Abstract
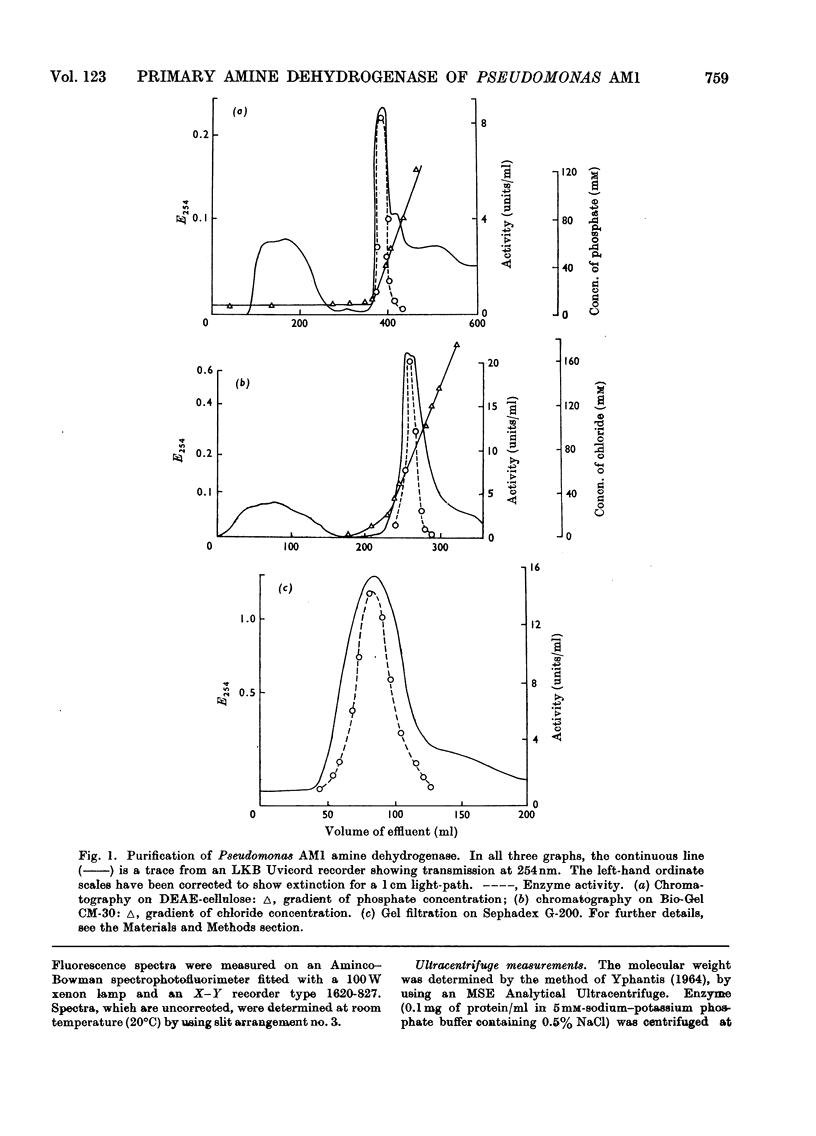
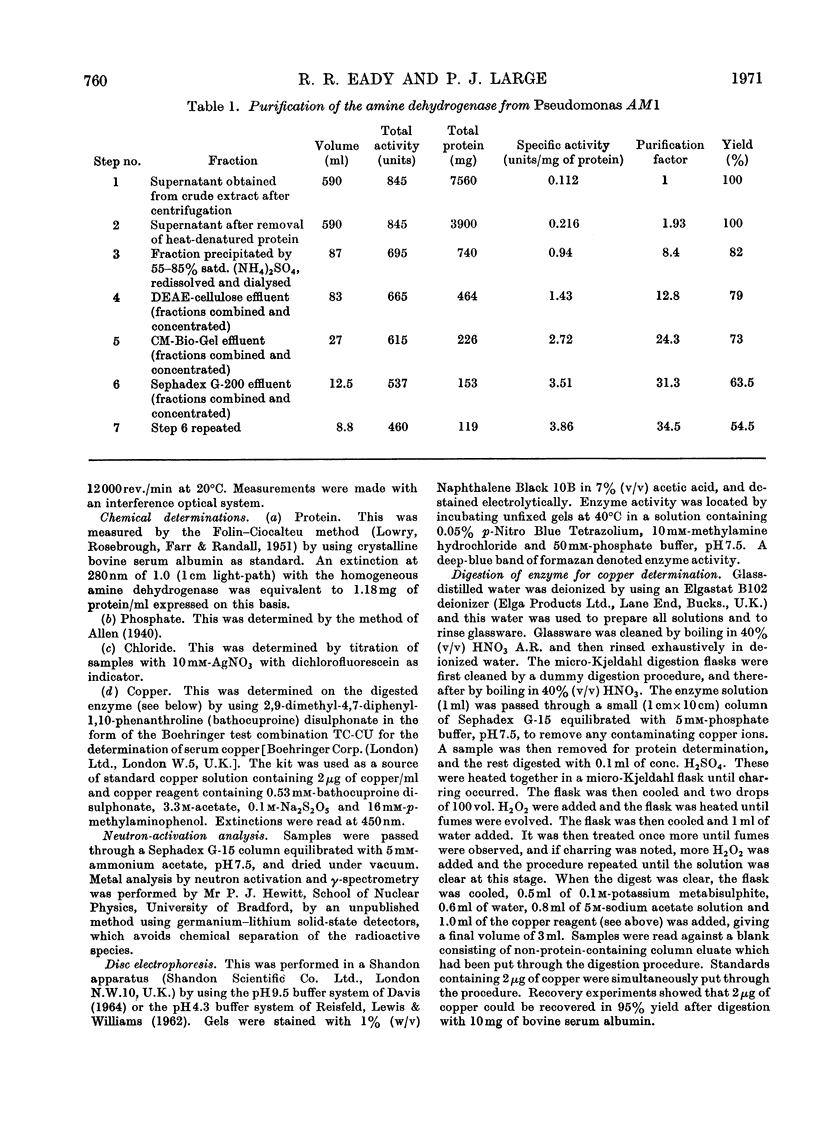
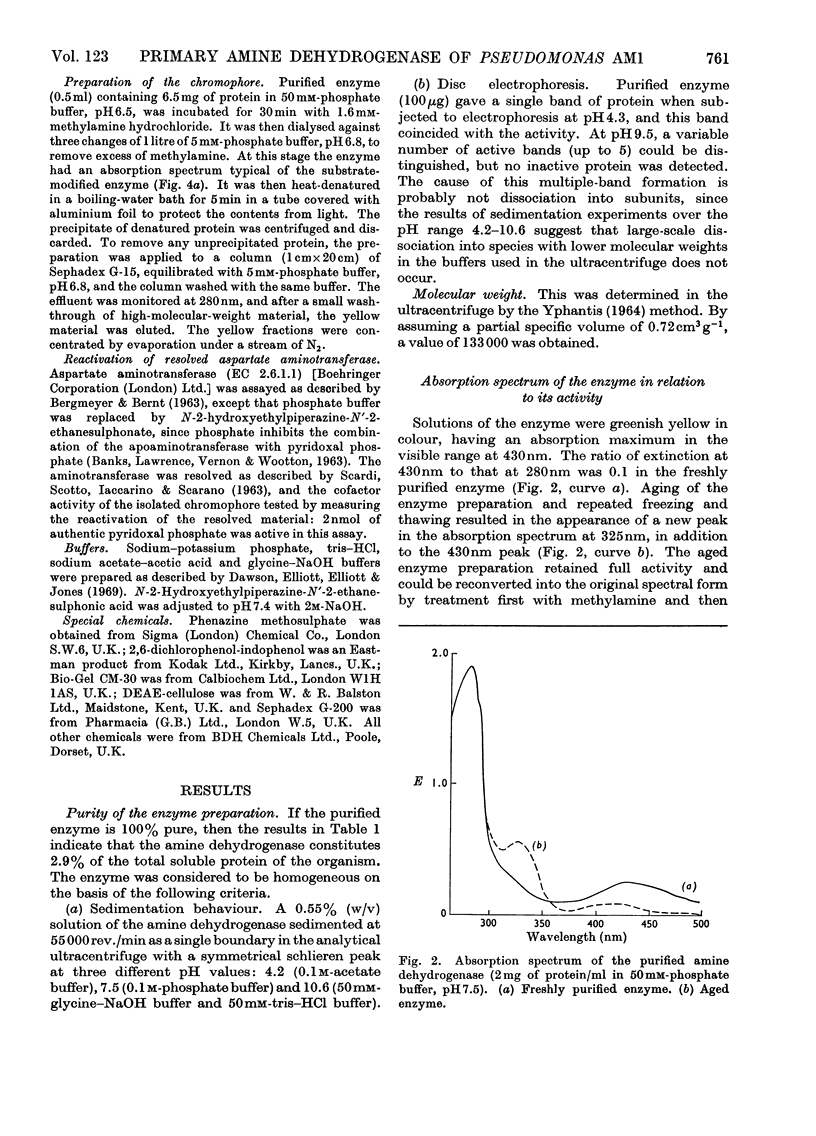
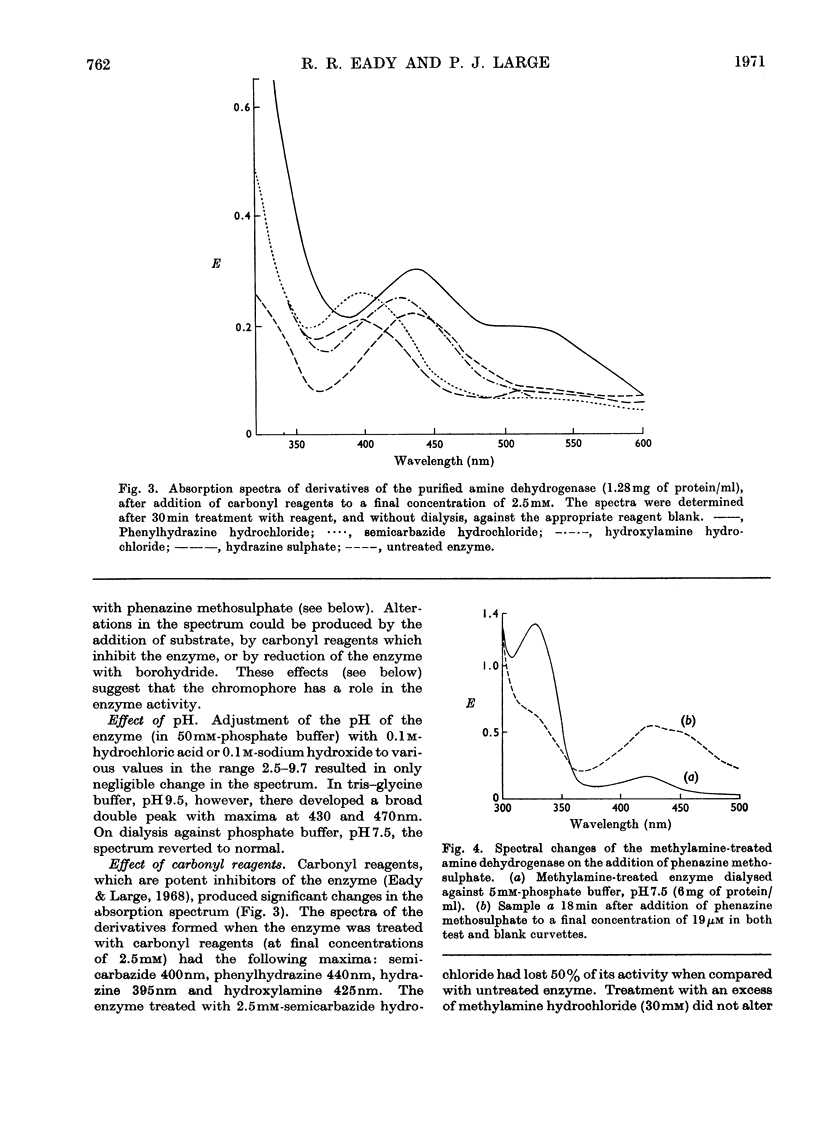
1. An improved procedure is reported for purification of the amine dehydrogenase from methylamine-grown Pseudomonas AM1 which yielded a product homogeneous by sedimentation and disc-electrophoretic analysis, with molecular weight of 133000. 2. The purified enzyme had absorption maxima at 280 and 430nm. On aging, a third peak appeared at 325nm, and the 430nm peak decreased in intensity. This spectrum was independent of pH. 3. Addition of 2.5mm-semicarbazide, phenylhydrazine, hydrazine or hydroxylamine produced modified spectra with maxima respectively at 400, 440, 395 and 425nm. 4. Aerobic addition of methylamine resulted in a bleaching of the 430nm peak and the appearance of a new one at 325nm. This spectral change was retained after removal of the methylamine by dialysis. The original spectrum could be restored on addition of phenazine methosulphate. 5. Addition of borohydride partially inactivated the enzyme and produced spectral changes similar to those observed with methylamine. Pre-treatment with methylamine prevented the inactivation by borohydride. The degree of inactivation could be increased by alternate phenazine methosulphate and borohydride treatments. 6. The addition of methylamine or borohydride each caused shifts in the fluorescence emission maximum from 348 to 380nm. 7. Lineweaver–Burk plots of reciprocal activity against reciprocal concentration of either of the substrates n-butylamine or phenazine methosulphate were consistent with a mechanism that involves interconversion of two free forms of the enzyme by the two substrates. 