Abstract
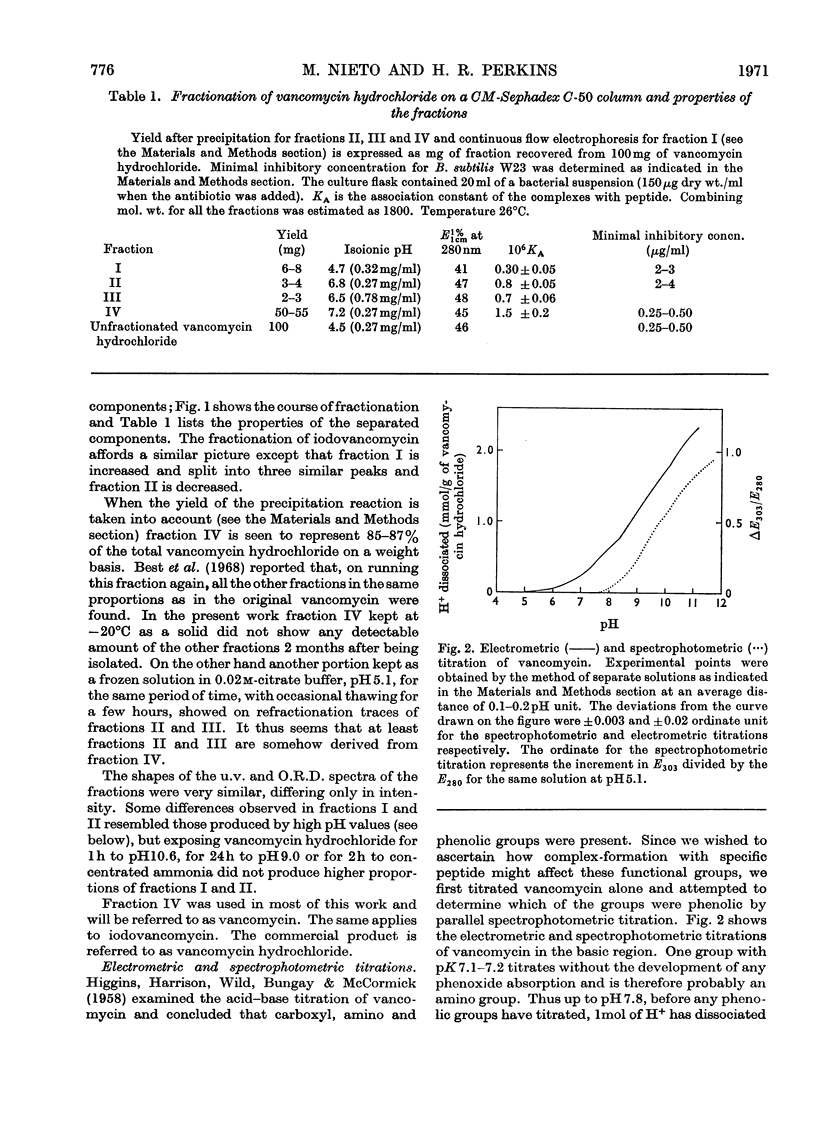
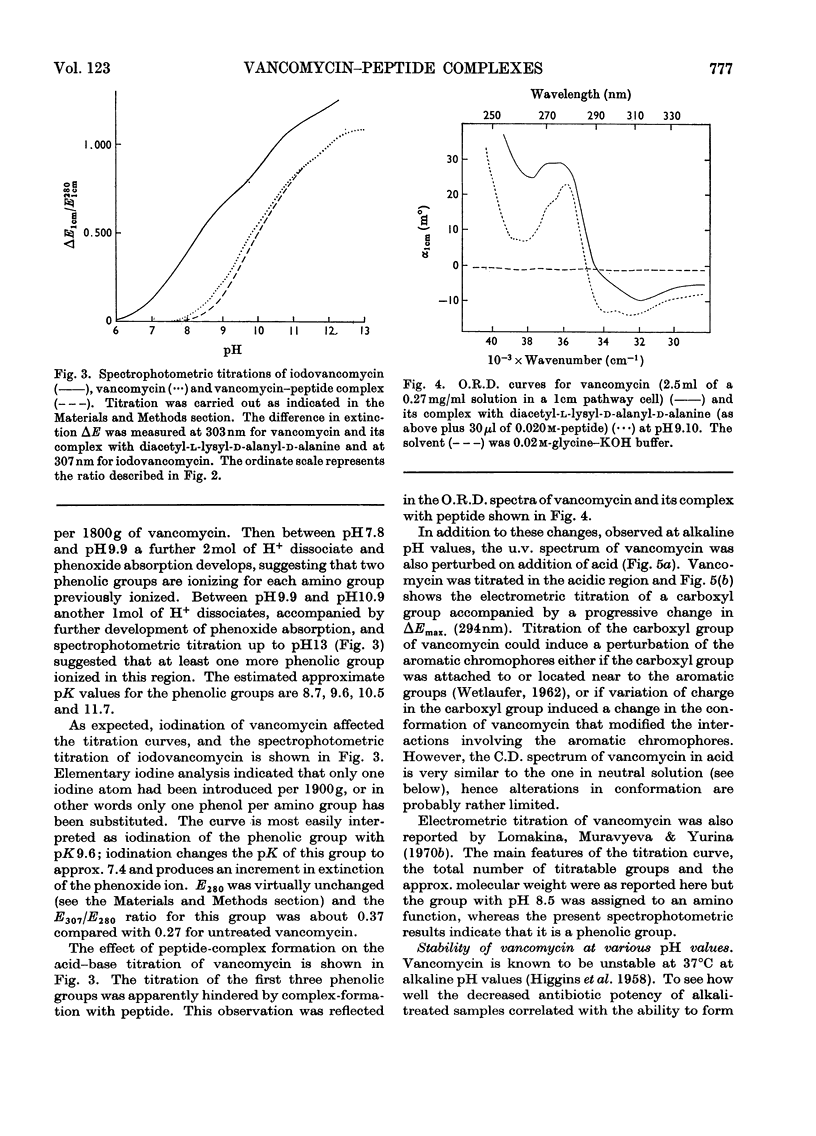
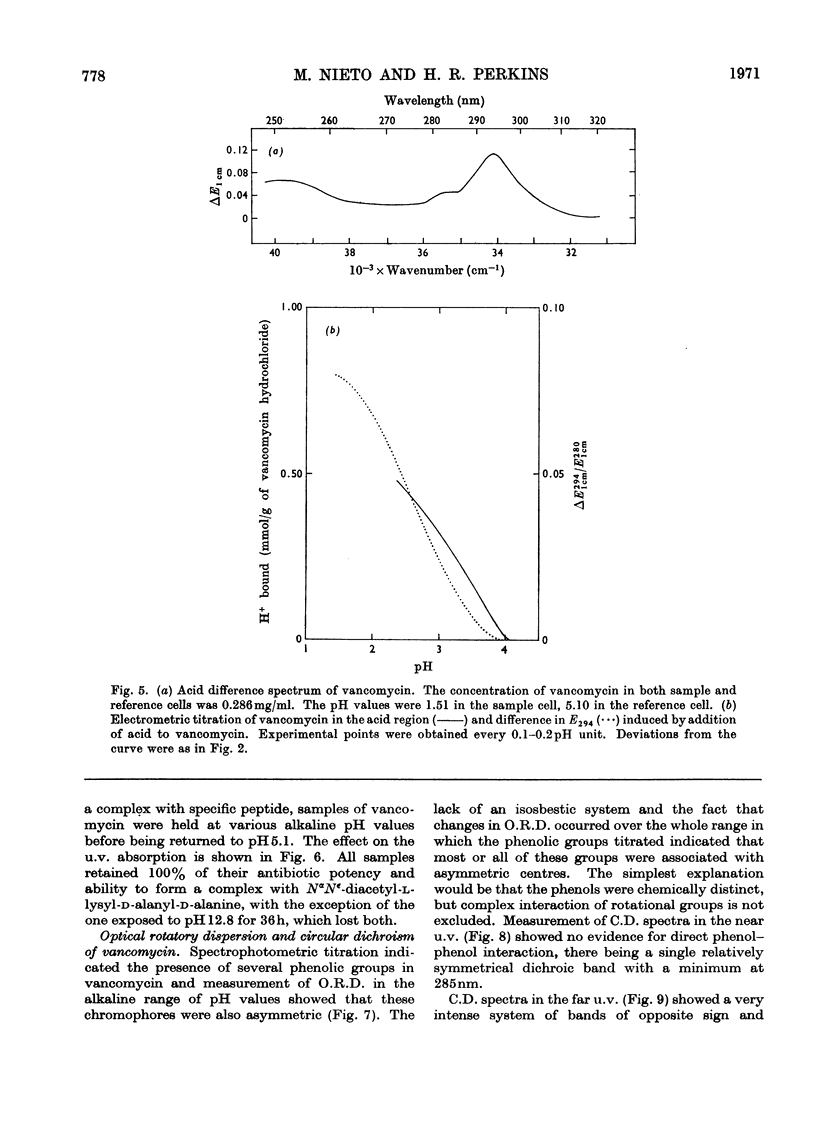
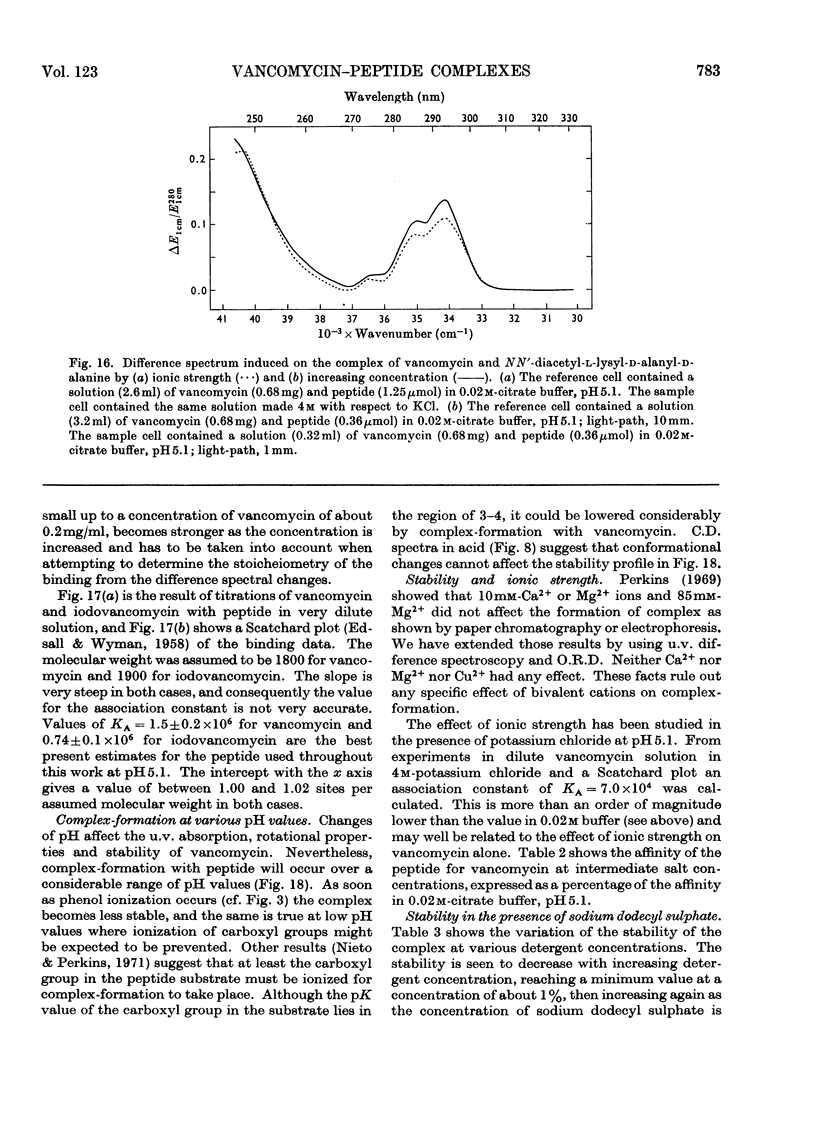
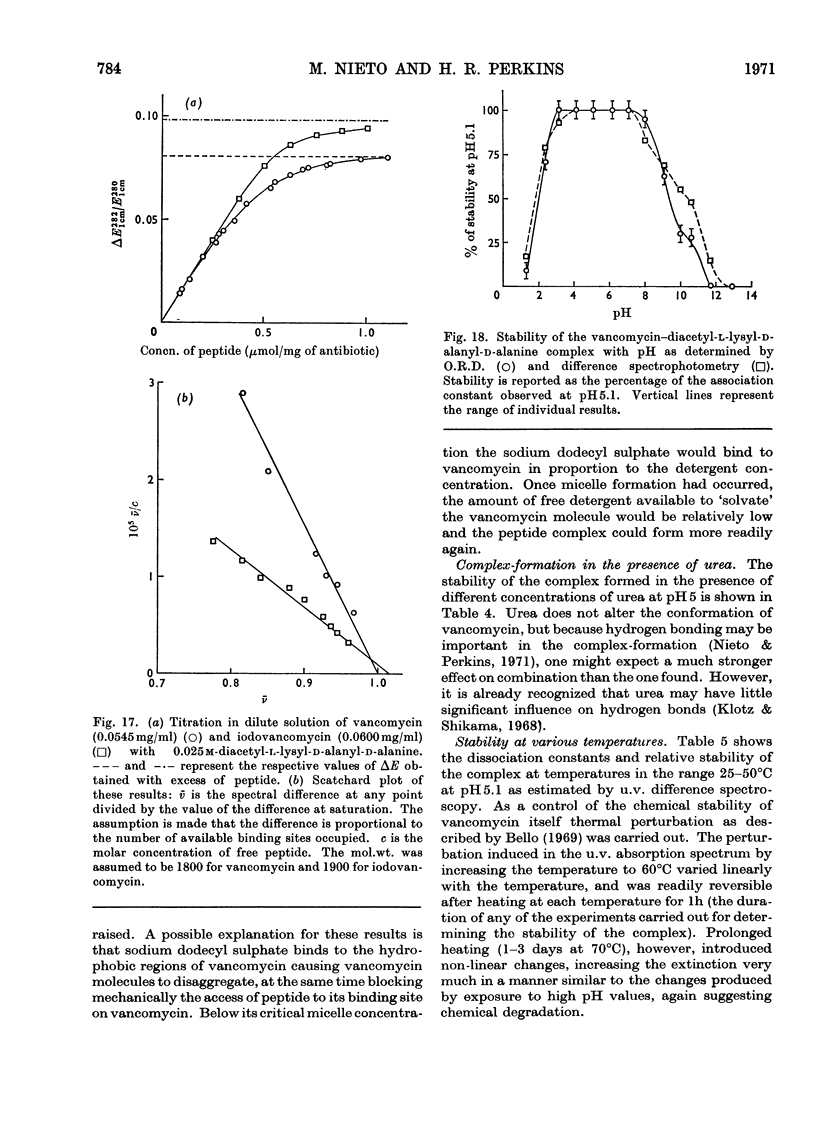
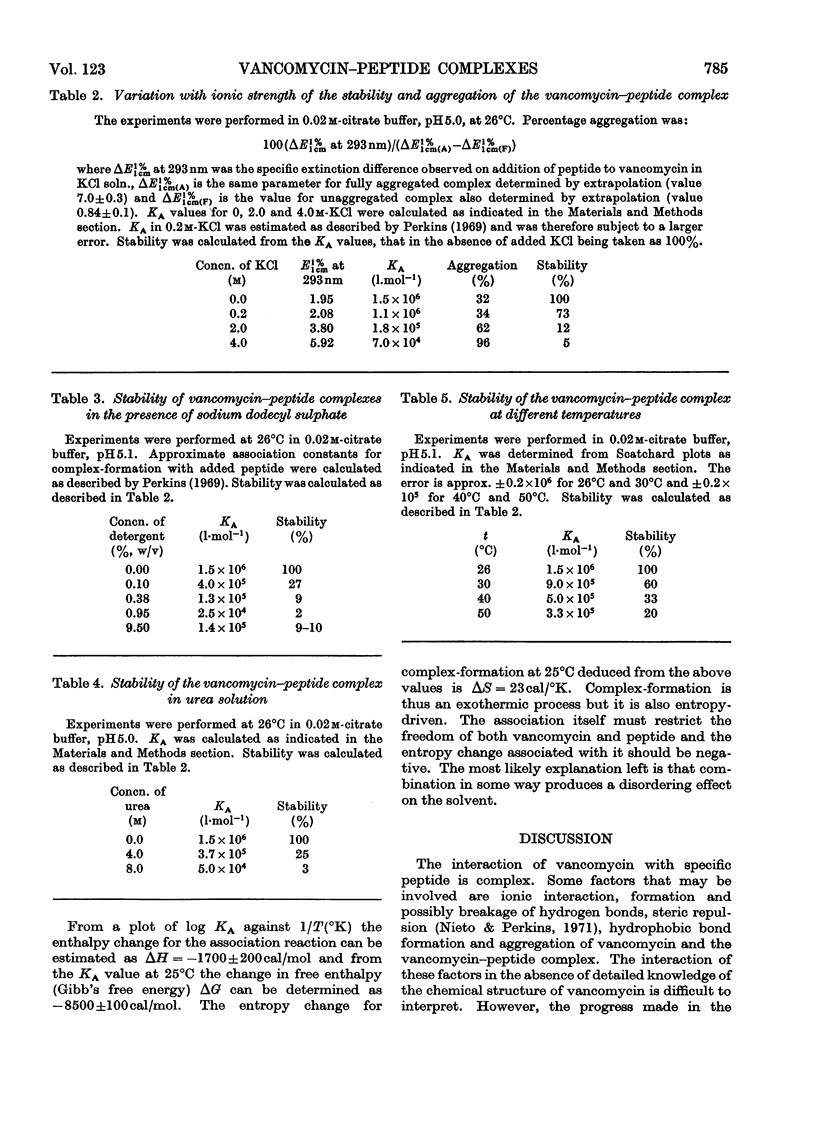
Electrometric and spectrophotometric titrations showed vancomycin to contain groups having pK values