Abstract
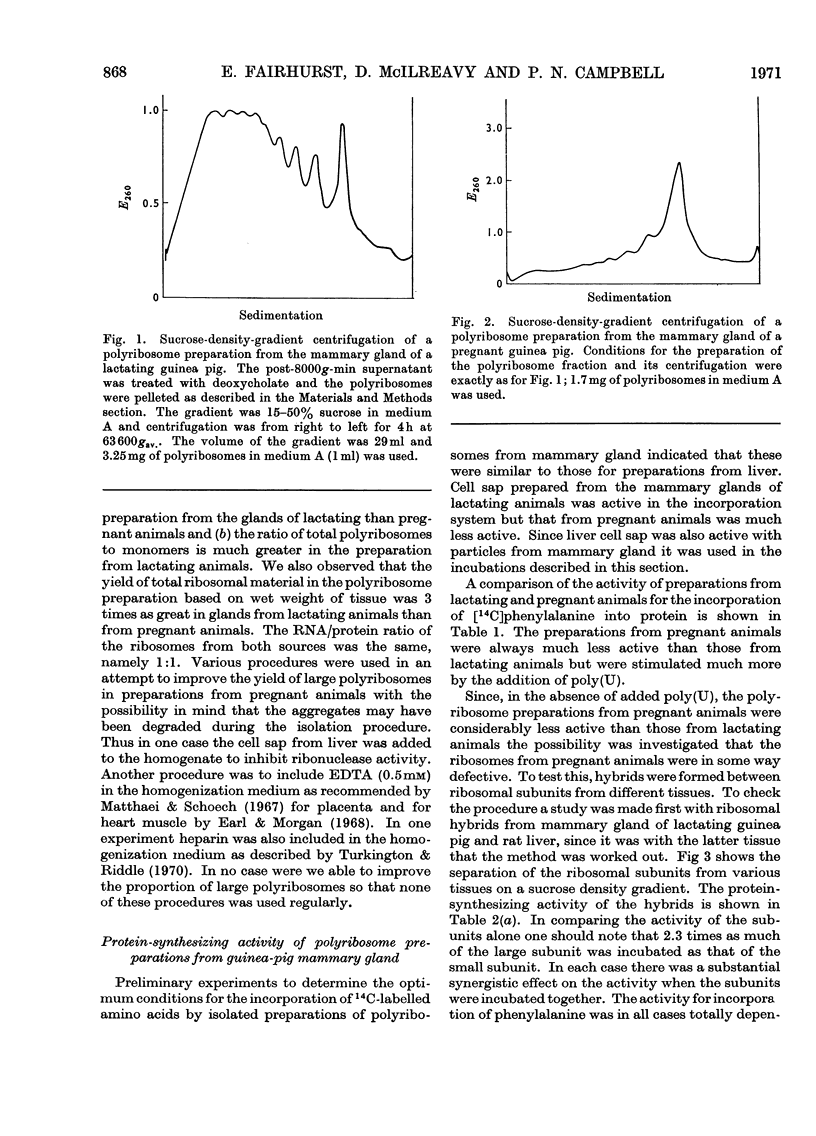
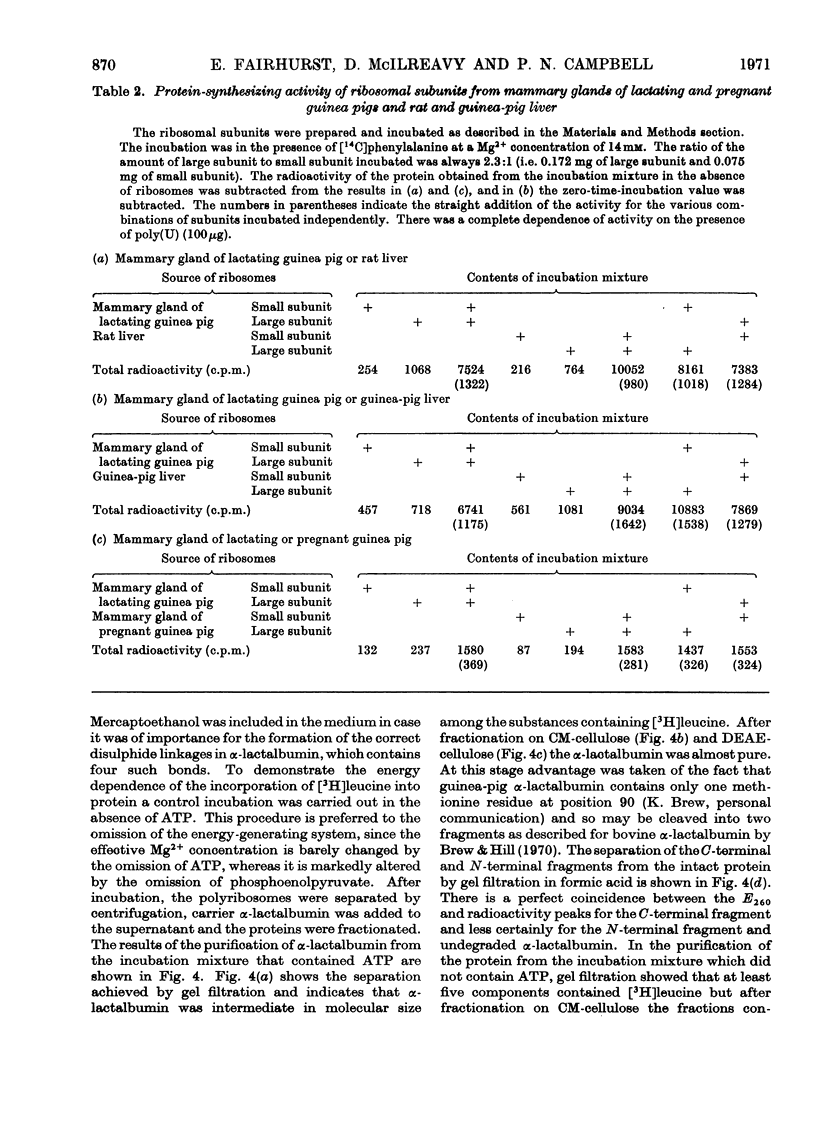
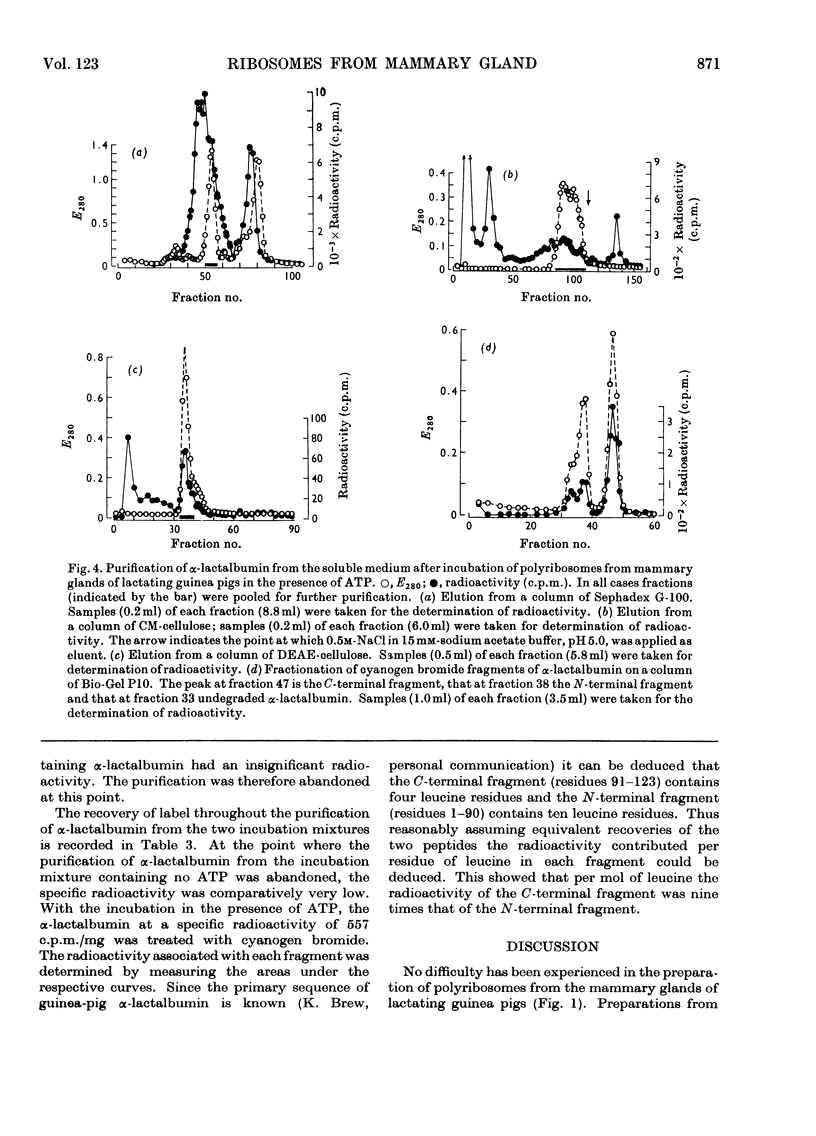
1. Polyribosome preparations were made from the deoxycholate-treated post-nuclear fractions obtained by the disruption of mammary glands from lactating and pregnant guinea pigs. 2. A high proportion of large polyribosomes was obtained from the glands of lactating animals whereas mainly small polyribosomes were obtained from the glands of pregnant animals. The isolated preparations incorporated [14C]phenylalanine into protein. The polyribosomes from the glands of pregnant animals were less active than those from the glands of lactating animals but the activity of the former was stimulated more by poly(U) than was the latter. 3. The ribosomes from mammary gland could be dissociated into subunits after incubation, under conditions necessary for protein synthesis, in the presence of puromycin. The subunits could be recombined to give a preparation that actively polymerized [14C]phenylalanine in the presence of poly(U). The subunits from guinea-pig mammary gland could be combined with subunits from liver of either guinea pig or rat. Hybrid ribosomes were also formed from subunits derived from glands of pregnant and lactating animals. The hybrids were as active as were the ribosomes formed by reassociation of subunits from the same tissue, suggesting that in this respect the ribosomes from pregnant animals were not defective. 