Abstract
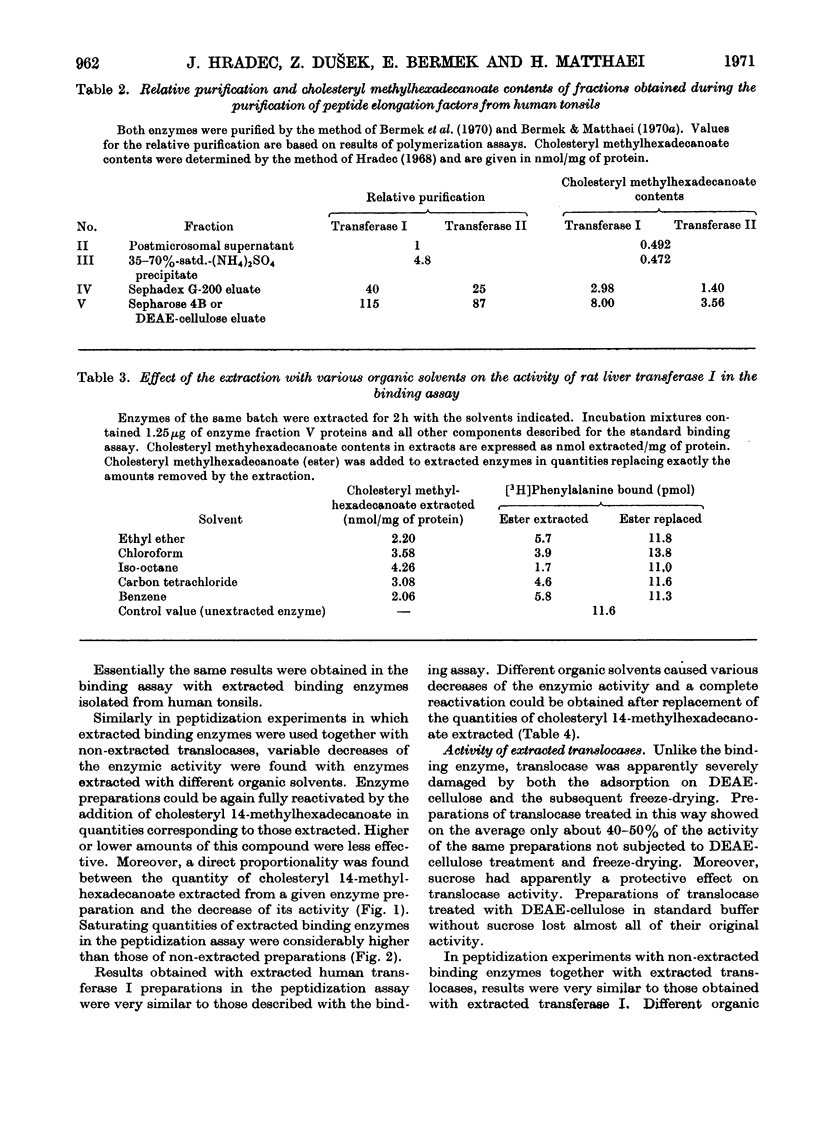
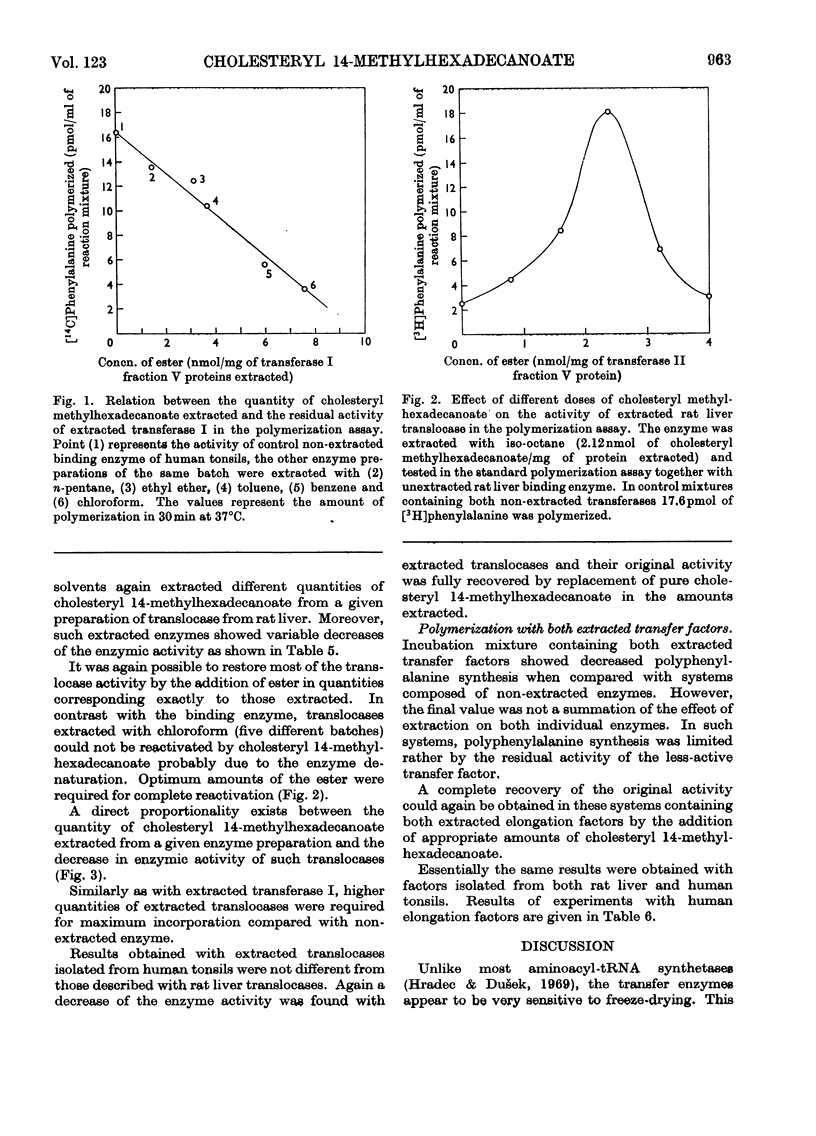
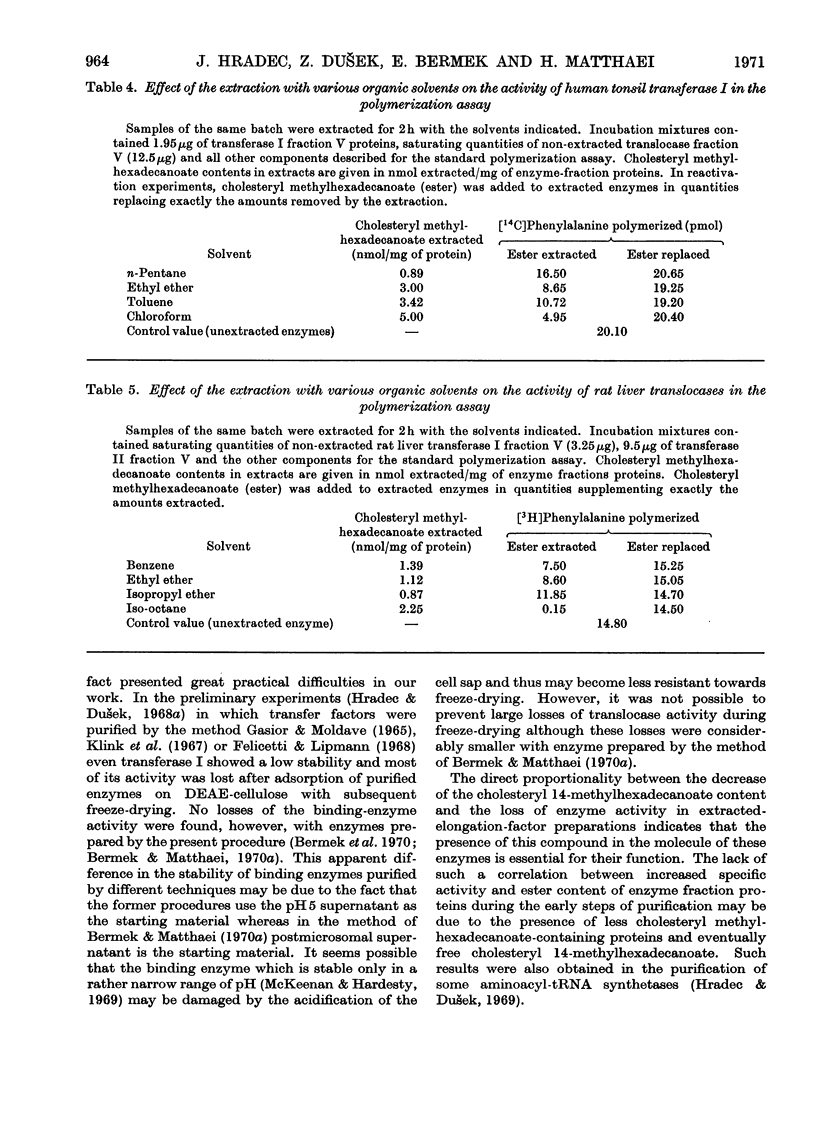
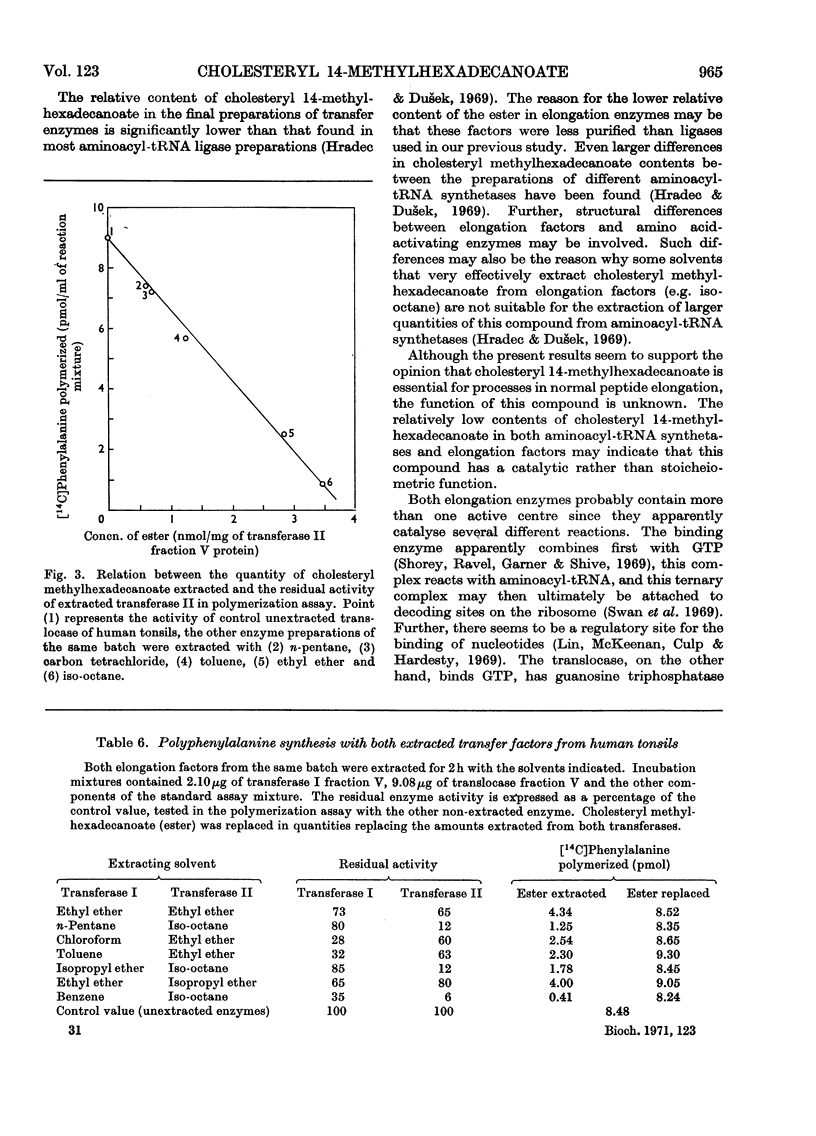
1. Peptide-elongation factors were purified from rat liver and human tonsils and the contents of cholesteryl 14-methylhexadecanoate were determined in fractions obtained during enzyme purification. The relative contents of this compound in purified enzyme preparations was several times higher than that in the crude starting material. Elongation factors from human tonsils contained a significantly larger quantity of the cholesteryl ester than enzyme from rat liver. 2. Transfer enzymes extracted with various organic solvents showed variable decreased activities in both binding and peptidization assay. The decrease of enzymic activity was proportional to the amount of cholesteryl 14-methylhexadecanoate extracted from a given enzymic preparation. In systems containing both extracted elongation factors the polyphenylalanine synthesis was limited by the residual activity of the less active transfer factor. 3. The original enzymic activity of extracted transferases was fully recovered by the addition of pure cholesteryl 14-methylhexadecanoate in quantities corresponding to those extracted. 4. Increase of the relative contents of this cholesteryl ester during enzyme purification, decrease of the enzymic activity after the extraction and its recovery by the addition of this compound indicates that the presence of this ester in elongation factors is essential for the normal function of these enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermek