Abstract
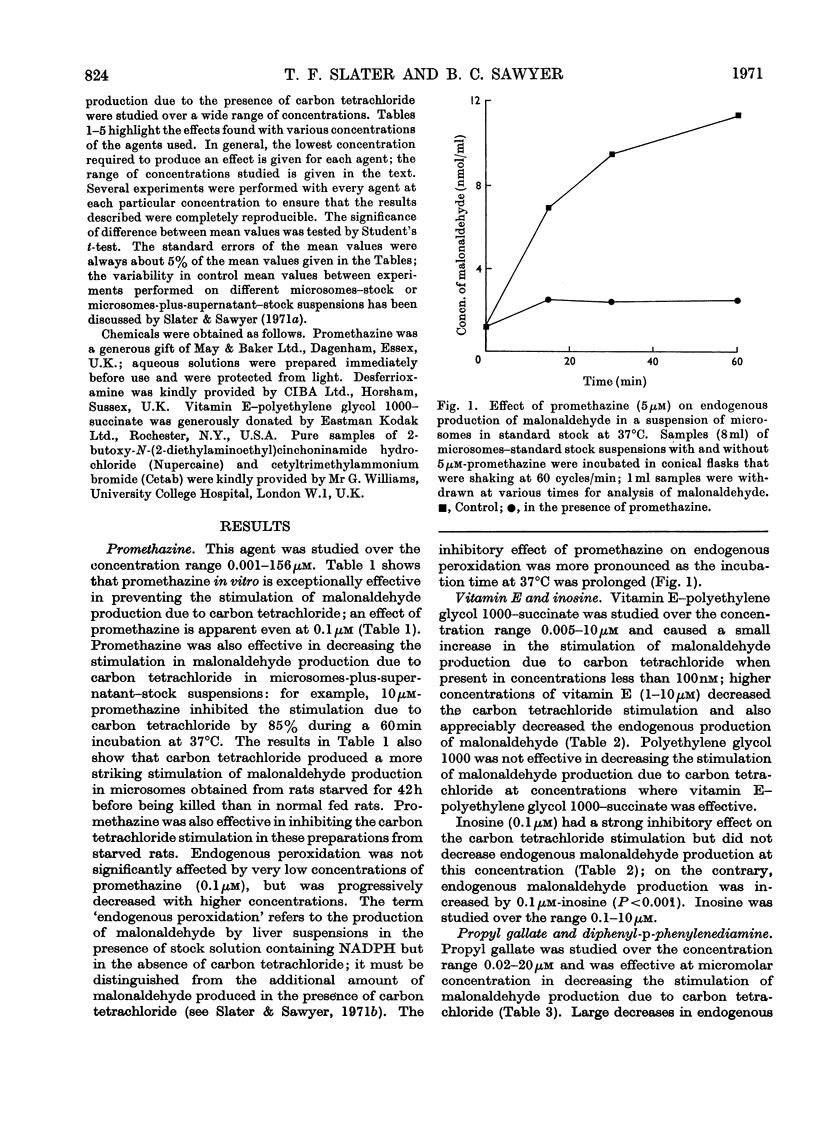
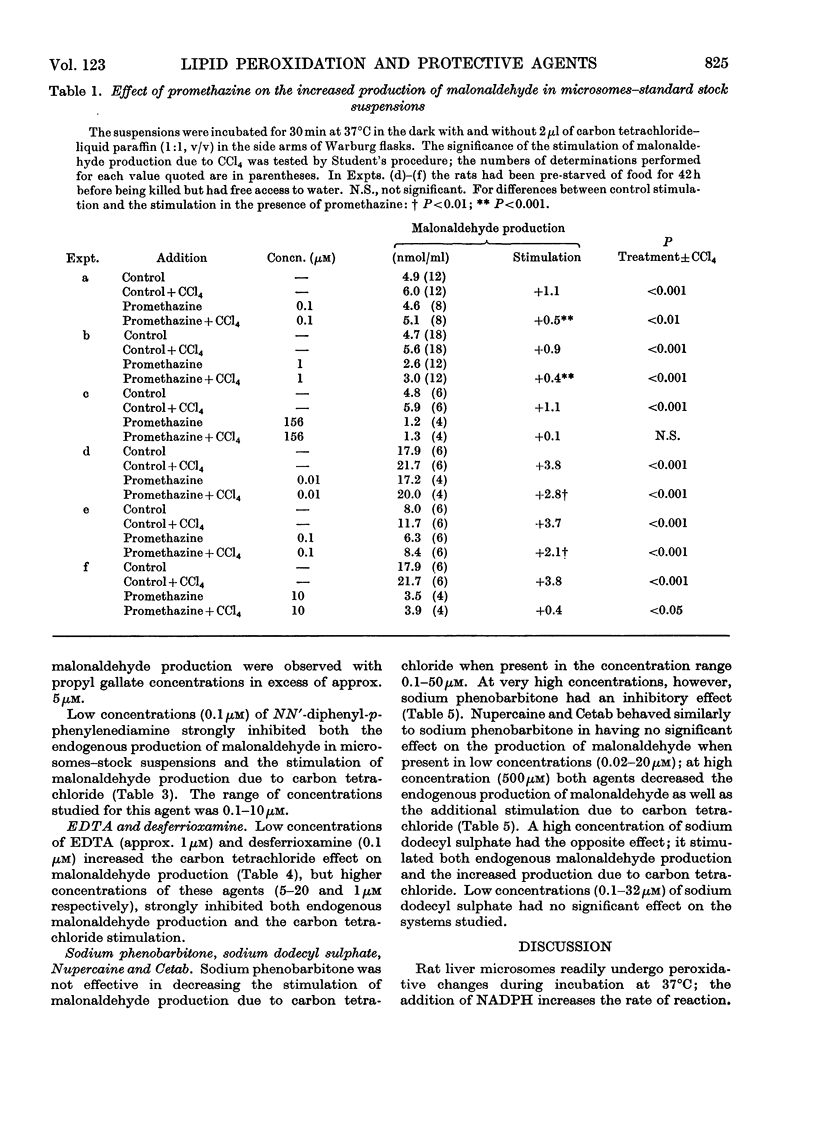
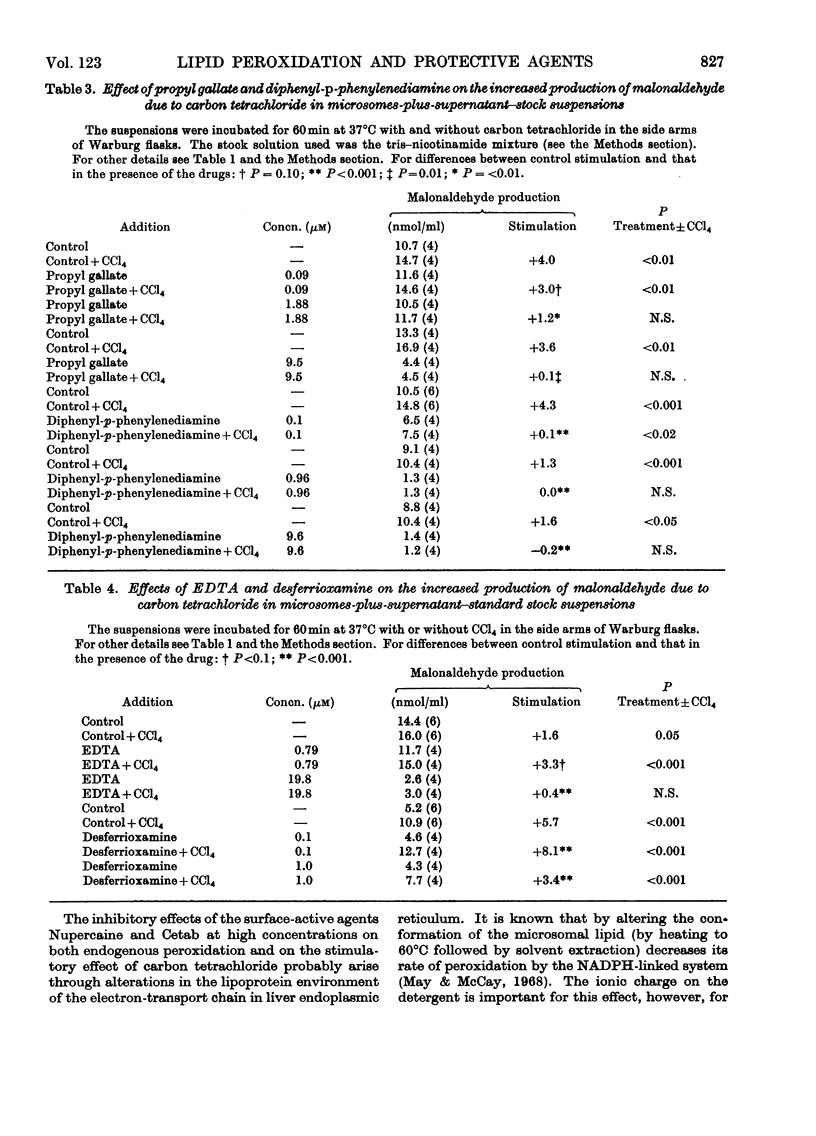
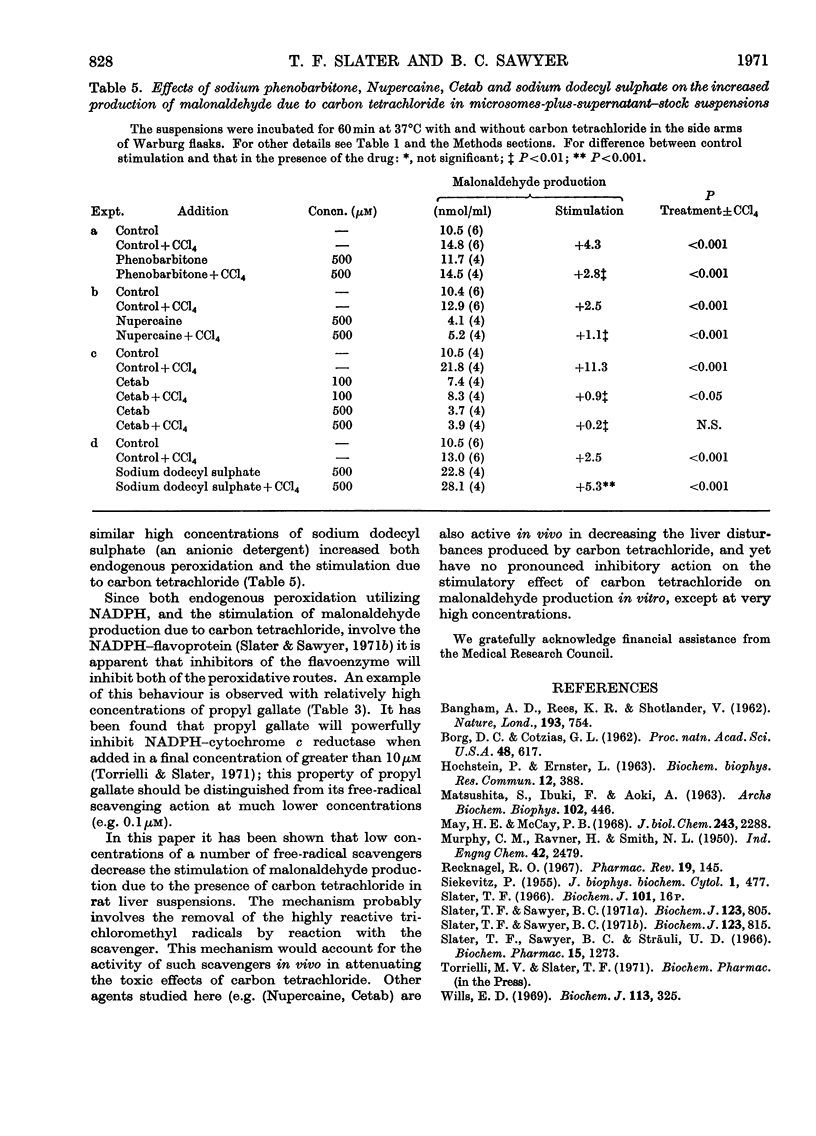
1. The effects of a number of free-radical scavengers and other agents on the stimulation of malonaldehyde production due to low concentrations of carbon tetrachloride have been studied in rat liver microsome suspensions. 2. Promethazine, propyl gallate and NN′-diphenyl-p-phenylenediamine were extremely active in inhibiting the stimulation of malonaldehyde production due to carbon tetrachloride; inhibitory effects were demonstrable with these agents at 0.1μm. 3. Low concentrations (1–100nm) of vitamin E–polyethylene glycol 1000–succinate increased the stimulation of malonaldehyde production due to carbon tetrachloride, but higher concentrations of the vitamin E preparation decreased both the stimulation due to carbon tetrachloride and the endogenous peroxidation that occurs in the absence of carbon tetrachloride. 