Abstract
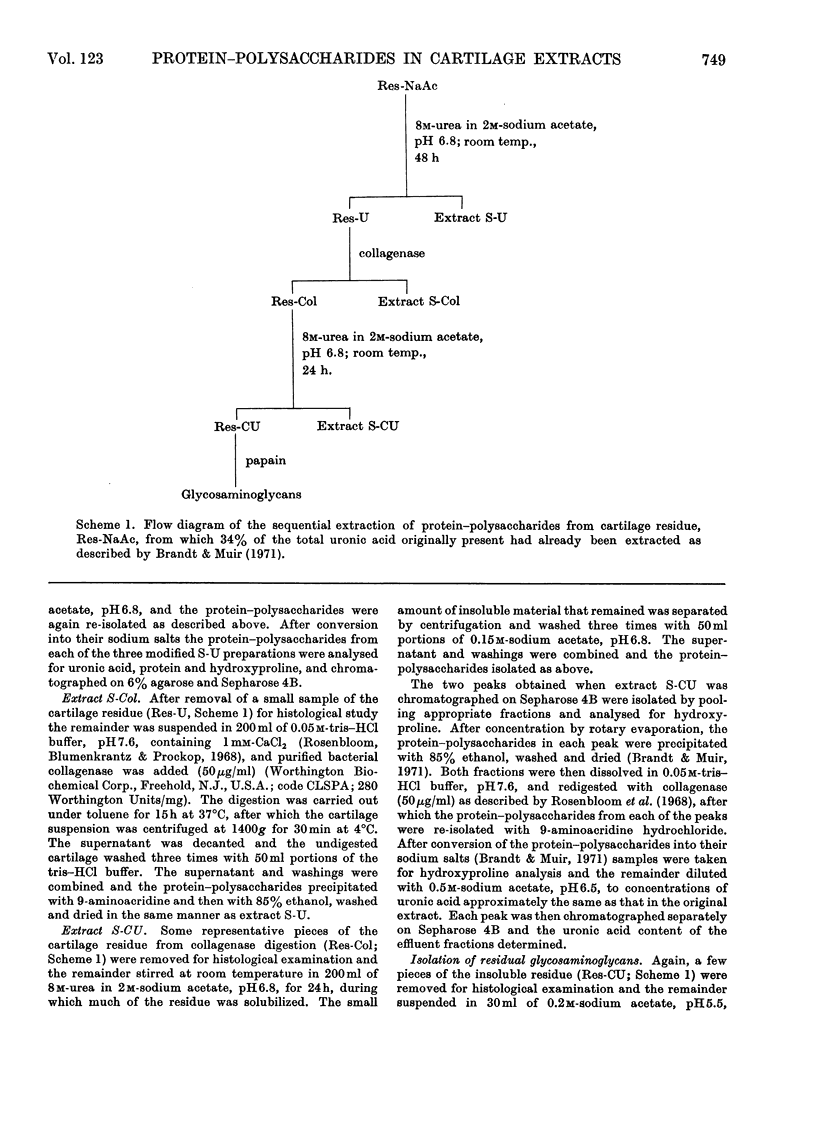
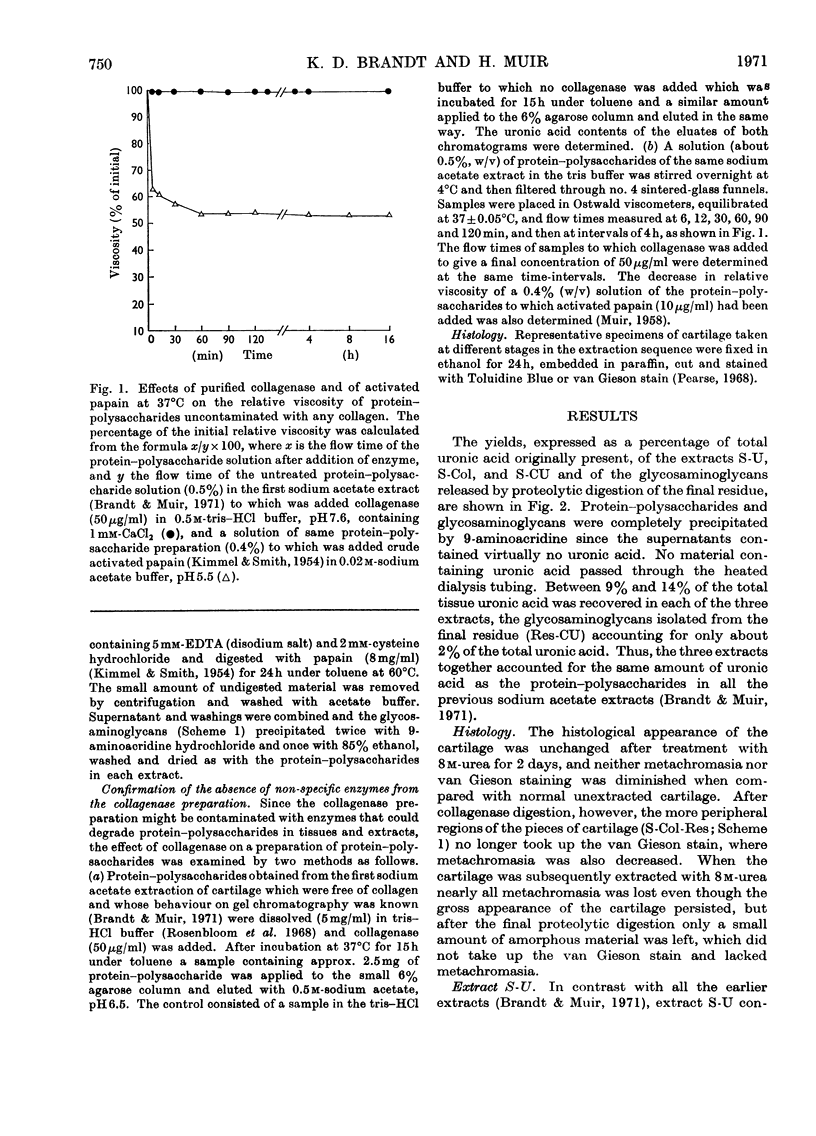
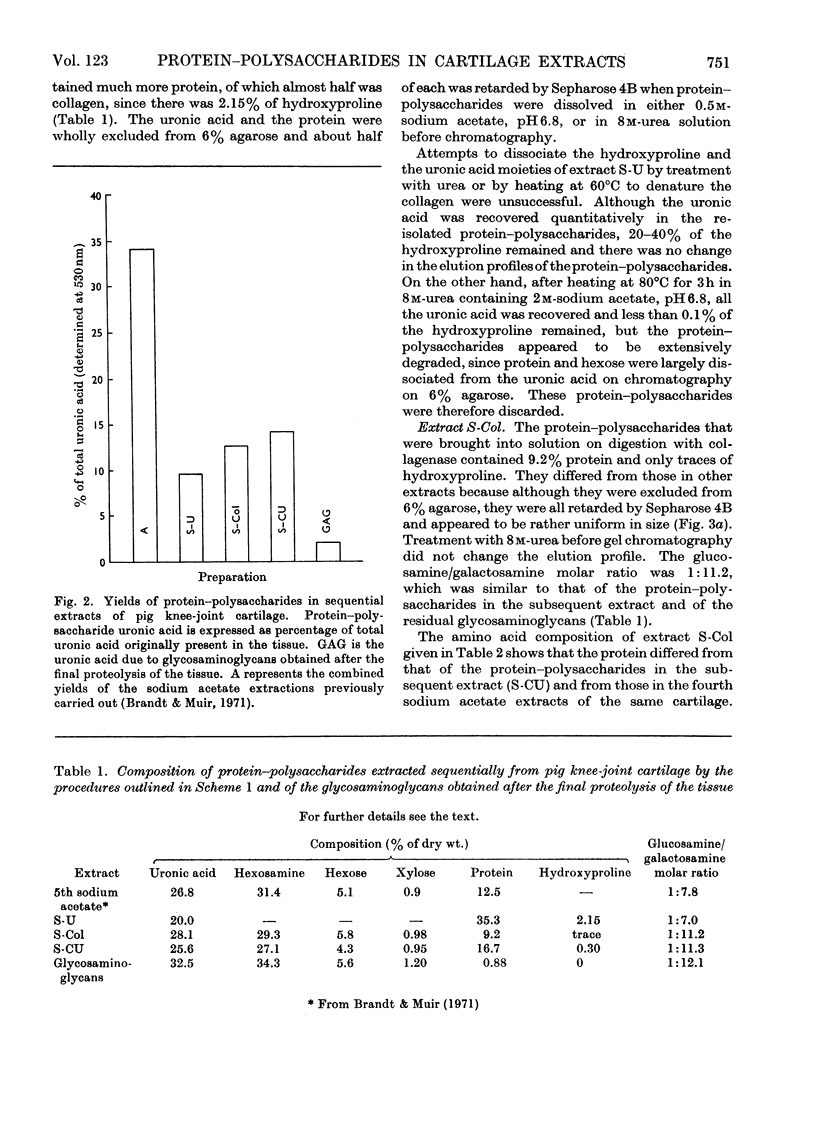
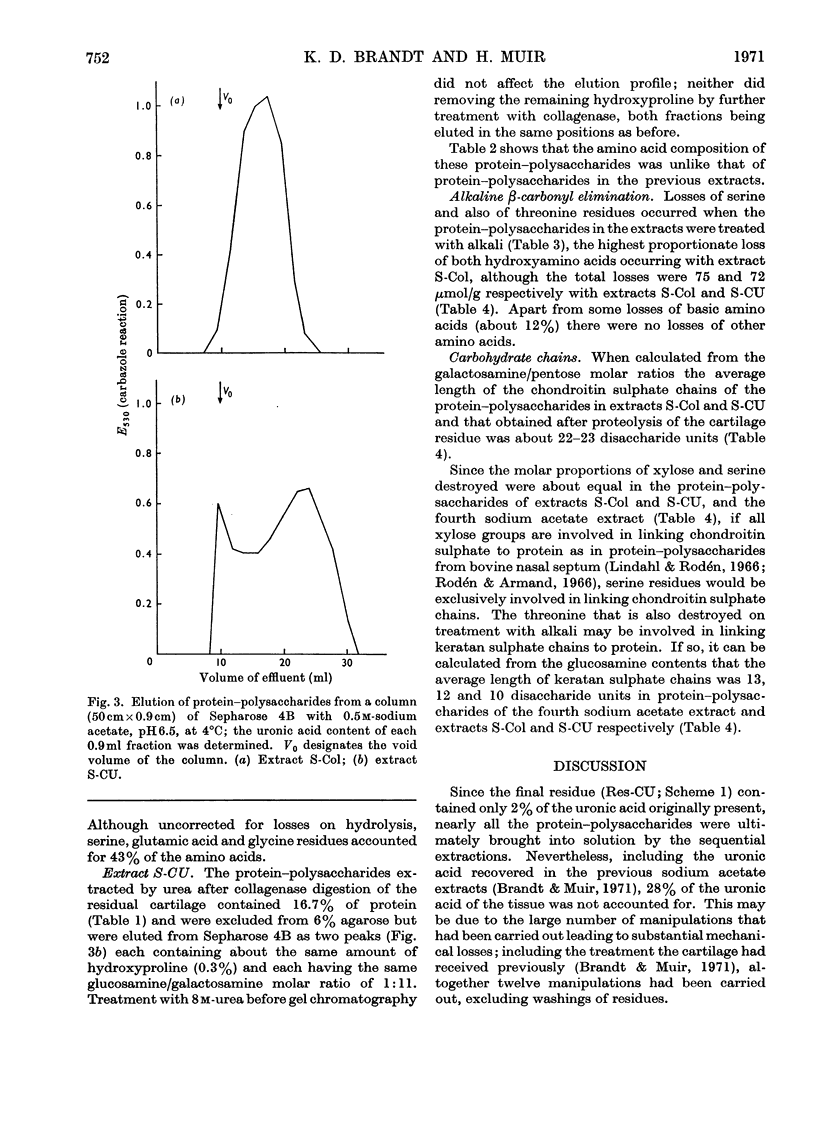
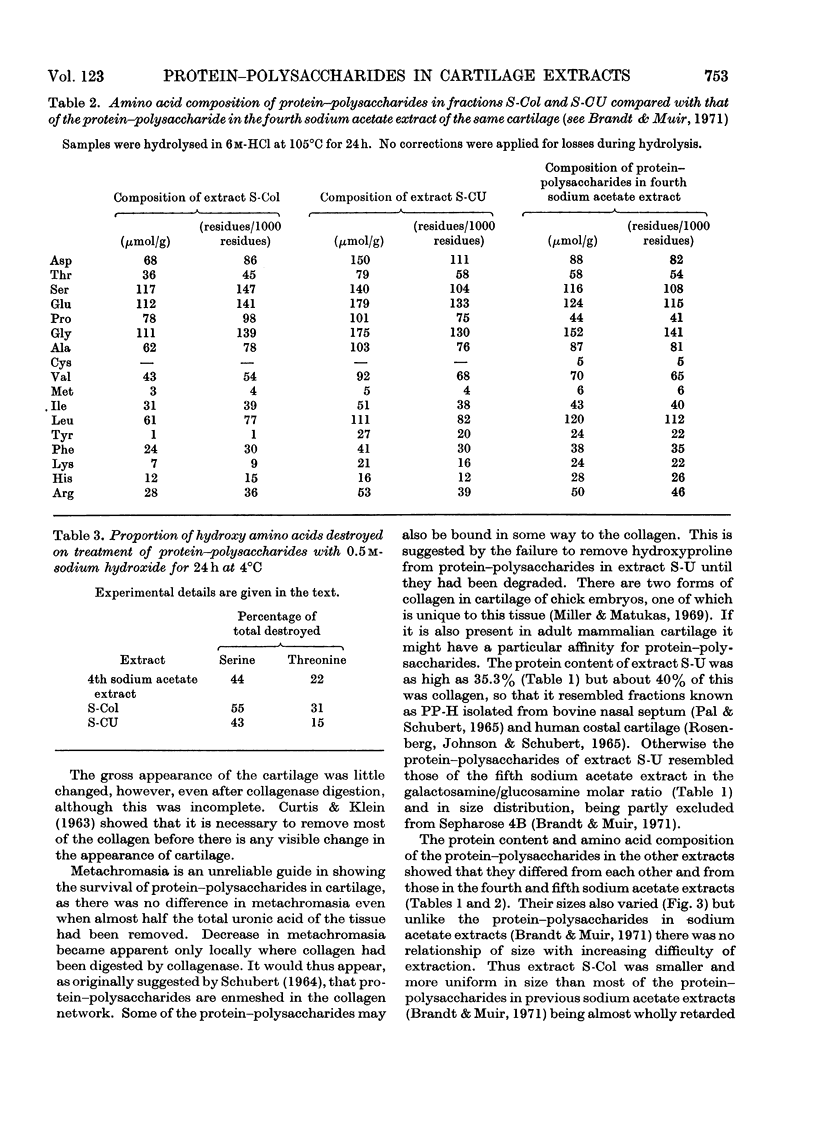
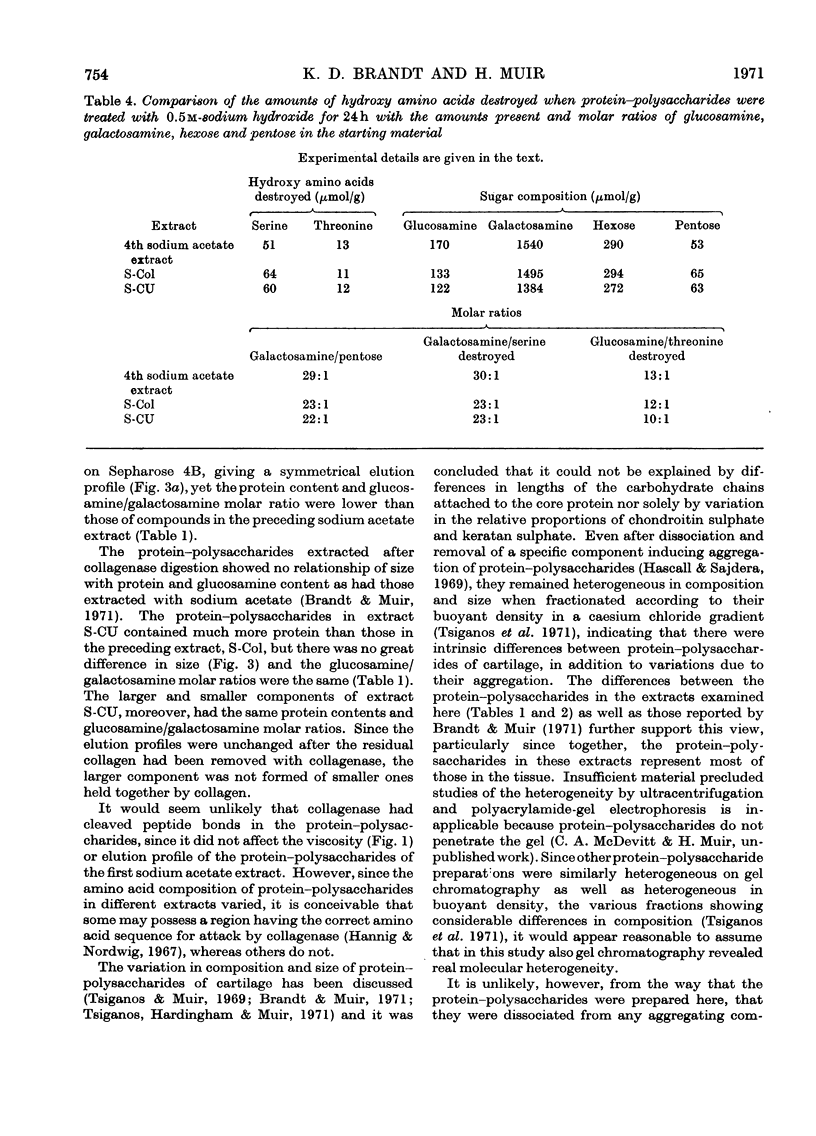
Pig articular cartilage, from which protein–polysaccharides soluble in iso-osmotic sodium acetate had been removed, was extracted in three further stages with 8m-urea in 2m-sodium acetate and with tris–HCl buffer after bacterial collagenase digestion, followed by the same urea–sodium acetate solution, thus leaving only 2% of the original uronic acid in the tissue. The histological appearance of the cartilage was unaltered until after collagenase digestion. The collagenase used did not affect the viscosity or molecular size of a protein–polysaccharide preparation obtained previously. The protein–polysaccharides in each extract