Abstract
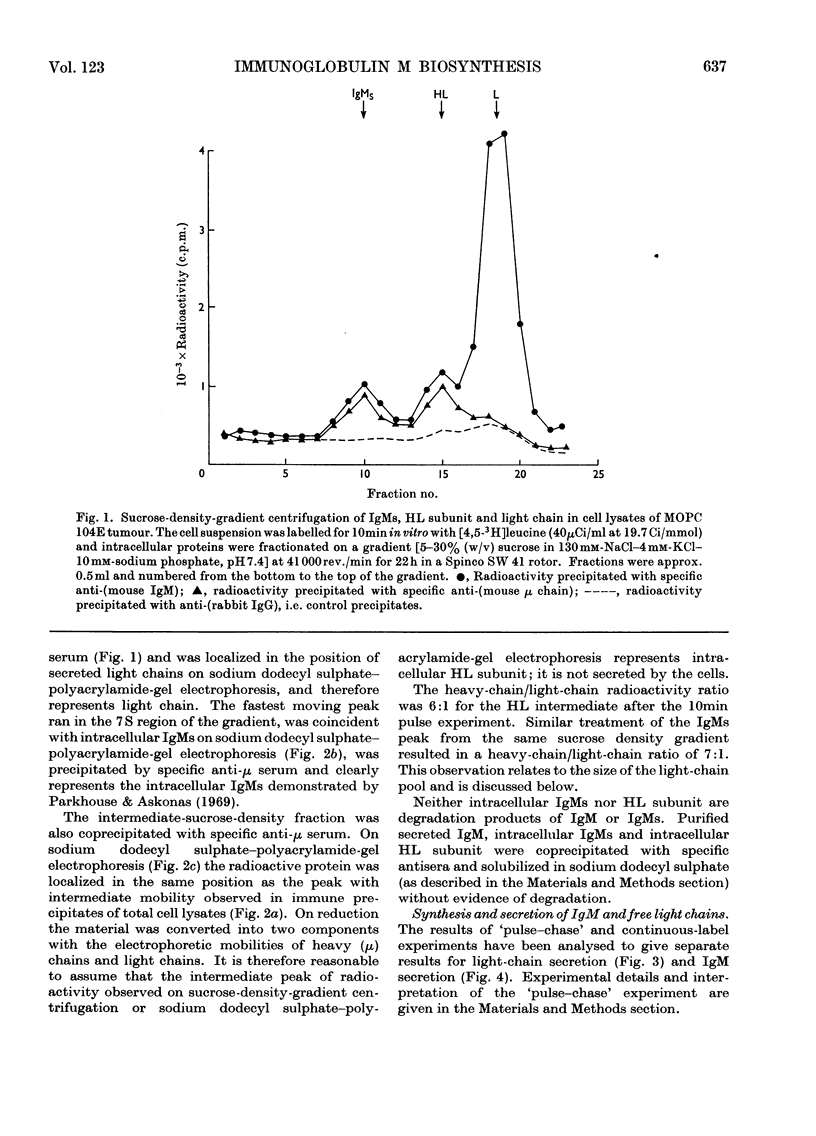
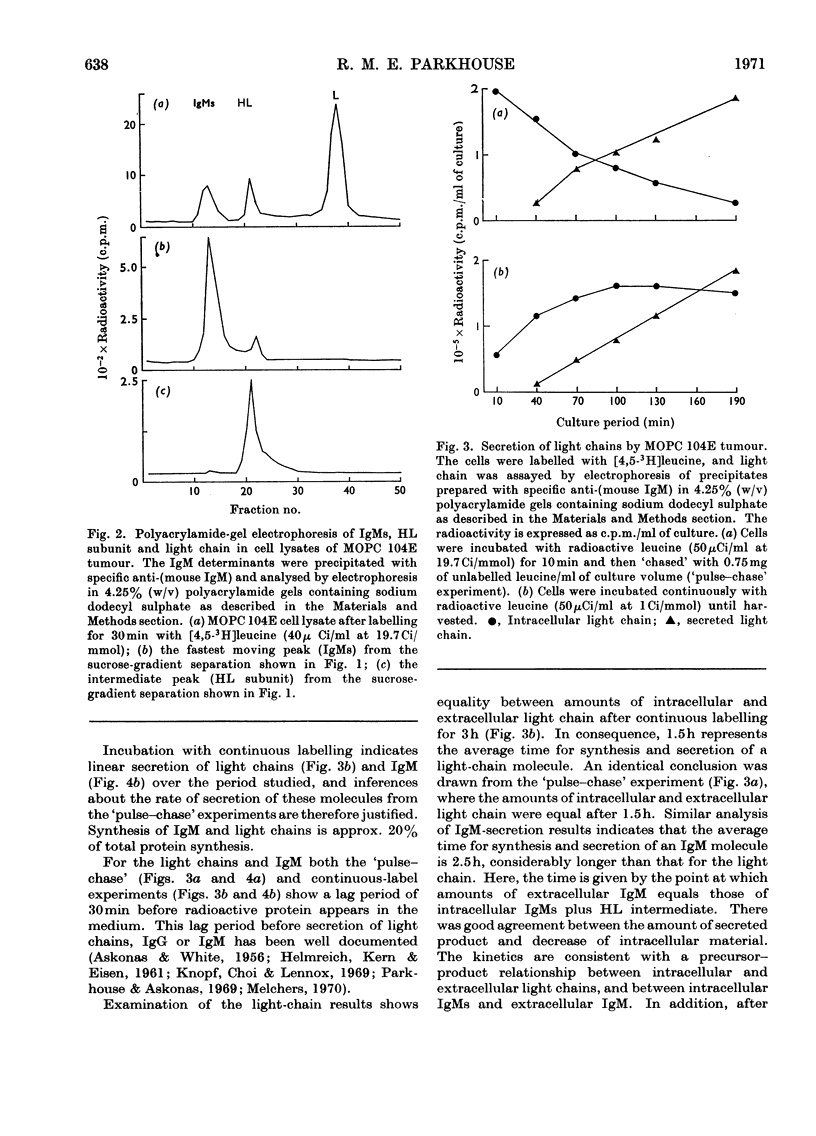
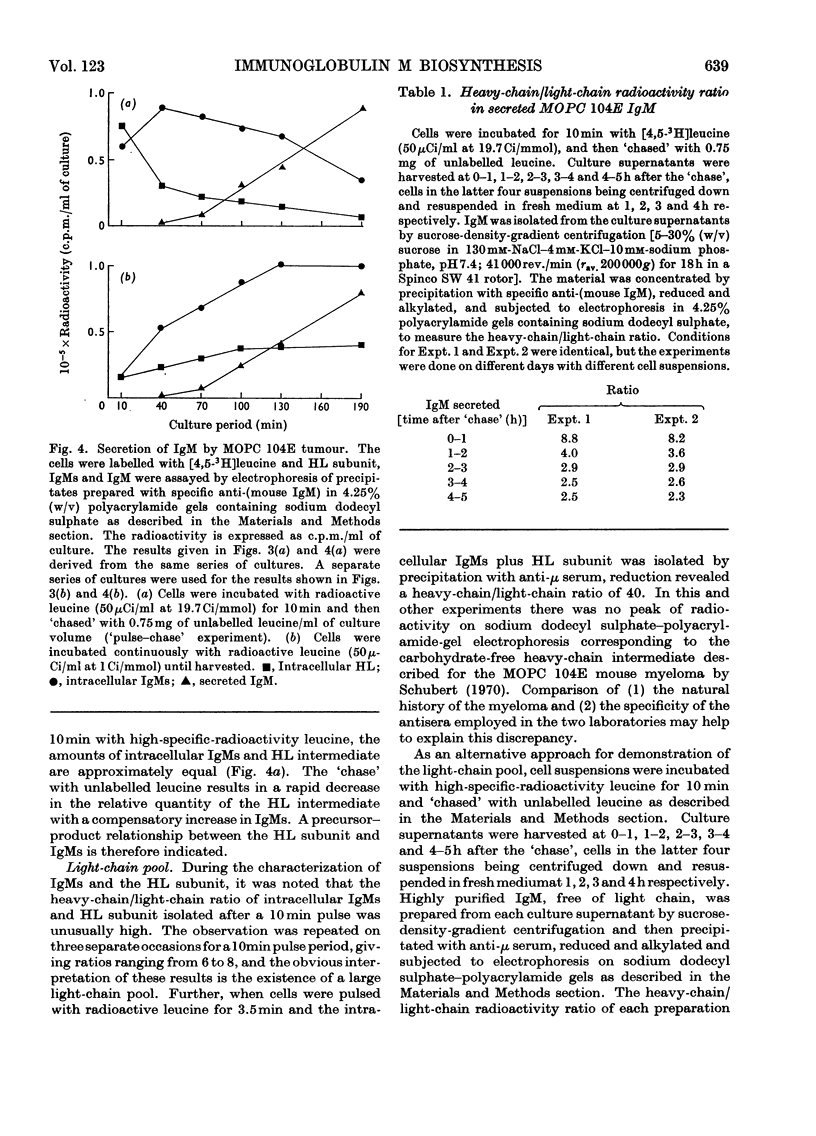
Immunoglobulin M (IgM) biosynthesis was studied with mouse plasma-cell tumour MOPC 104E as a model system. Cell suspensions prepared from solid tumours were incubated in vitro with [3H]leucine; the radioactivity incorporated into intracellular and secreted proteins was analysed by sucrose-density-gradient centrifugation and polyacrylamide-gel electrophoresis. The tumour secretes IgM and light chains. `Pulse–chase' experiments indicated average secretion times of 1.5h for light chain and 2.5h for IgM. The order of disulphide-bond assembly within the cell was shown to be heavy chain+light chain → heavy chain–light chain intermediate → IgMs. The 7S subunit (IgMs) was polymerized into IgM just before or at the time of secretion. Measurements of heavy-chain/light-chain radioactivity ratios in intracellular HL and IgMs and secreted IgM demonstrated the existence of a light-chain pool participating in IgM biosynthesis. The size of the light-chain pool, together with analysis of clones isolated in vivo, suggested that the tumour contains cells in which light-chain synthesis is in excess of heavy-chain production.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASKONAS B. A., WHITE R. G. Sites of antibody production in the guinea-pig; the relation between in vitro synthesis of anti-ovalbumin and gamma-globulin and distribution of antibody-containing plasma cells. Br J Exp Pathol. 1956 Feb;37(1):61–74. [PMC free article] [PubMed] [Google Scholar]