8. The enzyme, although spectrally modified, was not inactivated by dialysis against diethyldithiocarbamate, and contained about 0.27 g-atom of copper/mol, with small traces of cobalt, iron and zinc. 9. Conventional methods of resolution did not release the prosthetic group. Heat denaturation after treatment of the enzyme with methylamine liberated a yellow chromophore which did not reactivate resolved aspartate aminotransferase, and whose spectral, electrophoretic and fluorescence properties did not agree with any recognizable pyridoxal derivatives. 10. Despite the inconclusive results with the isolated chromophore, the observations on the enzyme suggest that it may contain a pyridoxal derivative bound as a Schiff's base which is converted into the pyridoxamine form on aerobic treatment with methylamine and reconverted into the pyridoxal form with phenazine methosulphate. 11. The copper detected is probably not involved in the enzyme mechanism, since most copper-chelating agents are not inhibitory, and since the enzyme does not react with oxygen.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
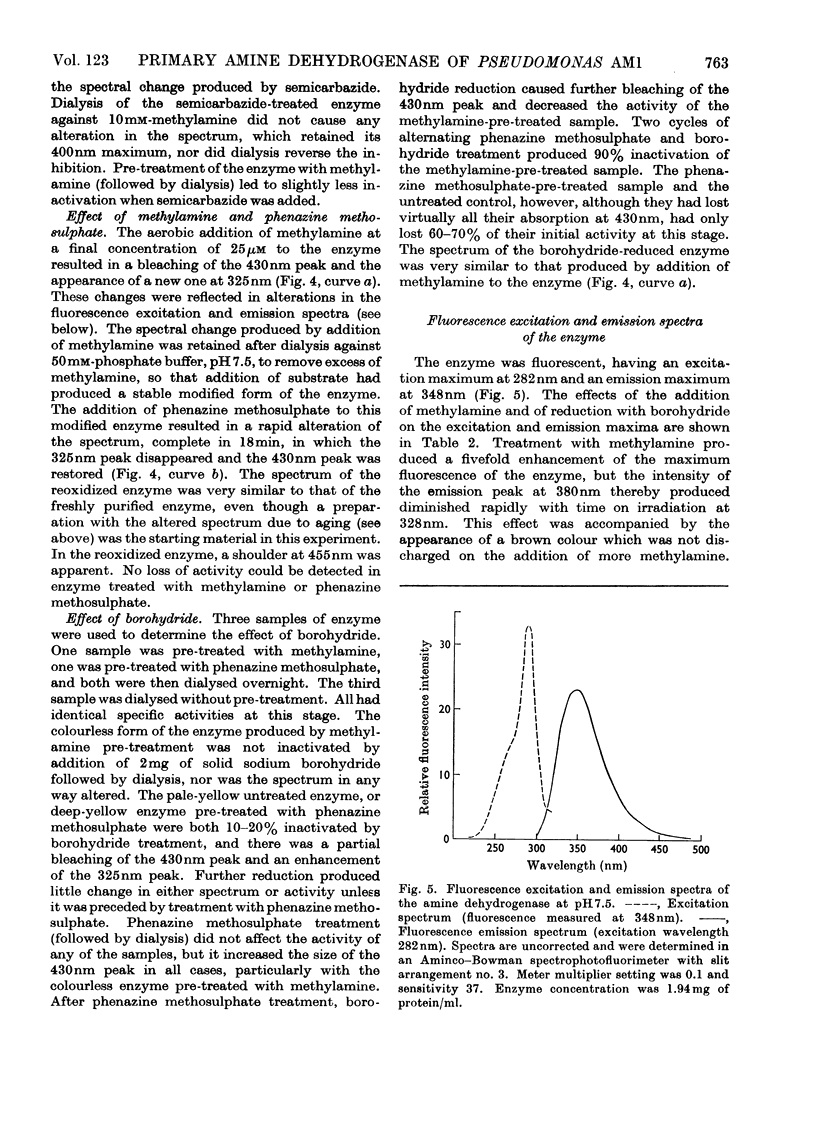
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
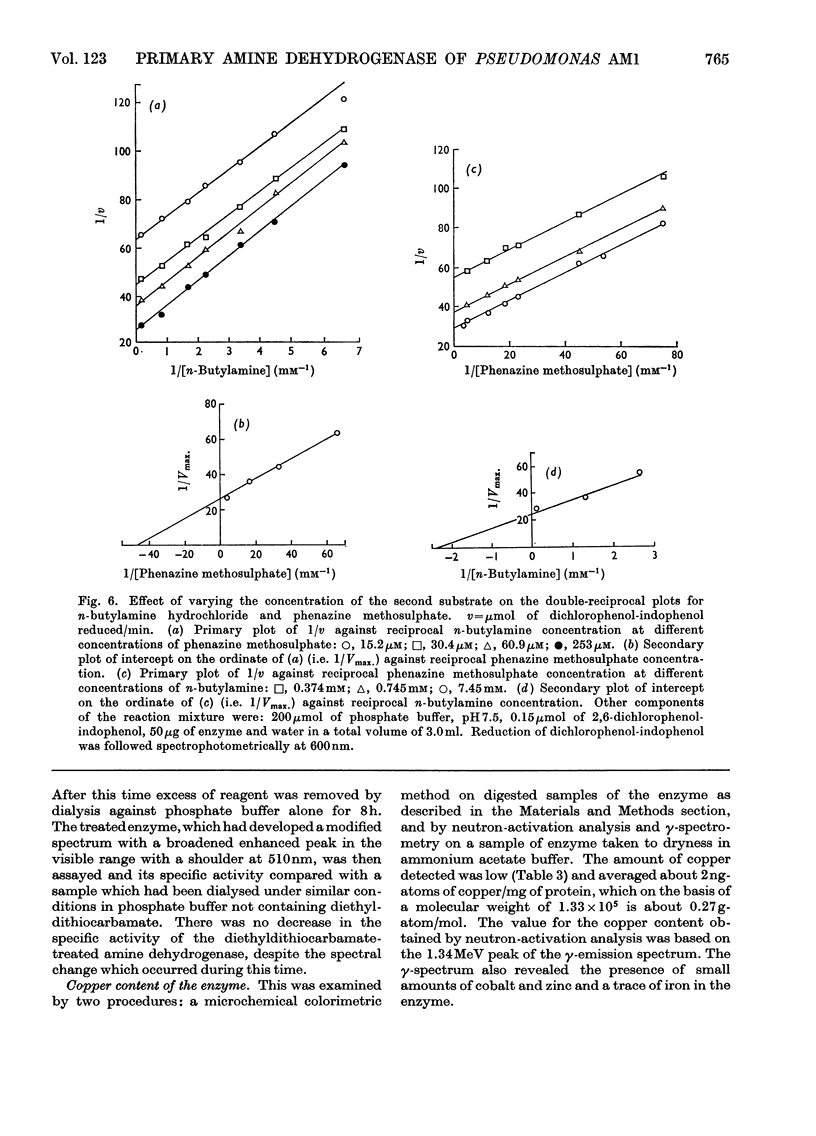
- Anthony C. Cytochrome c and the oxidation of C1 compounds in Pseudomonas AM1. Biochem J. 1970 Oct;119(5):54P–55P. [PMC free article] [PubMed] [Google Scholar]