4. Polyribosomes from mammary glands of lactating animals when incubated with cell sap from the same source were tested for their ability to synthesize α-lactalbumin. The polyribosomes were incubated in the presence of [3H]leucine and α-lactalbumin was isolated from the supernatant. The protein was finally treated with cyanogen bromide and the C-terminal and N-terminal fragments were separated and their radioactivity was determined. Both fragments were radioactive consistent with the synthesis of α-lactalbumin. 5. The results are discussed in relation to protein synthesis in the mammary gland after parturition.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird G. D., Herriman I. D. Amino acid incorporation into ribonucleoprotein particles prepared from rabbit mammary glands during pregnancy and lactation. Biochim Biophys Acta. 1968 Aug 23;166(1):162–174. doi: 10.1016/0005-2787(68)90500-5. [DOI] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. Studies on the biosynthesis of protein by lactating guinea-pig mammary gland. Characteristics of the synthesis of alpha-lactalbumin and total protein by slices and cell-free systems. Biochem J. 1967 Jan;102(1):265–274. doi: 10.1042/bj1020265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Campbell P. N. The characterization of the whey proteins of guinea-pig milk. The isolation and properties of alpha-lactalbumin. Biochem J. 1967 Jan;102(1):258–264. doi: 10.1042/bj1020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew K., Hill R. L. The isolation and characterization of the tryptic, chymotryptic, peptic, and cyanogen bromide peptides from bovine alpha-lactalbumin. J Biol Chem. 1970 Sep 10;245(17):4559–4569. [PubMed] [Google Scholar]
- Campbell P. N., Lowe E., Serck-Hanssen G. Protein synthesis by microsomal particles from regenerating rat liver. Biochem J. 1967 Apr;103(1):280–288. doi: 10.1042/bj1030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo F., Goldberger R. F., Steers E., Jr, Givol D., Anfinsen B. Purification and properties of an enzyme from beef liver which catalyzes sulfhydryl-disulfide interchange in proteins. J Biol Chem. 1966 Apr 10;241(7):1562–1567. [PubMed] [Google Scholar]
