Abstract
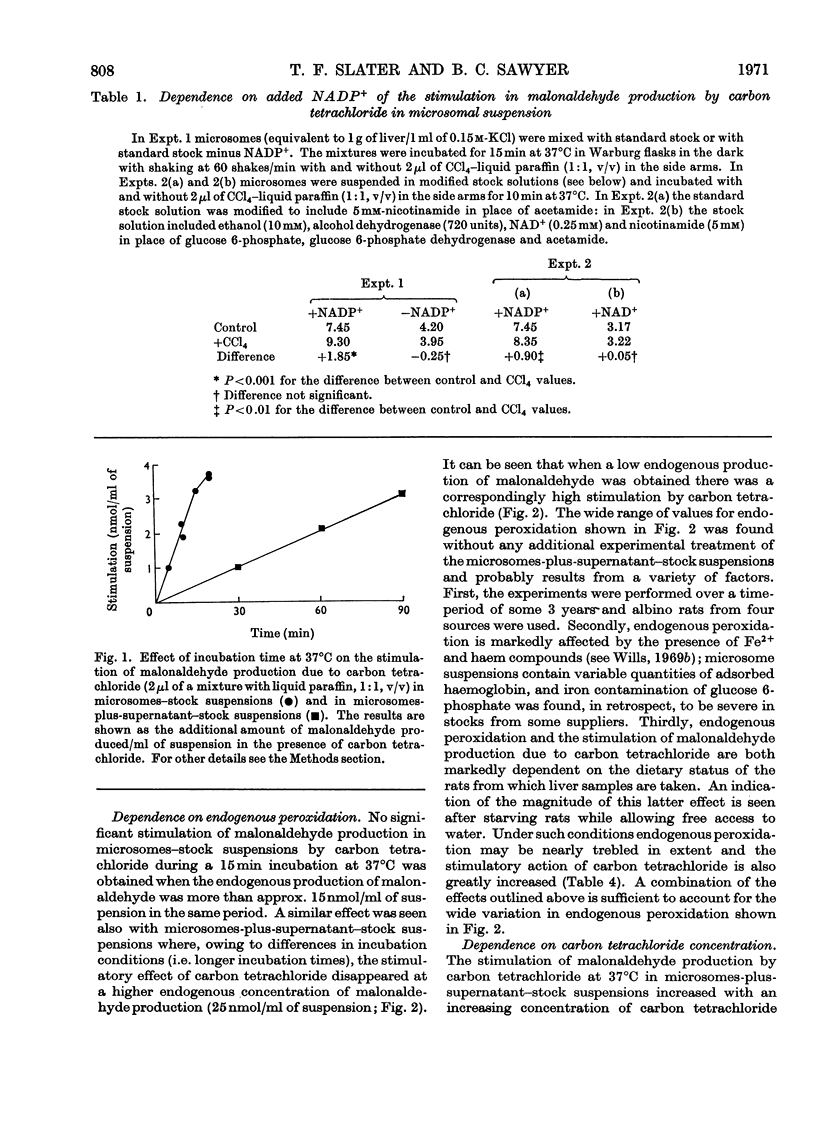
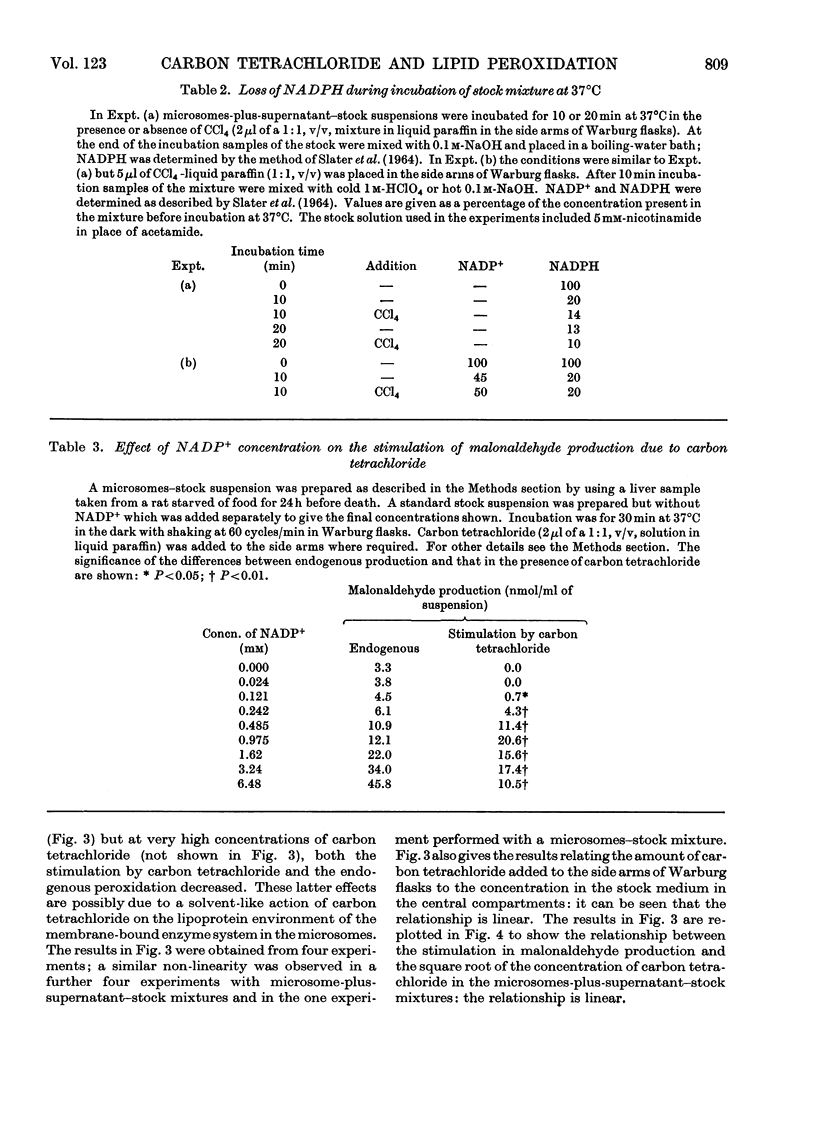
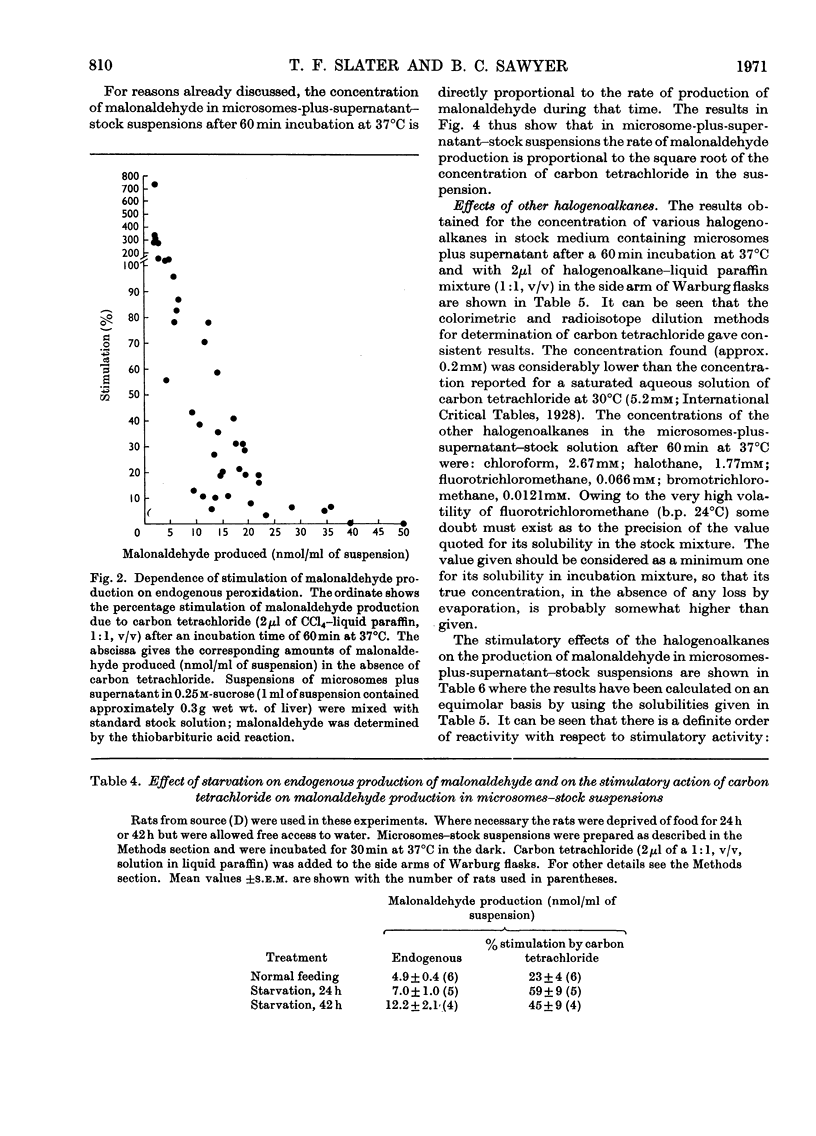
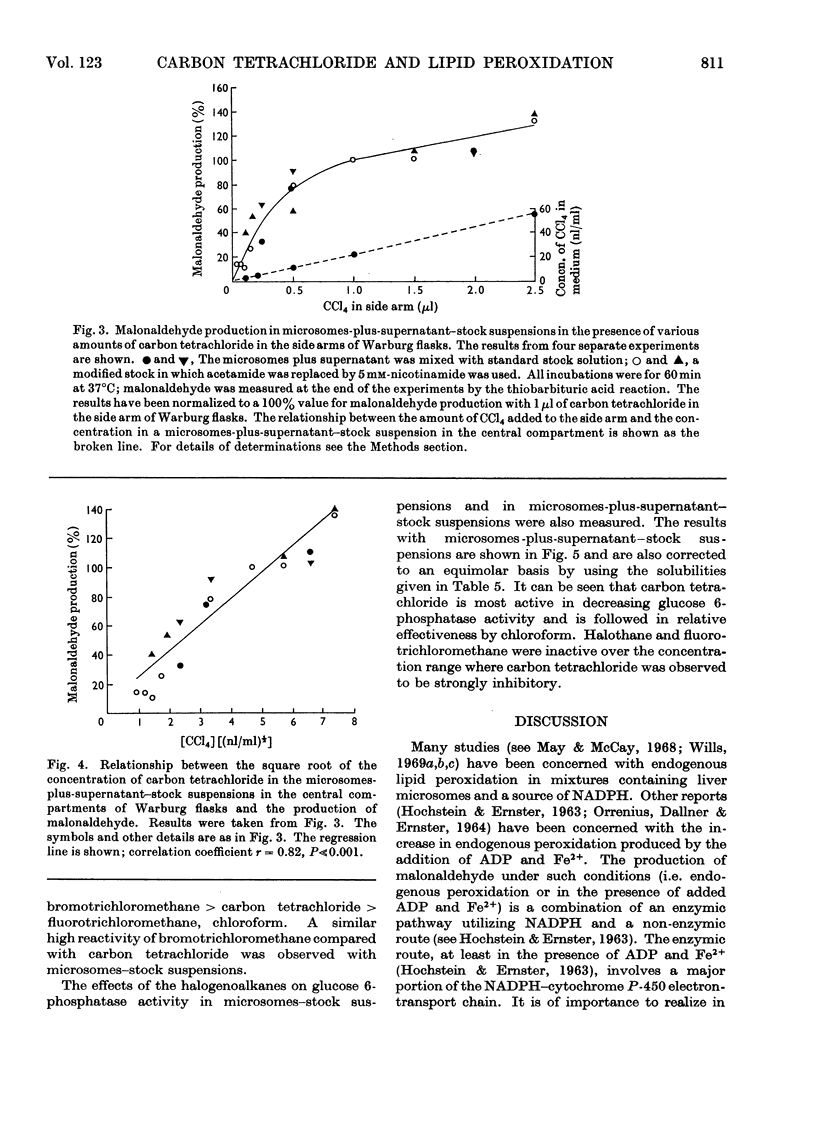
1. The general features of the reaction by which carbon tetrachloride stimulates lipid peroxidation have been elucidated in rat liver microsomal suspensions and in mixtures of microsomes plus cell sap. The production of lipid peroxides has been correlated with malonaldehyde production in the systems used. 2. The stimulation of malonaldehyde production by carbon tetrachloride requires a source of reduced NADP+ and is dependent on the extent of the endogenous peroxidation of the microsomal membranes: if extensive endogenous peroxidation occurs during incubation then no stimulation by carbon tetrachloride is apparent. 3. The stimulation of malonaldehyde production by carbon tetrachloride has been shown to be proportional to the square root of the carbon tetrachloride concentration in the incubation mixture. It is concluded that the stimulation of malonaldehyde production by carbon tetrachloride results from an initiation process that is itself dependent on the homolytic dissociation of carbon tetrachloride to free-radical products. 4. The increased production of malonaldehyde due to carbon tetrachloride is accompanied by a decreased activity of glucose 6-phosphatase in rat liver microsomal suspensions. 