti-hepatitis C antibody | (–) | |
| Aspartate aminotransferase | 66 IU/L | 13–30 IU/L | Immunological data | ||
| Alanine aminotransferase | 105 IU/L | 7–23 IU/L | Anti-thyroglobulin antibody | 302.8 IU/mL | 0–27.9 IU/mL |
| γ-Glutamyl transpeptidase | 83 IU/L | 9–32 IU/L | Anti-thyroid peroxidase antibody | 243.6 IU/mL | 0–15.9 IU/mL |
| Low-density lipoprotein-cholesterol | 143 mg/dL | 65–163 mg/dL | Anti-glutamic acid decarboxylase antibody | 16.0 U/mL | < 5.0 U/mL |
| High-density lipoprotein-cholesterol | 53 mg/dL | 48–103 mg/dL | |||
| Triglycerides | 147 mg/dL | 30–117 mg/dL | Urinalysis results | ||
| Uric acid | 4.9 mg/dL | 65–163 mg/dL | Protein | (-) | |
| Blood urea nitrogen | 9.8 mg/dL | 8–20 mg/dL | Albumin | 0 mg/g Creatinine | |
| Creatinine | 0.57 mg/dL | 0.46–0.79 mg/dL | Ketones | (–) | |
| Estimated glomerular filtration rate | 83 mL/min/1.73m2 | Occult blood | (–) | ||
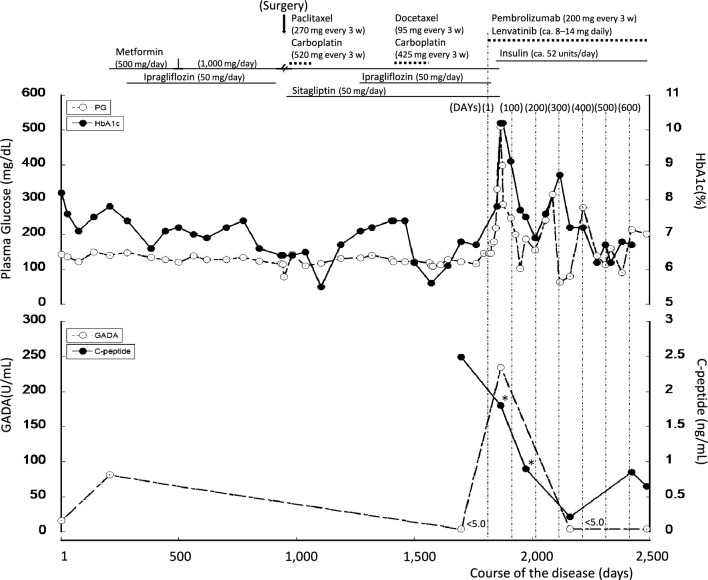
The patient was instructed to improve her diet and exercise more, and levothyroxine therapy was commenced. On Day 207, her GADA titer had further increased, to 81.7 U/mL. On the basis of the current diagnostic criteria of the Japan Diabetes Society [4], she was diagnosed with SPIDDM (probable). Metformin (500 mg/day, later increased to 1,000 mg/day) was administered from Day 207, and ipragliflozin (50 mg/day) was added on Day 281. As a consequence, her HbA1c was controlled at approximately 6.4%–7.5% (Fig. 1). Rosuvastatin (2.5 mg/day) was also initiated on Day 500.
Fig. 1.