4. Other agents tested that were effective in the range 1–20μm in decreasing the stimulation of malonaldehyde production due to carbon tetrachloride were inosine, desferrioxamine and EDTA. Agents tested that were not effective, except at very high concentrations (100μm or greater), were Nupercaine, Cetab and sodium phenobarbitone. 5. The results are discussed in terms of the mechanisms responsible for the observed inhibitions of malonaldehyde production, and of the relevance of the in vitro system to the liver damage produced by carbon tetrachloride in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., REES K. R., SHOTLANDER V. Penetration of lipid films by compounds preventing liver necrosis in rats. Nature. 1962 Feb 24;193:754–756. doi: 10.1038/193754a0. [DOI] [PubMed] [Google Scholar]
- BORG D. C., COTZIAS G. C. Interaction of trace metals with phenothiazine drug derivatives. I. Structure-reactivity correlations. Proc Natl Acad Sci U S A. 1962 Apr 15;48:617–623. doi: 10.1073/pnas.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- MATSUSHITA S., IBUKI F., AOKI A. CHEMICAL REACTIVITY OF THE NUCLEIC ACID BASES. I. ANTIOXIDATIVE ABILITY OF THE NUCLEIC ACIDS AND THEIR RELATED SUBSTANCES ON THE OXIDATION OF UNSATURATED FATTY ACIDS. Arch Biochem Biophys. 1963 Sep;102:446–451. doi: 10.1016/0003-9861(63)90253-4. [DOI] [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- Recknagel R. O. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967 Jun;19(2):145–208. [PubMed] [Google Scholar]
- SIEKEVITZ P. The distinctive occurrence of acid-soluble inosine in rat liver microsomal preparations. J Biophys Biochem Cytol. 1955 Sep 25;1(5):477–481. doi: 10.1083/jcb.1.5.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride on peroxidative reactions in rat liver fractions in vitro. Interaction sites in the endoplasmic reticulum. Biochem J. 1971 Aug;123(5):815–821. doi: 10.1042/bj1230815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D. Lipid peroxide formation in microsomes. The role of non-haem iron. Biochem J. 1969 Jun;113(2):325–332. doi: 10.1042/bj1130325. [DOI] [PMC free article] [PubMed] [Google Scholar]