of about 2.9, 7.2, 8.6, 9.6, 10.5 and 11.7. Of these the four last-named were phenolic. Titration above pH11 and below pH1 was irreversible and antibiotic potency was destroyed. Combination with the specific peptide diacetyl-l-lysyl-d-alanyl-d-alanine hindered the titration of the first three phenolic groups. Spectrophotometric titration of iodovancomycin showed that the phenolic group with pK 9.6 was the one iodinated. The stability of the vancomycin–peptide complex in the range pH1–13 showed that complex-formation occurred only when carboxyl groups were ionized and the phenolic groups were non-ionized. The complex was formed in concentrations of urea up to 8m, of potassium chloride up to 4m, of sodium dodecyl sulphate up to 1%, and at temperatures up to 60°C. From titration curves, organic chlorine and iodine analysis, and combination with peptide, a minimum molecular weight for vancomycin of 1700–1800 was estimated. Optical-rotatory-dispersion and circular-dichroism experiments suggested that vancomycin has only limited conformational flexibility. Both vancomycin and its complexes with peptide exhibited properties suggesting aggregation. Vancomycin and iodovancomycin can be fractionated into a main fraction and at least three minor components. The isolation of these fractions salt-free is described and their antibiotic properties are shown to correlate with their ability to form complexes with peptide.