- BUFFONI F., BLASCHKO H. BENZYLAMINE OXIDASE AND HISTAMINASE: PURIFICATION AND CRYSTALLIZATION OF AN ENZYME FROM PIG PLASMA. Proc R Soc Lond B Biol Sci. 1964 Dec 15;161:153–167. doi: 10.1098/rspb.1964.0086. [DOI] [PubMed] [Google Scholar]
- Blethen S. L., Boeker E. A., Snell E. E. Argenine decarboxylase from Escherichia coli. I. Purification and specificity for substrates and coenzyme. J Biol Chem. 1968 Apr 25;243(8):1671–1677. [PubMed] [Google Scholar]
- Bridges J. W., Davies D. S., Williams R. T. Fluorescence studies on some hydroxypyridines including compounds of the vitamin B6 group. Biochem J. 1966 Feb;98(2):451–468. doi: 10.1042/bj0980451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F., Corte L. D., Knowles P. F. The nature of copper in pig plasma benzylamine oxidase. Biochem J. 1968 Jan;106(2):575–576. doi: 10.1042/bj1060575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffoni F. Histaminase and related amine oxidases. Pharmacol Rev. 1966 Dec;18(4):1163–1199. [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- Churchich J. E., Harpring L. The effect of 8 M urea on fluorescence and activity properties of aspartate aminotransferase. Biochim Biophys Acta. 1965 Sep 20;105(3):575–582. doi: 10.1016/s0926-6593(65)80240-5. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Large P. J. Purification and properties of an amine dehydrogenase from Pseudomonas AM1 and its role in growth on methylamine. Biochem J. 1968 Jan;106(1):245–255. doi: 10.1042/bj1060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin