Abstract
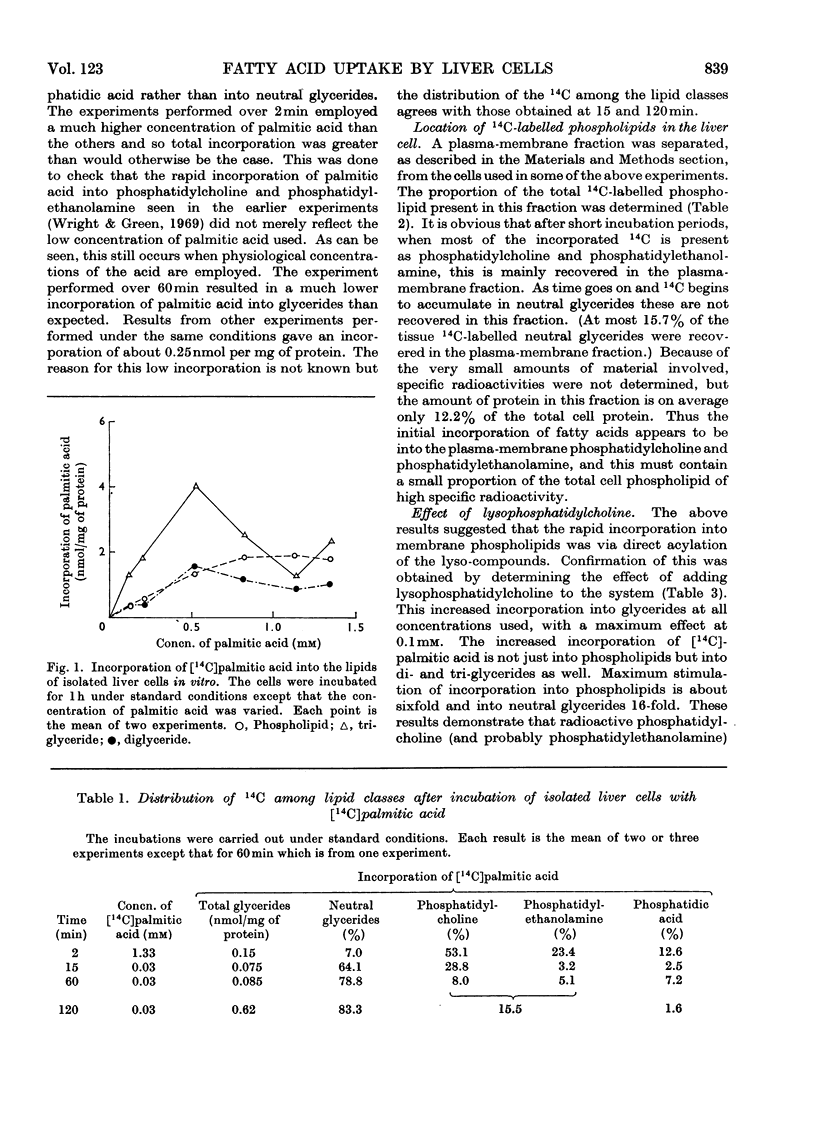
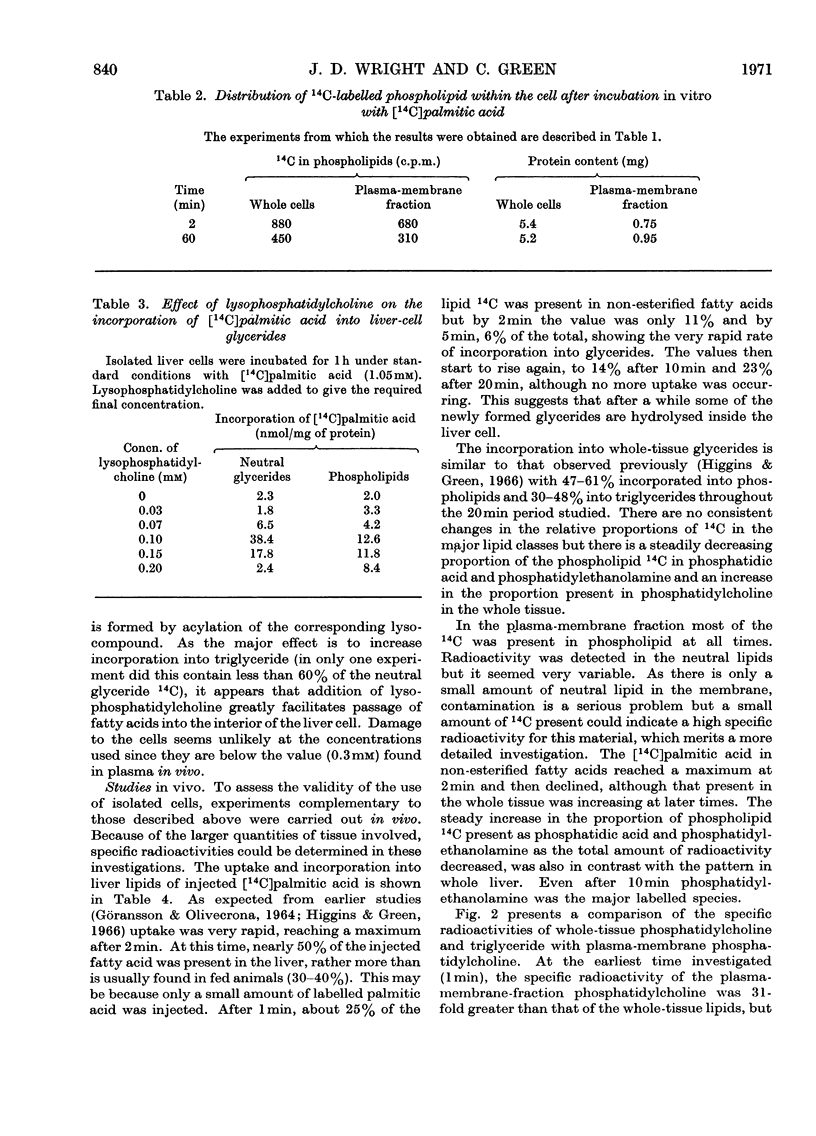
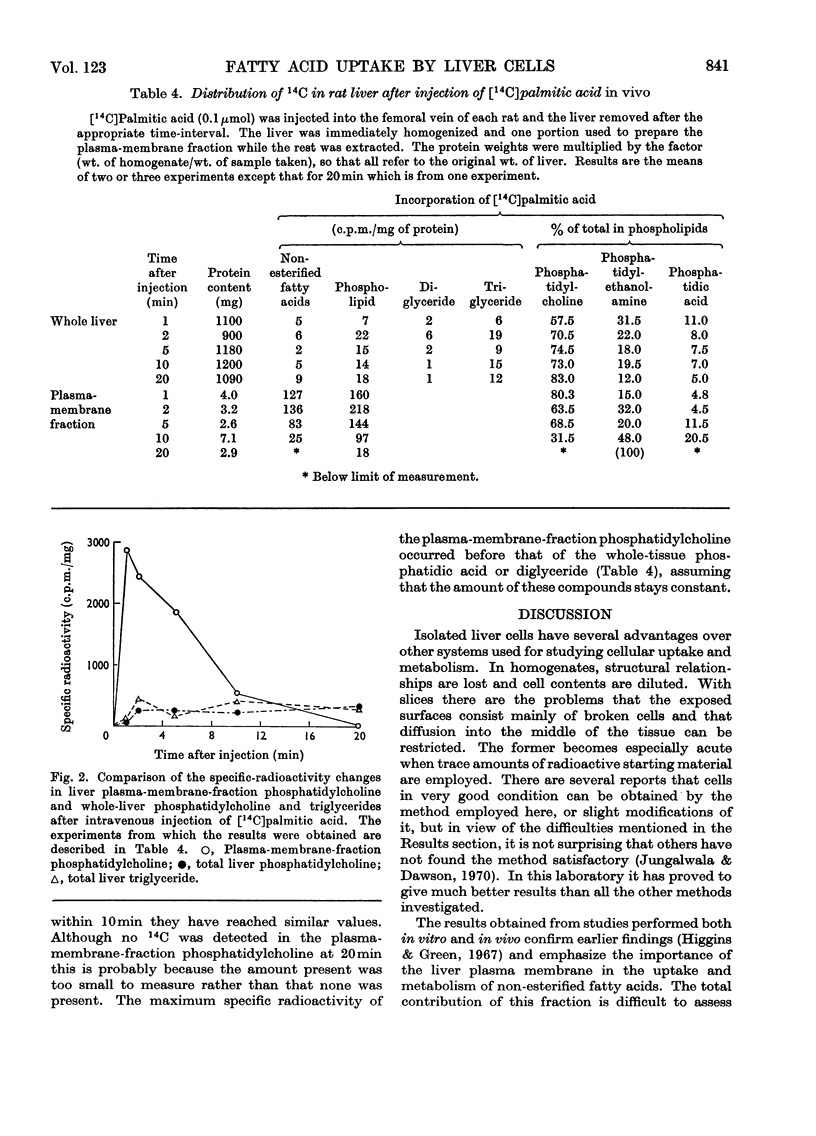
1. Suspensions of isolated rat liver parenchymal cells incorporate [14C]palmitic acid into glycerides at about 40% of the rate obtained with liver slices. 2. At short time-intervals most of the incorporation is into phosphatidylcholine and this is recovered mainly in the plasma-membrane fraction. 3. At later times (5min to 2h) the [14C]palmitic acid is mainly found in triglyceride, but this is not recovered in the plasma-membrane fraction. 4. Addition of lysophosphatidylcholine increases incorporation of palmitic acid into both phosphatidylcholine and triglyceride, with maximum effect at about 0.1mm. 5. In vivo, 1min after injection of [14C]palmitic acid, radioactive phosphatidylcholine is concentrated in the plasma-membrane fraction, but the proportion present in this fraction declines rapidly. 6. The phosphatidylcholine of the plasma-membrane fraction has, at 1min after injection, a specific radioactivity 30-fold greater than that of the whole tissue. 7. This phosphatidylcholine reaches its maximum specific radioactivity before the tissue phosphatidic acid or diglyceride. 8. The phosphatidylcholine of the plasma-membrane fraction has a very rapid turnover. 9. It is proposed that the rapid formation of phospholipids in the plasma membrane is by acylation of their lyso-derivatives and the role of this process in fatty acid uptake is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N., Schotz M. C. Quantitative aspects of free fatty acid metabolism in the fasted rat. J Lipid Res. 1967 Nov;8(6):646–660. [PubMed] [Google Scholar]
- Biezenski J. J. Efficient elution of rabbit liver and plasma phospholipids from thin-layer plates. J Lipid Res. 1967 Jul;8(4):409–410. [PubMed] [Google Scholar]
- Boberg J. Separation of labeled plasma and tissue lipids by thin-layer chromatography. A quantitative methodological study. Clin Chim Acta. 1966 Sep;14(3):325–334. doi: 10.1016/0009-8981(66)90109-4. [DOI] [PubMed] [Google Scholar]