differed in size, amino acid composition and protein content, but protein and keratan sulphate contents were not related to hydrodynamic size, in contrast with protein–polysaccharides extracted previously before collagenase digestion. Hydroxyproline could not be removed from those obtained by the first urea–sodium acetate extraction until degraded by heat. The galactosamine/pentose molar ratio agreed closely with the galactosamine/serine molar ratio that was destroyed on treatment with 0.5m-sodium hydroxide, showing that chondroitin sulphate was attached only to serine residues. From these molar ratios the chondroitin sulphate chains were calculated to be of the same average length in protein–polysaccharides in all three extracts although somewhat shorter than in protein–polysaccharides extracted previously. Some threonine residues were also destroyed on alkali treatment suggesting that keratan sulphate may be attached to threonine. These findings together with previous results show that differences in size, composition and physical state extend to all the protein–polysaccharides in cartilage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Meyer K. Studies on keratosulfates. Methylation, desulfation, and acid hydrolysis studies on old human rib cartilage keratosulfate. J Biol Chem. 1968 Mar 10;243(5):1052–1059. [PubMed] [Google Scholar]
- Brandt K. D., Muir H. Heterogeneity of protein-polysaccharides of porcine articular cartilage. The sequential extraction of chondroitin sulphate-proteins with iso-osmotic neutral sodium acetate. Biochem J. 1971 Jan;121(2):261–270. doi: 10.1042/bj1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLANAN M. J., CARROLL W. R., MITCHELL E. R. Physical and chemical properties of protamine from the sperm of salmon (Oncorhynchus tschawytscha). J Biol Chem. 1957 Nov;229(1):279–287. [PubMed] [Google Scholar]