Clinical course of the patient. C-peptide concentrations were measured in serum samples collected when the patient had fasted or not (*). 3 w 3 weeks. HbA1c glycated hemoglobin, GADA anti-glutamic acid decarboxylase antibody
On Day 941, the patient was diagnosed with endometrial cancer with pelvic lymph node metastasis. She underwent modified radical hysterectomy with pelvic lymph node dissection, para-aortic lymph node sampling, and omentectomy on Day 946, and then six rounds of combination therapy with carboplatin 520 mg and paclitaxel 270 mg every 3 weeks from Days 960 to 1,065. Eleven months later, computed tomography and positron emission tomography scans revealed new lymph node metastases in the para-aortic region and peritoneal dissemination. Therefore, she underwent six rounds of carboplatin 425 mg and docetaxel 95 mg treatment every 3 weeks, starting on Day 1,417. However, during the seventh round, an allergic reaction to carboplatin occurred; therefore, the treatment was discontinued. Eight months later (Day 1,786), imaging studies showed a marked reduction in peritoneal dissemination, but the para-aortic nodular lesion had enlarged and there was left ureteral compression. For further treatment of her diabetes, sitagliptin was added on Day 958. Her fasting serum C-peptide concentration was 2.49 ng/mL and her GADA titer was negative (< 5 U/mL) on Day 1,698.
Combination therapy with pembrolizumab and lenvatinib was initiated on Day 1,817 (DAY 1). Pembrolizumab 200 mg was administered every 3 weeks. Lenvatinib was initiated at 14 mg daily, but the patient developed symptoms and signs including hypertension, general malaise, fever, arthralgia and stomatitis, necessitating temporary dose modifications, as follows: 10 mg on DAYs 5–14, none on DAYs 15–39, 10 mg on DAYs 40–42, none on DAYs 43–49, 4 mg on DAYs 50–56, 8 mg on DAYs 57–70, 10 mg on DAYs 71–92, and 14 mg from DAY 93 onward. The dose was then adjusted as necessary within the range of 8–14 mg. On DAY 36, after the second course of pembrolizumab, her casual PG and HbA1c levels had increased to 330 mg/dL and 7.8%, respectively. At that time, it was not possible to clearly distinguish between the onset of T1D and fluctuations in the severity of her pre-existing diabetes; therefore, we introduced once-daily insulin (degludec) injection and the self-monitoring of blood glucose (SMBG). However, her blood glucose concentration rose sharply over a short period of time, with her PG and HbA1c levels reaching 509 mg/dL and 10.2%, respectively, on DAY 50. She was negative for urinary ketones. Her GADA titer rose again to 234 U/mL on DAY 50, when she was diagnosed with pembrolizumab-related T1D (Pem-T1D). At this time, her insulin therapy was changed to a basal–bolus regimen, which was adjusted according to the results of her SMBG (usually insulin aspart 12 units before each meal and degludec 16 units in the evening), and her oral hypoglycemic agents were discontinued. Pembrolizumab and lenvatinib were administered as scheduled and without interruption. Her C-peptide concentration was stable (1.80 ng/mL) on DAY 50, then gradually decreased over the next 10 months (DAY 156, casual C-peptide 0.90 ng/mL; DAY 345, fasting C-peptide 0.21 ng/mL). Her GADA titer had become negative by DAY 345, and remained negative on DAY 670. Her fasting C-peptide concentration subsequently increased (DAY 607, 0.85 ng/mL; DAY 670, 0.65 ng/mL). Her anti-insulinoma-associated antigen-2 antibody (IA-2A) titer was negative (< 0.6 U/mL) on DAY 691. Intermittently scanned continuous glucose monitoring commenced on DAY 303, and her daily insulin dose remained largely unchanged, but her PG and HbA1c levels improved and tended to stabilize (Fig. 1). Her thyroid function remained stable, and no other endocrine disturbances occurred after the initiation of pembrolizumab. Human leukocyte antigen (HLA) analysis revealed the presence of two susceptibility haplotypes for T1D (DRB1*04:05-DQB1*04:01-DPB1*02:01 and DRB1*04:05-DQB1*04:01-DPB1*05:01) [5].
At the time of preparation of this manuscript, the patient had undergone 31 rounds of pembrolizumab and lenvatinib therapy. Her para-aortic nodular lesion had slightly shrunk, and her left ureter had been decompressed. No local recurrence or new metastases of the cancer were identified. Therefore, she was assessed as having stable disease.