E., Krämer W., Mönkemeyer H., Matthaei H. Mechanisms in protein synthesis. XII. Sites of action of showdomycin in a cell-free polyphenylalanine synthesizing system from human lymphatic tissue: ribosomes and elongation factor TF II. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1311–1318. doi: 10.1016/0006-291x(70)90009-4. [DOI] [PubMed] [Google Scholar]
- Bermek E., Matthaei H. Elongation factors from human lymphatic tissue: Isolation and some properties. FEBS Lett. 1970 Sep 24;10(2):121–124. doi: 10.1016/0014-5793(70)80431-8. [DOI] [PubMed] [Google Scholar]
- Bermek E., Matthaei H. Human protein synthesis. V. The effect of antibiotics on an optimized polyphenylalanine synthesizing cell-free system from human lymphatic tissue. Hoppe Seylers Z Physiol Chem. 1970 Nov;351(11):1377–1383. doi: 10.1515/bchm2.1970.351.2.1377. [DOI] [PubMed] [Google Scholar]
- Felicetti L., Lipmann F. Comparison of amino acid polymerization factors isolated from rat liver and rabbit reticulocytes. Arch Biochem Biophys. 1968 May;125(2):548–557. doi: 10.1016/0003-9861(68)90613-9. [DOI] [PubMed] [Google Scholar]
- GASIOR E., MOLDAVE K. RESOLUTION OF AMINOACYL-TRANSFERRING ENZYMES FROM RAT LIVER BY MOLECULAR SIEVE CHROMATOGRAPHY. J Biol Chem. 1965 Aug;240:3346–3352. [PubMed] [Google Scholar]
- Hradec J. A chromatographic method for the quantitative determination of cholesterol 14-methylhexadecanoate (carcinolipin) in biological materials. J Chromatogr. 1968 Feb 6;32(3):511–518. doi: 10.1016/s0021-9673(01)80523-9. [DOI] [PubMed] [Google Scholar]