V. G., Hellerman L. Mitochondrial monoamine oxidase. I. Purification and characterization of the bovine kidney enzyme. J Biol Chem. 1967 Sep 25;242(18):4230–4238. [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- HILL J. M., MANN P. J. The inhibition of pea-seedling diamine oxidase by chelating agents. Biochem J. 1962 Oct;85:198–207. doi: 10.1042/bj0850198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton G. A. Mechanisms of two- and four-electron oxidations catalyzed by some metalloenzymes. Adv Enzymol Relat Areas Mol Biol. 1969;32:55–96. doi: 10.1002/9780470122778.ch3. [DOI] [PubMed] [Google Scholar]
- Hill J. M., Mann P. J. Further properties of the diamine oxidase of pea seedlings. Biochem J. 1964 Apr;91(1):171–182. doi: 10.1042/bj0910171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. M. The inactivation of pea-seedling diamine oxidase by peroxidase and 1,5-diaminopentane. Biochem J. 1967 Sep;104(3):1048–1055. doi: 10.1042/bj1041048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS W. T., SIZER I. W. Glutamic aspartic transaminase. IV. The mechanism of transamination. J Biol Chem. 1960 Mar;235:620–624. [PubMed] [Google Scholar]
- KENT A. B., KREBS E. G., FISCHER E. H. Properties of crystalline phosphorylase b. J Biol Chem. 1958 May;232(1):549–558. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system from Peptococcus glycinophilus. J Biol Chem. 1966 Jan 10;241(1):206–209. [PubMed] [Google Scholar]