- Boberg J. Turnover of H3-labelled palmitate in the unanesthetized rat. Acta Physiol Scand. 1969 Aug;76(4):495–502. doi: 10.1111/j.1748-1716.1969.tb04496.x. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Coleman R., Michell R. H., Finean J. B., Hawthorne J. N. A purified plasma membrane fraction isolated from rat liver under isotonic conditions. Biochim Biophys Acta. 1967 Sep 9;135(4):573–579. doi: 10.1016/0005-2736(67)90089-2. [DOI] [PubMed] [Google Scholar]
- Duncombe W. G. The colorimetric micro-determination of long-chain fatty acids. Biochem J. 1963 Jul;88(1):7–10. doi: 10.1042/bj0880007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eibl H., Hill E. E., Lands W. E. The subcellular distribution of acyltransferases which catalyze the synthesis of phosphoglycerides. Eur J Biochem. 1969 Jun;9(2):250–258. doi: 10.1111/j.1432-1033.1969.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Elovson J. Immediate fate of albumin-bound [I-14C]stearic acid following its intraportal injection into carbohydrate refed rats. Early course of desaturation and esterification in the liver. Biochim Biophys Acta. 1965 Dec 2;106(3):480–494. doi: 10.1016/0005-2760(65)90065-2. [DOI] [PubMed] [Google Scholar]
- GOERANSSON G., OLIVECRONA T. THE METABOLISM OF FATTY ACIDS IN THE RAT. I. PALMITIC ACID. Acta Physiol Scand. 1964 Nov;62:224–239. doi: 10.1111/j.1748-1716.1964.tb03970.x. [DOI] [PubMed] [Google Scholar]
- Graham J. M., Higgins J. A., Green C. The isolation of rat liver plasma membrane fragments. Biochim Biophys Acta. 1968 Mar 1;150(2):303–305. doi: 10.1016/0005-2736(68)90173-9. [DOI] [PubMed] [Google Scholar]
- Higgens J. A., Green C. The entry of palmitic acid into rat-liver cells. Biochem J. 1967 Aug;104(2):26P–26P. [PMC free article] [PubMed] [Google Scholar]
- Higgins J. A., Green C. The uptake of lipids by rat liver cells. Biochem J. 1966 Jun;99(3):631–639. doi: 10.1042/bj0990631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton R. H., Dobrota M., Fitzsimons J. T., Reid E. Preparation of a plasma membrane fraction from rat liver by zonal centrifugation. Eur J Biochem. 1970 Feb;12(2):349–359. doi: 10.1111/j.1432-1033.1970.tb00857.x. [DOI] [PubMed] [Google Scholar]
- Howard R. B., Pesch L. A. Respiratory activity of intact, isolated parenchymal cells from rat liver. J Biol Chem. 1968 Jun 10;243(11):3105–3109. [PubMed] [Google Scholar]