- Abel C. A., Grey H. M. Studies on the structure of mouse gamma-A myeloma proteins. Biochemistry. 1968 Jul;7(7):2682–2688. doi: 10.1021/bi00847a035. [DOI] [PubMed] [Google Scholar]
- Askonas B. A., Parkhouse R. M. Assembly of immunoglobulin M. Blocked thiol groups of intracellular 7S subunits. Biochem J. 1971 Jul;123(4):629–634. doi: 10.1042/bj1230629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askonas B. A., Williamson A. R. Interchain disulphide-bond formation in the assembly of immunoglobulin G. Heavy-chain dimer as an intermediate. Biochem J. 1968 Oct;109(4):637–643. doi: 10.1042/bj1090637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman J. B. Synthesis of the gamma-G heavy chain in rabbit lymph node cells. Biochemistry. 1967 May;6(5):1311–1320. doi: 10.1021/bi00857a013. [DOI] [PubMed] [Google Scholar]
- HELMREICH E., KERN M., EISEN H. N. The secretion of antibody by isolated lymph node cells. J Biol Chem. 1961 Feb;236:464–473. [PubMed] [Google Scholar]
- Laskov R., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. I. Adaptation of the Merwin plasma cell tumor-11 to culture, cloning, and characterization of gamma globulin subunits. J Exp Med. 1970 Mar 1;131(3):515–541. doi: 10.1084/jem.131.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr, White D. O., Scharff M. D. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968 Sep;36(1):115–125. doi: 10.1016/0042-6822(68)90121-9. [DOI] [PubMed] [Google Scholar]