- Earl D. C., Morgan H. E. An improved preparation of ribosomes and polysomes from cardiac muscle. Arch Biochem Biophys. 1968 Nov;128(2):460–469. doi: 10.1016/0003-9861(68)90052-0. [DOI] [PubMed] [Google Scholar]
- FRASER M. J., GUTFREUND H. Steps in amino-acid incorporation into mammary tissue. Proc R Soc Lond B Biol Sci. 1958 Dec 17;149(936):392–400. doi: 10.1098/rspb.1958.0078. [DOI] [PubMed] [Google Scholar]
- Gaye P., Denamur R. Preferential synthesis of beta lactoglobulin by the bound polyribosomes of the mammary gland. Biochem Biophys Res Commun. 1970 Oct 9;41(1):266–272. doi: 10.1016/0006-291x(70)90498-5. [DOI] [PubMed] [Google Scholar]
- Herrington M. D., Hawtrey A. O. Differences in the ribosomes prepared from lactating and non-lactating bovine mammary gland. Biochem J. 1971 Jan;121(2):279–285. doi: 10.1042/bj1210279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington M. D., Hawtrey A. O. Studies on subcellular fractions of non-lactating bovine mamm- ary gland. S Afr J Med Sci. 1969 Jul;34(2):49–58. [PubMed] [Google Scholar]
- Lawford G. R. The effect of incubation with puromycin on the dissociation of rat liver ribosomes into active subunits. Biochem Biophys Res Commun. 1969 Sep 24;37(1):143–150. doi: 10.1016/0006-291x(69)90892-4. [DOI] [PubMed] [Google Scholar]
- Martin T. E., Wool I. G. Active hybrid 80 s particles formed from subunits of rat, rabbit and protozoan (Tetrahymena pyriformis) ribosomes. J Mol Biol. 1969 Jul 14;43(1):151–161. doi: 10.1016/0022-2836(69)90085-0. [DOI] [PubMed] [Google Scholar]