- Jungalwala F. B., Dawson R. M. Phospholipid synthesis and exchange in isolated liver cells. Biochem J. 1970 Apr;117(3):481–490. doi: 10.1042/bj1170481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDS W. E., MERKL I. Metabolism of glycerolipids. III. Reactivity of various acyl esters of coenzyme A with alpha'-acylglycerophosphorylcholine, and positional specificities in lecithin synthesis. J Biol Chem. 1963 Mar;238:898–904. [PubMed] [Google Scholar]
- LANDS W. E. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J Biol Chem. 1958 Apr;231(2):883–888. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laudat M. H., Koenig J., Laudat P. Incorporation "in vitro" du palmitate de sodium 1-14C dans les fractions lipidiques du foie de rat (tranches et hépatocytes isolés) Bull Soc Chim Biol (Paris) 1969 Jun 4;51(1):105–115. [PubMed] [Google Scholar]
- Lippel K., Robinson J., Trams E. G. Intracellular distribution of palmitoyl-CoA synthetase in rat liver. Biochim Biophys Acta. 1970 Apr 22;206(1):173–177. doi: 10.1016/0005-2744(70)90094-x. [DOI] [PubMed] [Google Scholar]
- MERKL I., LANDS W. E. Metabolism of glycerolipids. IV. Synthesis of phosphatidylethanolamine. J Biol Chem. 1963 Mar;238:905–906. [PubMed] [Google Scholar]
- Nachbaur J., Colbeau A., Vignais P. M. Incorporation of fatty acids into the outer and inner membranes of isolated rat liver mitochondria. FEBS Lett. 1969 Apr;3(2):121–124. doi: 10.1016/0014-5793(69)80113-4. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Mead J. F. Long chain fatty acid activation in subcellular preparations from rat liver. J Biol Chem. 1968 Jan 25;243(2):352–361. [PubMed] [Google Scholar]
- Portman O. W., Alexander M., Osuga T. Heterogeneity of lipid composition of microsome subfractions from aorta and liver. Biochim Biophys Acta. 1969 Oct 28;187(3):435–438. doi: 10.1016/0005-2760(69)90017-4. [DOI] [PubMed] [Google Scholar]
- Reed C. F. Phospholipid exchange between plasma and erythrocytes in man and the dog. J Clin Invest. 1968 Apr;47(4):749–760. doi: 10.1172/JCI105770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshef L., Shapiro B. Depletion and regeneration of fatty acid-absorbing capacity of adipose tissue and liver particles. Biochim Biophys Acta. 1966 Dec 7;125(3):456–464. doi: 10.1016/0005-2760(66)90034-8. [DOI] [PubMed] [Google Scholar]