- Klein S. M., Sagers R. D. Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase from Peptococcus glycinophilus. J Biol Chem. 1967 Jan 25;242(2):301–305. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Large P. J., Eady R. R., Murden D. J. An enzymic method for the micro estimation of methylamine, ethylamine, and n-propylamine. Anal Biochem. 1969 Dec;32(3):402–407. doi: 10.1016/s0003-2697(69)80007-2. [DOI] [PubMed] [Google Scholar]
- MANN P. J. Further purification and properties of the amine oxidase of pea seedlings. Biochem J. 1961 Jun;79:623–631. doi: 10.1042/bj0790623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUO Y., GREENBERG D. M. A crystalline enzyme that cleaves homoserine and cystathionine. II. Prosthetic group. J Biol Chem. 1958 Feb;230(2):561–571. [PubMed] [Google Scholar]
- Martinez-Carrion M., Jenkins W. T. D-Alanine-D-glutamate transaminase. I. Purification and characterization. J Biol Chem. 1965 Sep;240(9):3538–3546. [PubMed] [Google Scholar]
- Mondovì B., Costa M. T., Agrò A. F., Rotilio G. Pyridoxal phosphate as a prosthetic group of pig kidney diamine oxidase. Arch Biochem Biophys. 1967 Mar;119(1):373–381. doi: 10.1016/0003-9861(67)90468-7. [DOI] [PubMed] [Google Scholar]
- Mondovì B., Rotilio G., Costa M. T., Finazzi-Agrò A., Chiancone E., Hansen R. E., Beinert H. Diamine oxidase from pig kidney. Improved purification and properties. J Biol Chem. 1967 Mar 25;242(6):1160–1167. [PubMed] [Google Scholar]
- Morino Y., Snell E. E. The subunit structure of tryptophanase. I. The effect of pyridoxal phosphate on the subunit structure and physical properties of tryptophanase. J Biol Chem. 1967 Dec 10;242(23):5591–5601. [PubMed] [Google Scholar]