Discussion
The median time to onset of ICI–T1D has been reported to be 12 weeks or 4.5 cycles after the initiation of an ICI [1, 2]. T1D autoantibodies, and predominantly GADA, have been shown to be present in 40.4%–53.0% of patients and are significantly associated with an earlier onset of ICI–T1D and the onset of DKA [1, 2]. It has also been suggested that patients who have islet autoantibodies, SPIDDM, or latent autoimmune diabetes mellitus in adults (LADA) before undergoing ICI may be at extremely high risk of developing ICI–T1D for a brief period of time after the start of treatment [6]. Furthermore, the allele and haplotype frequencies of HLA-DRB1*04:05, DQB1*04:01, and most importantly, DPB1*05:01, are high in Japanese patients with ICI–T1D [7]. The clinical features of the present patient are consistent with those of the other patients with ICI–T1D described above. However, given that the prevalence of DKA is 69.7% in patients at the onset of ICI–T1D [1], the present patient was somewhat atypical. This may have been because she was able to start insulin therapy early, given that her pre-existing diabetes had been closely monitored, and this may have prevented DKA. On the basis of these findings, we diagnosed her with pembrolizumab-related T1D. Although there has been one previous case report of lenvatinib-associated T1D [8], there have been few reports of TKI-related autoimmune T1D, and therefore such a diagnosis seemed to be less appropriate than one of ICI–T1D.
It has also been reported that in ICI–T1D, the serum C-peptide concentrations of patients at presentation are < 0.4 nmol/L (< 1.2 ng/mL) in 91.6% of instances [1] and low in 84% [2], and that the C-peptide concentrations of patients 1 week to 10 months later are 0.02 ± 0.04 (mean ± SD) nmoL/L [1]. Of 22 Japanese patients with ICI–T1D, 50% were found to meet the criteria for fulminant T1D, and the remaining 50% met the criteria for acute-onset T1D [3]. The serum C-peptide concentrations of most patients decrease over the 2–3 weeks following the development of ICI–T1D [3]. The clinical course of the present patient differed markedly from those of previously reported patients, in that her serum C-peptide concentration was stable at the time of onset of ICI–T1D (DAY 50, 1.80 ng/mL), then gradually decreased over the following 10 months, but had not disappeared 620 days after the onset of the condition (DAY 670, 0.65 ng/mL). Although several cases of ICI–T1D have been reported in which the serum C-peptide concentrations of the patients were maintained to some extent or increased after the withdrawal of the ICI [3, 9–11], the present patient was able to continue to undergo pembrolizumab therapy, without interruption, even after the onset of T1D.
It would be interesting to know why the Pem-T1D progressed slowly in the present patient. We speculate that the following factors may explain this slow progression: 1) genetic variation, 2) the use of lenvatinib, 3) the use of sitagliptin and metformin, and 4) the use of insulin therapy.
Initially, we hypothesized that the present patient may have had a genotype that was protective against autoimmune T1D, regardless of the etiology. Indeed, one patient has been reported who had both susceptibility and protective HLA genotypes and who showed a worsening of their pre-existing diabetes after starting nivolumab, but whose C-peptide concentration was somewhat preserved 40 days after the discontinuation of nivolumab and the start of dexamethasone for chemotherapeutic purposes [10]. However, the present patient was found to be homozygous for a T1D susceptibility HLA haplotype (HLA-DRB1*04:05-DQB1*04:01) [5]. This genotype has been reported to be associated with not only SPIDDM, but also acute-onset, fulminant T1D, with a higher odds ratio than for SPIDDM [5]. Therefore, this hypothesis seems unlikely to be correct, but we cannot exclude the possibility that genetic factors other than the HLA haplotype described may be involved [12].