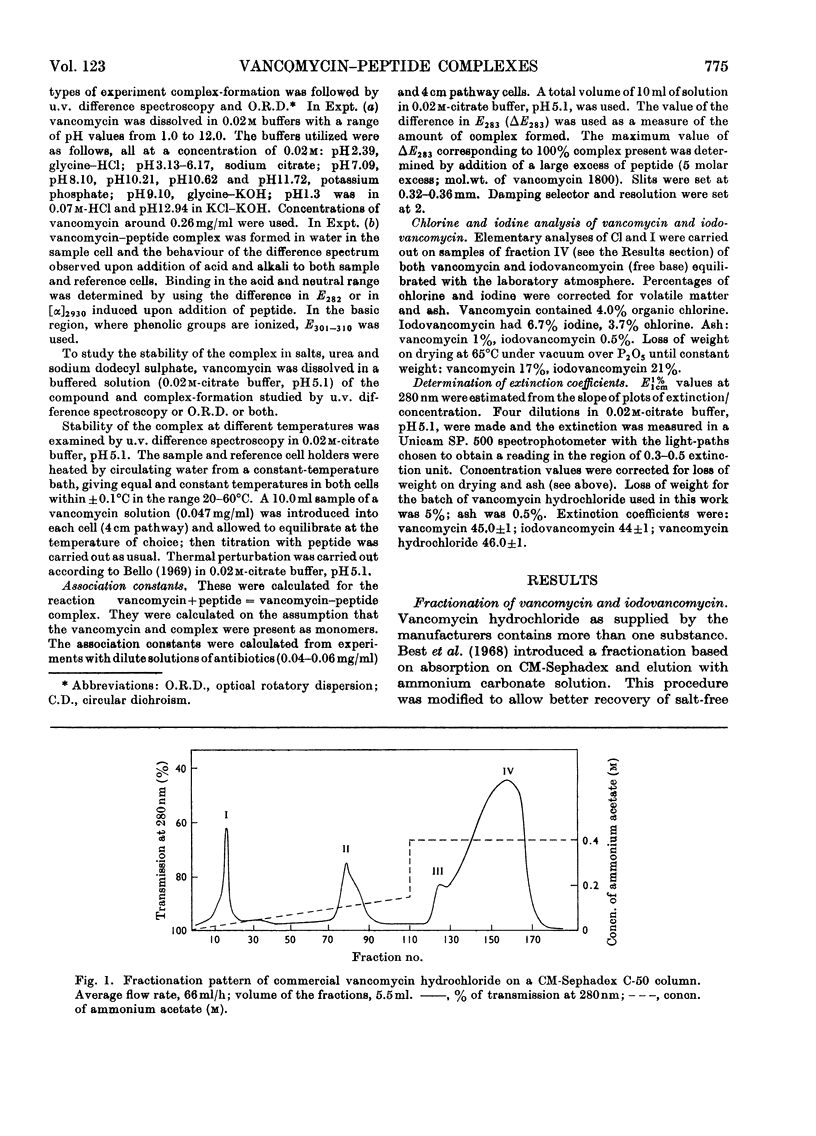
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON J. S., MATSUHASHI M., HASKIN M. A., STROMINGER J. L. LIPID-PHOSPHOACETYLMURAMYL-PENTAPEPTIDE AND LIPID-PHOSPHODISACCHARIDE-PENTAPEPTIDE: PRESUMED MEMBRANE TRANSPORT INTERMEDIATES IN CELL WALL SYNTHESIS. Proc Natl Acad Sci U S A. 1965 Apr;53:881–889. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Matsuhashi M., Haskin M. A., Strominger J. L. Biosythesis of the peptidoglycan of bacterial cell walls. II. Phospholipid carriers in the reaction sequence. J Biol Chem. 1967 Jul 10;242(13):3180–3190. [PubMed] [Google Scholar]
- Bayley P. M., Nielsen E. B., Schellman J. A. The rotatory properties of molecules containing two peptide groups: theory. J Phys Chem. 1969 Jan;73(1):228–243. doi: 10.1021/j100721a038. [DOI] [PubMed] [Google Scholar]
- Bello J. The thermal perturbation method for the estimation of exposed tyrosines of proteins. I. Ribonuclease in aqueous glycol, glycerol, and enaturants. Biochemistry. 1969 Nov;8(11):4542–4550. doi: 10.1021/bi00839a047. [DOI] [PubMed] [Google Scholar]
- Best G. K., Best N. H., Durham N. N. Chromatographic separation of the vancomycin complex. Antimicrob Agents Chemother (Bethesda) 1968;8:115–119. [PubMed] [Google Scholar]
- Best G. K., Grastie M. K., McConnell R. D. Relative affinity of vancomycin and ristocetin for cell walls and uridine diphosphate-N-acetylmuramyl pentapeptide. J Bacteriol. 1970 May;102(2):476–482. doi: 10.1128/jb.102.2.476-482.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N., Perkins H. R. Compounds formed between nucleotides related to the biosynthesis of bacterial cell wall and vancomycin. Biochem Biophys Res Commun. 