- NISHIMURA J. S., GREENBERG D. M. Purification and properties of L-threonine dehydrase of sheep liver. J Biol Chem. 1961 Oct;236:2684–2691. [PubMed] [Google Scholar]
- Oi S., Inamasu M., Yasunobu K. T. Mechanistic studies of beef plasma amine oxidase. Biochemistry. 1970 Aug 18;9(17):3378–3383. doi: 10.1021/bi00819a013. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- SCARDI V., SCOTTO P., IACCARINO M., SCARANO E. The binding of pyridoxal 5-phosphate to aspartate aminotransferase of pig heart. Biochem J. 1963 Jul;88:172–175. doi: 10.1042/bj0880172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUKUYA R., SCHWERT G. W. Glutamic acid decarboxylase. II. The spectrum of the enzyme. J Biol Chem. 1960 Jun;235:1653–1657. [PubMed] [Google Scholar]
- Shaltiel S., Hedrick J. L., Fischer E. H. On the role of pyridoxal 5'-phosphate in phosphorylase. II. Resolution of rabbit muscle phosphorylase b. Biochemistry. 1966 Jun;5(6):2108–2116. doi: 10.1021/bi00870a044. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. The ultraviolet fluorescence of proteins in neutral solution. Biochem J. 1960 Aug;76:381–388. doi: 10.1042/bj0760381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- WADA H., SNELL E. E. Enzymatic transamination of pyridoxamine. I. With oxaloacetate and alpha-ketoglutarate. J Biol Chem. 1962 Jan;237:127–132. [PubMed] [Google Scholar]