Lenvatinib is a multi-targeting TKI that inhibits tyrosine kinases, including vascular endothelial growth factor receptor (VEGFR1-3), fibroblast growth factor receptor (FGFR1-4), platelet-derived growth factor receptor α (PDGFRα), stem cell factor receptor (c-Kit), and rearranged during transfection (RET) [13]. It has been reported that TKIs may not only ameliorate, but also prevent the clinical manifestations of both T1D and T2D [14–16]. Their antidiabetic effects are thought to be mediated through the inhibition of tyrosine kinases such as c-Abl, PDGFR, VEGFR2, epidermal growth factor receptor (EGFR), and c-Kit [14–16]. In non-obese diabetic mice, the treatment of prediabetic and newly diabetic mice with imatinib (principal targets: c-Abl, PDGFR, c-Kit, and discoidal domain receptor-1 and 2 [14, 16]) or sunitinib (principal targets: c-Kit, PDGFR, and VEGFR [14, 16]) prevents or ameliorates T1D [17], with an efficacy that is dependent on the inhibition of PDGFR and, to some extent, c-Kit [17]. Treatment with sunitinib or imatinib reduces or removes the requirements for insulin in patients with recent-onset or long-standing T1D [18–20]. In addition, a phase 2 clinical trial [21] showed that 26 weeks of treatment with imatinib in patients with recent-onset T1D slowed the decline in beta-cell function over 12 months. Imatinib-treated participants had a significantly larger area under the curve of C-peptide concentration during a mixed-meal tolerance test than those who were placebo treated, but this effect was not sustained over 24 months [21]. The present patient started taking lenvatinib at an earlier stage of T1D (before the onset of Pem-T1D) and continued to take it at the time of writing. Therefore, the use of lenvatinib may be associated with a slower progression of Pem-T1D. To the best of our knowledge, this is the first reported case of T1D related to combination therapy with an ICI and a TKI.
Sitagliptin and metformin were administered to the present patient until DAY 50. It has been reported that both DPP-4 inhibitors and metformin may be effective means of preventing the loss of beta-cell function in patients with SPIDDM or LADA [12, 22–25]. Although these medications may have affected the clinical course of the present patient while they were being taken, it seems unlikely that they would have had a persistent effect after their discontinuation [24].
It has previously been reported that intensive insulin therapy, or even small doses of insulin administered by injection, may preserve beta-cell function in T1D [12, 26], and the present patient may have started injecting insulin earlier than the previously reported patients. However, because insulin therapy is usually initiated soon after diagnosis in patients with ICI–T1D, the differences in the clinical courses of the present and previously reported patients are unlikely to be the result of the insulin therapy.
A limitation of the present study was that the changes in the titers of anti-islet autoantibodies other than GADA were not characterized. Similarly, the patient’s serum C-peptide concentration was not measured during the course of the probable SPIDDM. Although continued therapy with sitagliptin and metformin after the onset of Pem-T1D may have had a protective effect on beta-cell function, the patient’s diagnosis was changed to T1D after the onset of Pem-T1D for medical insurance purposes; therefore, the use of these medications was discontinued.
In conclusion, the present patient was noteworthy in that her Pem-T1D progressed more slowly than that of previously reported patients, and because lenvatinib administration may have contributed to this slower progression. This case may have implications for the use of ICI combination therapy and the prevention of T1D.
Acknowledgements
We thank Mark Cleasby, PhD, from Edanz (https://jp.edanz.com/ac) for editing drafts of this manuscript.
Author contributions
All the authors contributed to the study’s conception and design. The first draft of the manuscript was written by IH. NI contributed to discussion. Both authors commented on drafts of the manuscript and approved the final version.
Declarations
Conflicts of interest
Iwaho Hazekawa and Norio Ishii declare that they have no conflicts of interest.