- Hradec J., Dolejs L. The chemical constitution of carcinolipin. Biochem J. 1968 Mar;107(2):129–134. doi: 10.1042/bj1070129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradec J., Dusek Z. Effect of cholesteryl 14-methylhexadecanoate on the activity of some amino acid-transfer ribonucleic acid ligases from mammalian tissues. Biochem J. 1969 Dec;115(5):873–880. doi: 10.1042/bj1150873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hradec J., Dusek Z. Effect of lipids, in particular cholesteryl 14-methylhexadecanoate, on the incorporation of labelled amino acids into transfer ribonucleic acid in vitro. Biochem J. 1968 Nov;110(1):1–8. doi: 10.1042/bj1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki F., Moldave K. Evidence for the enzymatic binding of aminoacyl transfer ribonucleic acid to rat liver ribosomes. J Biol Chem. 1968 Feb 25;243(4):791–798. [PubMed] [Google Scholar]
- Lin S. Y., McKeehan W. L., Culp W., Hardesty B. Partial characterization of the enzymatic properties of the aminoacyl transfer ribonucleic acid binding enzyme. J Biol Chem. 1969 Aug 25;244(16):4340–4350. [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthaei H., Sander G., Swan D., Kreuzer T., Caffier H., Parmeggiani A. Reaktionsschritte der Polypeptidsynthese an Ribosomen. Mechanismen der Proteinsynthese X. Naturwissenschaften. 1968 Jun;55(6):281–294. doi: 10.1007/BF00591706. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Hardesty B. Purification and partial characterization of the aminoacyl transfer ribonucleic acid binding enzyme from rabbit reticulocytes. J Biol Chem. 1969 Aug 25;244(16):4330–4339. [PubMed] [Google Scholar]