5. The relative activities of bromotrichloromethane, fluorotrichloromethane and chloroform have been evaluated in comparison with the effects of carbon tetrachloride in increasing malonaldehyde production and in decreasing glucose 6-phosphatase activity. Bromotrichloromethane was more effective, and fluorotrichloromethane and chloroform were less effective, than carbon tetrachloride in producing these two effects. It is concluded that homolytic bond fission of the halogenomethanes is a requisite for the occurrence of the two effects observed in the endoplasmic reticulum.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER T. C. Reduction of carbon tetrachloride in vivo and reduction of carbon tetrachloride and chloroform in vitro by tissues and tissue constituents. J Pharmacol Exp Ther. 1961 Dec;134:311–319. [PubMed] [Google Scholar]
- Comporti M., Saccocci C., Dianzani M. U. Effect of CCl-4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia. 1965 Nov 6;29(3):185–204. [PubMed] [Google Scholar]
- Delaney V. B., Slater T. F. A filtration method for the separation of cell sap from the large-particle fraction of rat liver suspensions, enabling the rapid measurement of nucleotide distributions. Biochem J. 1970 Jan;116(2):299–302. doi: 10.1042/bj1160299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANZ J., COLE B. T. Effects of ultraviolet-irradiated methyl linolenate on cell division and respiration in Saccharomyces cerevisiae. Arch Biochem Biophys. 1962 Feb;96:382–385. doi: 10.1016/0003-9861(62)90424-1. [DOI] [PubMed] [Google Scholar]