Research involving human participants and/or animals
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and/or with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent or substitute for it was obtained from all patients for inclusion in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu L, Tsang V, Menzies AM, Sasson SC, Carlino MS, Brown DA, Clifton-Bligh R, Gunton JE. Risk factors and characteristics of checkpoint inhibitor-associated autoimmune diabetes mellitus (CIADM): a systematic review and delineation from type 1 diabetes. Diabetes Care. 2023;46:1292–9. [DOI] [PubMed] [Google Scholar]
- 2.de Filette JMK, Pen JJ, Decoster L, Vissers T, Bravenboer B, Van der Auwera BJ, Gorus FK, Roep BO, Aspeslagh S, Neyns B, Velkeniers B, Kharagjitsingh AV. Immune checkpoint inhibitors and type 1 diabetes mellitus: a case report and systematic review. Eur J Endocrinol. 2019;181:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden MY, Imagawa A, Abiru N, Awata T, Ikegami H, Uchigata Y, Oikawa Y, Osawa H, Kajio H, Kawasaki E, Kawabata Y, Kozawa J, Shimada A, Takahashi K, Tanaka S, Chujo D, Fukui T, Miura J, Yasuda K, Yasuda H, Kobayashi T, Hanafusa T, Consultation of the Japan Diabetes Society Committee on Type 1 Diabetes Mellitus Research. Characteristics and clinical course of type 1 diabetes mellitus related to anti-programmed cell deth-1 therapy. Diabetes Int. 2019;10:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimada A, Kawasaki E, Abiru N, Awata T, Oikawa Y, Osawa H, Kajio H, Kozawa J, Takahashi K, Chujo D, Noso S, Fukui T, Miura J, Yasuda K, Yasuda H, Imagawa A, Ikegami H. New diagnostic criteria (2023) for slowly progressive type 1 diabetes (SPIDDM): report from Committee on Type 1 Diabetes in Japan Diabetes Society (English version). Diabetol Int. 2024;15:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabata Y, Ikegami H, Awata T, Imagawa A, Maruyama T, Kawasaki E, Tanaka S, Shimada A, Osawa H, Kobayashi T, Hanafusa T, Tokunaga K, Makino H, Committee on Type 1 Diabetes, Japan Diabetes Society. Differential association of HLA with three subtypes of type 1 diabetes: fulminant, slowly progressive and acute-onset. Diabetologia. 2009;52:2513–21. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi H, Miyoshi Y, Uehara Y, Fujii K, Nagata S, Obata Y, Kosugi M, Hazama Y, Yasuda T. Case of slowly progressive type 1 diabetes mellitus with drastically reduced insulin secretory capacity after immune checkpoint inhibitor treatment for advanced renal cell carcinoma. Diabetol Int. 2020;12:234–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inaba H, Morita S, Kosugi D, Asai Y, Kaido Y, Ito S, Hirobata T, Inoue G, Yamamoto Y, Jinnin M, Kimura H, Ota M, Okudaira Y, Nakatani H, Kobayashi T, Iwama S, Arima H, Matsuoka T. Amino acid polymorphisms in human histocompatibility leukocyte antigen class II and proinsulin epitope have impacts on type 1 diabetes mellitus induced by immune-checkpoint inhibitors. Front Immunol. 2023;14:1165004. 10.3389/fimmu.2023.1165004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao PF, Peng TR, Wu TW, Hu YH. Lenvatinib-associated fulminant type 1 diabetes mellitus. Am J Ther. 2023;30:e561–3. [DOI] [PubMed] [Google Scholar]
- 9.Hansen E, Sahasrabudhe D, Sievert L. A case report of insulin-dependent diabetes as immune-related toxicity of pembrolizumab: presentation, management and outcome. Cancer Immunol Immunother. 2016;65:765–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumura K, Nagasawa K, Oshima Y, Kikuno S, Hayashi K, Nishimura A, Okubo M, Uruga H, Kishi K, Kobayashi T, Mori Y. Aggravation of diabetes, and incompletely deficient insulin secretion in a case with type 1 diabetes-resistant human leukocyte antigen DRB1*15:02 treated with nivolumab. J Diabetes Investig. 