- Melchers F. Biosynthesis of the carbohydrate portion of immunoglobulins. Kinetics of synthesis and secretion of [3H] leucine-, [3H] galactose- and [3H] mannose-labelled myeloma protein by two plasma-cell tumours. Biochem J. 1970 Oct;119(4):765–772. doi: 10.1042/bj1190765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba Y., Hanaoka M. Immunoglobulin synthesis by cultured mouse myeloma cells. J Immunol. 1969 Jun;102(6):1486–1497. [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A., Dourmashkin R. R. Electron microscopic studies of mouse immunoglobulin M; structure and reconstitution following reduction. Immunology. 1970 Apr;18(4):575–584. [PMC free article] [PubMed] [Google Scholar]
- Parkhouse R. M., Askonas B. A. Immunoglobulin M biosynthesis. Intracellular accumulation of 7S subunits. Biochem J. 1969 Nov;115(2):163–169. doi: 10.1042/bj1150163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Cohn M. Immunoglobulin biosynthesis. 3. Blocks in defective synthesis. J Mol Biol. 1968 Dec;38(3):273–288. doi: 10.1016/0022-2836(68)90386-0. [DOI] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin assembly in a mouse myeloma. Proc Natl Acad Sci U S A. 1968 Jun;60(2):683–690. doi: 10.1073/pnas.60.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Scharff M. D., Maizel J. V., Jr, Uhr J. W. Polyribosomal synthesis and assembly of the H and L chains of gamma globulin. Proc Natl Acad Sci U S A. 1966 Jul;56(1):216–221. doi: 10.1073/pnas.56.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland E. W., 3rd, Zimmerman D. H., Kern M. Synthesis and secretion of gamma-globulin by lymph node cells, VIII. Order of synthesis of the interchain disulfide linkages of immunoglobulins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):987–994. doi: 10.1073/pnas.66.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]