- Matthaei J. H., Schoech G. K. Human gene expression. I. An aminoacyl-RNA binding system from human placenta. Biochem Biophys Res Commun. 1967 Jun 23;27(6):638–643. doi: 10.1016/s0006-291x(67)80082-2. [DOI] [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel M., Lelong J. C., Brawerman G., Gros F. Function of three protein factors and ribosomal subunits in the initiation of protein synthesis in E. coli. Nature. 1968 Sep 7;219(5158):1016–1021. doi: 10.1038/2191016a0. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Riddle M. Hormone-dependent formation of polysomes in mammary cells in vitro. J Biol Chem. 1970 Oct 10;245(19):5145–5152. [PubMed] [Google Scholar]
- Vanaman T. C., Wakil S. J., Hill R. L. The preparation of tryptic, peptic, thermolysin, and cyanogen bromide peptides from the acyl carrier protein of Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6409–6419. [PubMed] [Google Scholar]
- WETTSTEIN F. O., STAEHELIN T., NOLL H. Ribosomal aggregate engaged in protein synthesis: characterization of the ergosome. Nature. 1963 Feb 2;197:430–435. doi: 10.1038/197430a0. [DOI] [PubMed] [Google Scholar]
- Williams D. J., Gurari D., Rabin B. R. The effects of ribosomes on the activity of a membrane bound enzyme catalysing thiol-disulphide interchange. FEBS Lett. 1968 Dec;2(2):133–135. doi: 10.1016/0014-5793(68)80123-1. [DOI] [PubMed] [Google Scholar]
- von der Decke A., Ashby P., McIlreavy D., Campbell P. N. The reverible dissociation and activity ofribosoma subunits of liver and skeletal muscle from rats. Biochem J. 1970 Dec;120(4):815–819. doi: 10.1042/bj1200815. [DOI] [PMC free article] [PubMed] [Google Scholar]