- GHOSHAL A. K., RECKNAGEL R. O. POSITIVE EVIDENCE OF ACCELERATION OF LIPOPEROXIDATION IN RAT LIVER BY CARBON TETRACHLORIDE: IN VITRO EXPERIMENTS. Life Sci. 1965 Aug;4:1521–1530. doi: 10.1016/0024-3205(65)90173-6. [DOI] [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. II. Enzymic properties and stoichiometry. J Biol Chem. 1968 May 10;243(9):2296–2305. [PubMed] [Google Scholar]
- Orrenius S., Dallner G., Ernster L. Inhibition of the TPNH-linked lipid peroxidation of liver microsomes by drugs undergoing oxidative demethylation. Biochem Biophys Res Commun. 1964;14:329–334. doi: 10.1016/s0006-291x(64)80005-x. [DOI] [PubMed] [Google Scholar]
- RECKNAGEL R. O., LITTERIA M. Biochemical changes in carbon tetrachloride fatty liver: concentration of carbon tetrachloride in liver and blood. Am J Pathol. 1960 May;36:521–531. [PMC free article] [PubMed] [Google Scholar]
- Recknagel R. O. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967 Jun;19(2):145–208. [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- Saslaw L. D., Corwin L. M., Waravdekar V. S. Production of chromophoric substances in the thiobarbituric acid test. Arch Biochem Biophys. 1966 Apr;114(1):61–66. doi: 10.1016/0003-9861(66)90305-5. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Necrogenic action of carbon tetrachloride in the rat: a speculative mechanism based on activation. Nature. 