- WEBER G. Enumeration of components in complex systems by fluorescence spectrophotometry. Nature. 1961 Apr 1;190:27–29. doi: 10.1038/190027a0. [DOI] [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. MONOAMINE OXIDASE. IV. NATURE OF THE SECOND PROSTHETIC GROUP OF PLASMA MONOAMINE OXIDASE. J Biol Chem. 1963 Aug;238:2669–2675. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. I. Purification, crystallization, and properties of plasma monoamine oxidase. J Biol Chem. 1962 May;237:1511–1516. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K. T. Monoamine oxidase. II. Copper, one of the prosthetic groups of plasma monoamine oxidase. J Biol Chem. 1962 Oct;237:3077–3082. [PubMed] [Google Scholar]
- YAMADA H., YASUNOBU K., YAMANO T., MASON H. S. Copper in plasma amine oxidase. Nature. 1963 Jun 15;198:1092–1093. doi: 10.1038/1981092a0. [DOI] [PubMed] [Google Scholar]
- Yamada H., Kumagai H., Kawasaki H., Matsui H., Ogata K. Crystallization and properties of diamine oxidase from pig kidney. Biochem Biophys Res Commun. 1967 Dec 15;29(5):723–727. doi: 10.1016/0006-291x(67)90277-x. [DOI] [PubMed] [Google Scholar]
- Yamasaki E. F., Swindell R., Reed D. J. Some aspects of catalysis by the amine oxidase of pea seedlings. Biochemistry. 1970 Mar 3;9(5):1206–1210. doi: 10.1021/bi00807a022. [DOI] [PubMed] [Google Scholar]
- Yasunobu K. T., Igaue I., Gomes B. The purification and properties of beef liver mitochondrial monoamine oxidase. Adv Pharmacol. 1968;6(Pt A):43–59. doi: 10.1016/s1054-3589(08)61155-2. [DOI] [PubMed] [Google Scholar]
- Youdim M. B., Sourkes T. L. Properties of purified, soluble monoamine oxidase. Can J Biochem. 1966 Oct;44(10):1397–1400. doi: 10.1139/o66-158. [DOI] [PubMed] [Google Scholar]