1966 Aug 12;24(3):489–494. doi: 10.1016/0006-291x(66)90188-4. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Shikama K. Nature of urea effects on anion binding by macromolecules. Arch Biochem Biophys. 1968 Mar 11;123(3):551–557. doi: 10.1016/0003-9861(68)90176-8. [DOI] [PubMed] [Google Scholar]
- Lomakina N. N., Murav'eva L. I., Iurina M. S. Molekuliarnyi ves i chislo ionogennykh grupp ristomitsinov i blizkikh k nim antibiotikov. Antibiotiki. 1970 Jan;15(1):21–24. [PubMed] [Google Scholar]
- Lomakina N. N., Murav'eva L. I., Pushkash M. Ristomitsiny A i B. Stroenie uglevodoni chasti molekuly. Antibiotiki. 1968 Oct;13(10):867–871. [PubMed] [Google Scholar]
- Lomakina N. N., Spiridonova I. A., Bognár R., Puskás M., Sztaricskai F. O vydelenii i svoistvakh dezoksiaminosakhara iz ristomitsina. Antibiotiki. 1968 Nov;13(11):975–978. [PubMed] [Google Scholar]
- Lomakina N. N., Zenkova V. A., Bognár R., Sztaricskai F., Sheinker Iu N., Turchin K. F. Struktura aminokislot iz antibiotika ristomitsina. Aminokislota A. Antibiotiki. 1968 Aug;13(8):675–682. [PubMed] [Google Scholar]
- MCCORMICK M. H., MCGUIRE J. M., PITTENGER G. E., PITTENGER R. C., STARK W. M. Vancomycin, a new antibiotic. I. Chemical and biologic properties. Antibiot Annu. 1955;3:606–611. [PubMed] [Google Scholar]
- Nieto M., Perkins H. R. Modifications of the acyl-D-alanyl-D-alanine terminus affecting complex-formation with vancomycin. Biochem J. 1971 Aug;123(5):789–803. doi: 10.1042/bj1230789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The preparation of iodinated vancomycin and its distribution in bacteria treated with the antibiotic. Biochem J. 1970 Jan;116(1):83–92. doi: 10.1042/bj1160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]


