Abstract
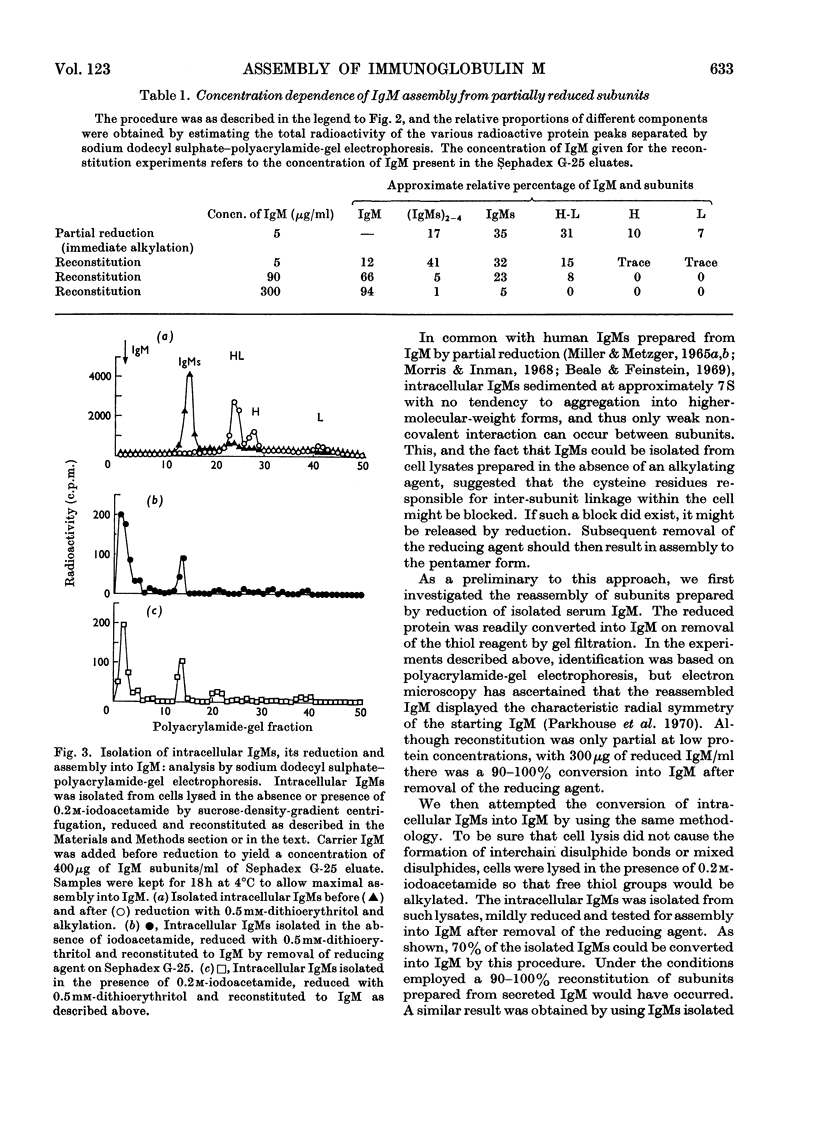
We have shown previously that immunoglobulin M (IgM) is present within IgM-forming cells mainly in its 7S subunit form (IgMs), whereas only fully assembled IgM pentamers are secreted. There is no spontaneous polymerization of intracellular IgMs in cell lysates, suggesting that the 7S subunits had blocked cysteine residues. This suggestion was explored and confirmed in the present paper. Radioactive IgM (secreted) and IgMs (intracellular) were prepared by sucrose-density-gradient centrifugation after incubation of cells of the IgM-producing mouse myeloma MOPC 104E with [3H]leucine. We investigated the susceptibility to reduction of fully assembled mouse IgM and its reconstitution from subunits by analysis by polyacrylamide-gel electrophoresis under dissociating conditions. With increasing concentrations of dithioerythritol, interchain disulphide bonds were cleaved in the following order: inter-IgMs subunit, intra-IgMs subunit H-H, intra-IgMs subunit H-L. Removal of the reducing agent from IgM-reduction mixtures by filtration through Sephadex G-25 caused partial reconstitution of IgM at low protein concentrations (5–100μg/ml) and total reconstitution at higher protein concentrations (300μg/ml or more). Isolated radioactive intracellular IgMs showed no tendency to polymerize unless first treated with a reducing agent; under optimum conditions removal of the reducing agent caused 70% of the subunits to be assembled into IgM. Similar assembly occurred when IgMs was isolated from cells that had been lysed in the presence of an irreversible alkylating reagent (iodoacetamide). The intracellular IgMs cysteine residues responsible for inter-IgMs linkage therefore appear to be reversibly blocked within the cells. Assembly into IgM is thus controlled by removal of this block during secretion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale D., Feinstein A. Studies on the reduction of a human 19S immunoglobulin M. Biochem J. 1969 Apr;112(2):187–194. doi: 10.1042/bj1120187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Preparation of mammalian polyribosomes with the detergent Nonidet P-40. Biochim Biophys Acta. 1967 Nov 21;149(1):302–304. doi: 10.1016/0005-2787(67)90715-0. [DOI] [PubMed] [Google Scholar]
- Brownstone A. D. A versatile system for preparative electrophoresis in acrylamide gel. Anal Biochem. 1969 Jan;27(1):25–46. doi: 10.1016/0003-2697(69)90216-4. [DOI] [PubMed] [Google Scholar]
- FERDINAND W., STEIN W. H., MOORE S. AN UNUSUAL DISULFIDE BOND IN STREPTOCOCCAL PROTEINASE. J Biol Chem. 1965 Mar;240:1150–1155. [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- McIntimif K. R., Asofsky R. M., Potter M., Kuff E. L. Macroglobulin-Producing Plasma-Cell Tumor in Mice: Identification of a New Light Chain. Science. 1965 Oct 15;150(3694):361–363. doi: 10.1126/science.150.3694.361. [DOI] [PubMed] [Google Scholar]
- Metzger H. Structure and function of gamma M macroglobulins. Adv Immunol. 1970;12:57–116. doi: 10.1016/s0065-2776(08)60168-6. [DOI] [PubMed] [Google Scholar]
- Miller F., Metzger H. Characterization of a human macroglobulin. II. Distribution of the disulfide bonds. J Biol Chem. 1965 Dec;240(12):4740–4745. [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M. Immunoglobulin M biosynthesis. Production of intermediates and excess of light-chain in mouse myeloma MOPC 104E. Biochem J. 1971 Jul;123(4):635–641. doi: 10.1042/bj1230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR J. F., ANTONINI E., WYMAN J. THE OXYGEN EQUILIBRIUM OF CYSTINE-TREATED HUMAN HEMOGLOBIN WITHOUT FREE SULFHYDRYL GROUPS. J Biol Chem. 1963 Aug;238:2660–2662. [PubMed] [Google Scholar]