2018;9:438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai G, Saito D, Nakajima R, Hatano M, Noguchi Y, Kurihara S, Katayama S, Inoue I, Noda M, Shimada A. Intrinsic insulin secretion capacity might be preserved by discontinuing anti-programmed cell death protein 1 antibody treatment in ‘anti-programmed cell death protein 1 antibody-induced’ fulminant type 1 diabetes. J Diabetes Investig. 2018;9:448–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura A, Matsumura K, Kikuno S, Nagasawa K, Okubo M, Mori Y, Kobayashi T. Slowly progressive type 1 diabetes mellitus: current knowledge and future perspectives. Diabetes Metab Syndr Obes. 2019;12:2461–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suyama K, Iwase H. Lenvatinib: a promising molecular targeted agent for multiple cancers. Cancer Control. 2018;25:1073274818789361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fountas A, Diamantopoulos LN, Tsatsoulis A. Tyrosine kinase inhibitors and diabetes: a novel treatment paradigm? Trends Endocrinol Metab. 2015;26:643–56. [DOI] [PubMed] [Google Scholar]
- 15.Althubiti M. Tyrosine kinase targeting: a potential therapeutic strategy for diabetes. Saudi J Med Med Sci. 2022;10:183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ak S, Patel SS, Patel S, Parikh P. Future treatment of diabetes - tyrosine kinase inhibitors. J Diabetes Metab Disord. 2022;22:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louvet C, Szot GL, Lang J, Lee MR, Martinier N, Bollag G, Zhu S, Weiss A, Bluestone JA. Tyrosine kinase inhibitors reverse type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:18895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Templeton A, Brändle M, Cerny T, Gillessen S. Remission of diabetes while on sunitinib treatment for renal cell carcinoma. Ann Oncol. 2008;19:824–5. [DOI] [PubMed] [Google Scholar]
- 19.Huda MS, Amiel SA, Ross P, Aylwin SJ. Tyrosine kinase inhibitor sunitinib allows insulin independence in long-standing type 1 diabetes. Diabetes Care. 2014;37:e87–8. [DOI] [PubMed] [Google Scholar]
- 20.Salaroli A, Loglisci G, Serrao A, Alimena G, Breccia M. Fasting glucose level reduction induced by imatinib in chronic myeloproliferative disease with TEL-PDGFRβ rearrangement and type 1 diabetes. Ann Hematol. 2012;91:1823–4. [DOI] [PubMed] [Google Scholar]
- 21.Gitelman SE, Bundy BN, Ferrannini E, Lim N, Blanchfield JL, DiMeglio LA, Felner EI, Gaglia JL, Gottlieb PA, Long SA, Mari A, Mirmira RG, Raskin P, Sanda S, Tsalikian E, Wentworth JM, Willi SM, Krischer JP, Bluestone JA, Greevec Trial Study Group. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomized, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awata T, Shimada A, Maruyama T, Oikawa Y, Yasukawa N, Kurihara S, Miyashita Y, Hatano M, Ikegami Y, Matsuda M, Niwa M, Kazama Y, Tanaka S, Kobayashi T. Possible long-term efficacy of sitagliptin, a dipeptidyl peptidase-4 inhibitor, for slowly progressive type 1 diabetes (SPIDDM) in the stage of non-insulin-dependency: an open-label randomized controlled pilot trial (SPAN-S). Diabetes Ther. 2017;8:1123–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Zheng P, Huang G, Yang L, Zhou Z. Dipeptidyl peptidase-4 inhibitors: promising new agents for autoimmune diabetes. Clin Exp Med. 2018;18:473–80. [DOI] [PubMed] [Google Scholar]
- 24.Gurgel Penaforte-Saboia J, Cour CEB, Vasconcelos Albuquerque N, Lauanna Lima Silva V, da Cunha Bitar, Olegario N, Oliveira Fernandes V, Montenegro Junior RM. Emerging roles of dipeptidyl peptidase-4 inhibitors in delaying the progression of type 1 diabetes mellitus. Diabetes Metab Syndr Obes. 2021;14:565–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirata T, Shimada A, Morimoto J, Maruyama T. Slowly progressive type 1 diabetes treated with metformin for five years after onset. Intern Med. 2013;52:2635–7. [DOI] [PubMed] [Google Scholar]
- 26.The Diabetes Control and Complication Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the diabetes control and complication trial. Ann Intern Med. 1998;128:517–23. [DOI] [PubMed] [Google Scholar]