- Moldave K., Galasinski W., Rao P., Siler J. Studies on the peptidyl tRNA translocase from rat liver. Cold Spring Harb Symp Quant Biol. 1969;34:347–356. doi: 10.1101/sqb.1969.034.01.041. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmimoff H., Arnstein H. R. The initiation of haemoglobin synthesis in rabbit reticulocytes. Biochem J. 1969 Oct;115(1):113–124. doi: 10.1042/bj1150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorey R. L., Ravel J. M., Garner C. W., Shive W. Formation and properties of the aminoacyl transfer ribonucleic acid-guanosine triphosphate-protein complex. J Biol Chem. 1969 Sep 10;244(17):4555–4564. [PubMed] [Google Scholar]
- Siler J., Moldave K. Studies on the kinetics of peptidyl transfer RNA translocase from rat liver. Biochim Biophys Acta. 1969 Nov 19;195(1):138–144. doi: 10.1016/0005-2787(69)90610-8. [DOI] [PubMed] [Google Scholar]
- Skogerson L., Moldave K. Evidence for the role of aminoacyltransferase II in peptidyl transfer ribonucleic acid translocation. J Biol Chem. 1968 Oct 25;243(20):5361–5367. [PubMed] [Google Scholar]
- Swan D., Sander G., Bermek E., Krämer W., Kreuzer T., Arglebe C., Zöllner R., Eckert K., Mathaei H. On the mechanism of coded binding of aminoacyl-tRNA to ribosomes: number and properties of sites. Cold Spring Harb Symp Quant Biol. 1969;34:179–196. doi: 10.1101/sqb.1969.034.01.025. [DOI] [PubMed] [Google Scholar]
- Vazquez D., Battaner E., Neth R., Heller G., Monro R. E. The function of 80 S ribosomal subunits and effects of some antibiotics. Cold Spring Harb Symp Quant Biol. 1969;34:369–375. doi: 10.1101/sqb.1969.034.01.043. [DOI] [PubMed] [Google Scholar]