1966 Jan 1;209(5018):36–40. doi: 10.1038/209036a0. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The effects of carbon tetrachloride on rat liver microsomes during the first hour of poisoning in vivo, and the modifying actions of promethazine. Biochem J. 1969 Feb;111(3):317–324. doi: 10.1042/bj1110317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro. Inhibitory effects of free-radical scavengers and other agents. Biochem J. 1971 Aug;123(5):823–828. doi: 10.1042/bj1230823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro. Interaction sites in the endoplasmic reticulum. Biochem J. 1971 Aug;123(5):815–821. doi: 10.1042/bj1230815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B., Sträuli U. An assay procedure for nicotinamide-adenine dinucleotides in rat liver and other tissues. Arch Int Physiol Biochim. 1964 Jun;72(3):427–447. doi: 10.3109/13813456409065351. [DOI] [PubMed] [Google Scholar]
- WIRTSCHAFTER Z. T., CRONYN M. W. FREE RADICAL MECHANISM FOR SOLVENT TOXICITY. Arch Environ Health. 1964 Aug;9:186–191. doi: 10.1080/00039896.1964.10663818. [DOI] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. General considerations. Biochem J. 1969 Jun;113(2):315–324. doi: 10.1042/bj1130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J. 1969 Jun;113(2):333–341. doi: 10.1042/bj1130333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]