- Scherphof G. L., van Deenen L. L. On the pathways of fatty acid incorporation into the lipids of subcellular particles of rat liver and into erythrocytes. Biochim Biophys Acta. 1966 Feb 14;113(2):417–420. doi: 10.1016/s0926-6593(66)80087-5. [DOI] [PubMed] [Google Scholar]
- Shohet S. B., Nathan D. G., Karnovsky M. L. Stages in the incorporation of fatty acids into red blood cells. J Clin Invest. 1968 May;47(5):1096–1108. doi: 10.1172/JCI105799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohet S. B. Release of phospholipid fatty acid from human erythrocytes. J Clin Invest. 1970 Sep;49(9):1668–1678. doi: 10.1172/JCI106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W. L., Trams E. G. Synthesis of lipids by liver plasma membranes. Incorporation of acyl-coenzyme A derivatives into membrane lipids in vitro. Biochim Biophys Acta. 1968 Dec 10;163(4):459–471. doi: 10.1016/0005-2736(68)90075-8. [DOI] [PubMed] [Google Scholar]
- Stein Y., Stein O. Metabolism of labeled lysolecithin, lysophosphatidyl ethanolamine and lecithin in the rat. Biochim Biophys Acta. 1966 Feb 1;116(1):95–107. doi: 10.1016/0005-2760(66)90095-6. [DOI] [PubMed] [Google Scholar]
- Stein Y., Widnell C., Stein O. Acylation of lysophosphatides by plasma membrane fractions of rat liver. J Cell Biol. 1968 Oct;39(1):185–192. doi: 10.1083/jcb.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G., Fales H. A., Gal A. E. S-palmityl pantetheine as an intermediate in the metabolism of palmityl Coenzyme A by rat liver plasma membrane preparations. Biochem Biophys Res Commun. 1968 Jun 28;31(6):973–976. doi: 10.1016/0006-291x(68)90548-2. [DOI] [PubMed] [Google Scholar]
- Wright J. D., Green C. Palmitic acid uptake and metabolism by isolated rat liver cells. Biochem J. 1969 Oct;114(4):65P–66P. doi: 10.1042/bj1140065pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch H., van Deenen L. L. Chemical structure and biochemical significance of lysolecithins from rat liver. Biochim Biophys Acta. 1965 Oct 4;106(2):326–337. doi: 10.1016/0005-2760(65)90041-x. [DOI] [PubMed] [Google Scholar]