- CESSI C., PILIEGO F. The determination of amino sugars in the presence of amino acids and glucose. Biochem J. 1960 Dec;77:508–510. doi: 10.1042/bj0770508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M. F., Muir H. The characterization of a protein-polysaccharide isolated from Kurloff cells of the guinea pig. Biochem J. 1970 Aug;118(5):783–790. doi: 10.1042/bj1180783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kivirikko K. I., Laitinen O., Prockop D. J. Modifications of a specific assay for hydroxyproline in urine. Anal Biochem. 1967 May;19(2):249–255. doi: 10.1016/0003-2697(67)90160-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindahl U., Rodén L. The chondroitin 4-sulfate-protein linkage. J Biol Chem. 1966 May 10;241(9):2113–2119. [PubMed] [Google Scholar]
- MUIR H. The nature of the link between protein and carbohydrate of a chondroitin sulphate complex from hyaline cartilage. Biochem J. 1958 Jun;69(2):195–204. doi: 10.1042/bj0690195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Matukas V. J. Chick cartilage collagen: a new type of alpha 1 chain not present in bone or skin of the species. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1264–1268. doi: 10.1073/pnas.64.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- PAL S., SCHUBERT M. THE ACTION OF HYDROXYLAMINE ON THE PROTEINPOLYSACCHARIDES OF CARTILAGE. J Biol Chem. 1965 Aug;240:3245–3248. [PubMed] [Google Scholar]
- Rosenberg L., Johnson B., Schubert M. Proteinpolysaccharides from human articular and costal cartilage. J Clin Invest. 1965 Oct;44(10):1647–1656. doi: 10.1172/JCI105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom J., Blumenkrantz N., Prockop D. J. Sequential hydroxylation of lysine and glycosylation of hydroxylysine during the biosynthesis of collagen in isolated cartilage. Biochem Biophys Res Commun. 1968 Jun 10;31(5):792–797. doi: 10.1016/0006-291x(68)90632-3. [DOI] [PubMed] [Google Scholar]
- SCHUBERT M. INTERCELLULAR MACROMOLECULES CONTAINING POLYSACCHARIDES. Biophys J. 1964 Jan;4:SUPPL119–SUPPL138. doi: 10.1016/s0006-3495(64)86933-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Estimation of pentoses and methylpentoses in biopolymers, in particular of fucose and xylose. Anal Biochem. 1966 Dec;17(3):495–501. doi: 10.1016/0003-2697(66)90184-9. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Muir H. Studies on protein-polysaccharides from pig laryngeal cartilage. Heterogeneity, fractionation and characterization. Biochem J. 1969 Aug;113(5):885–894. doi: 10.1042/bj1130885. [DOI] [PMC free article] [PubMed] [Google Scholar]


