Abstract
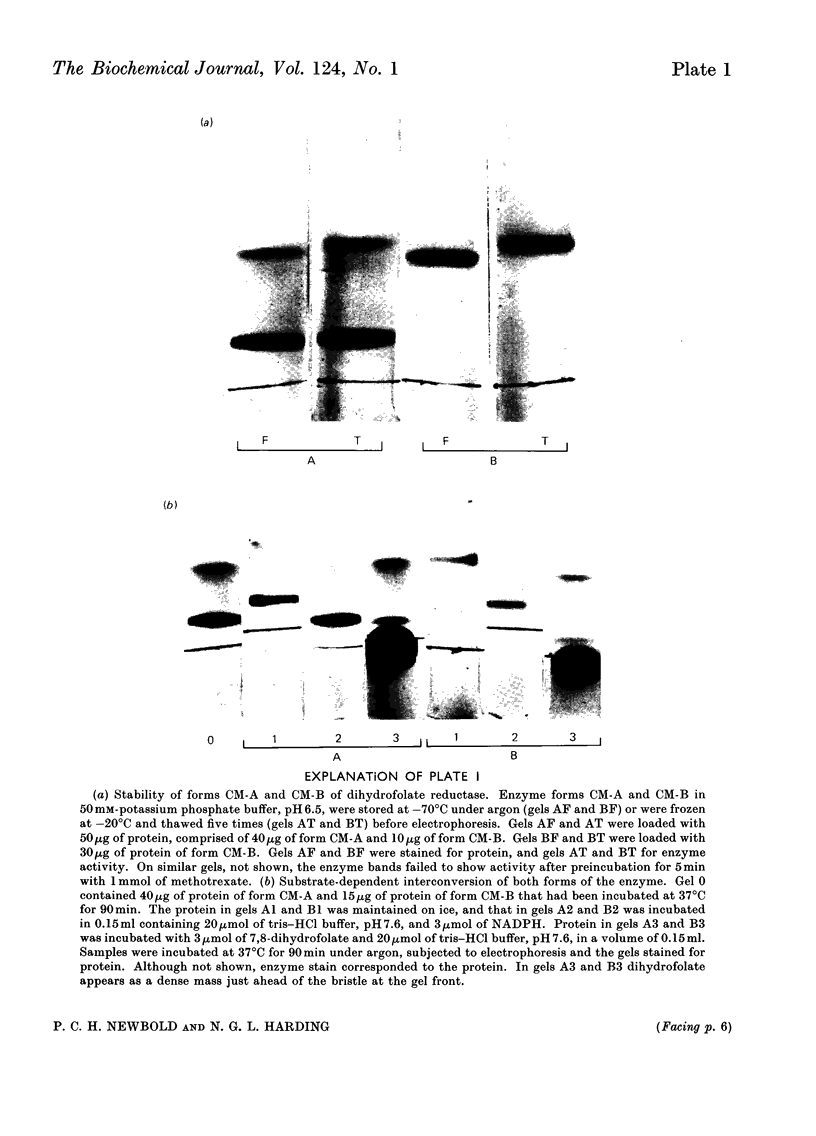
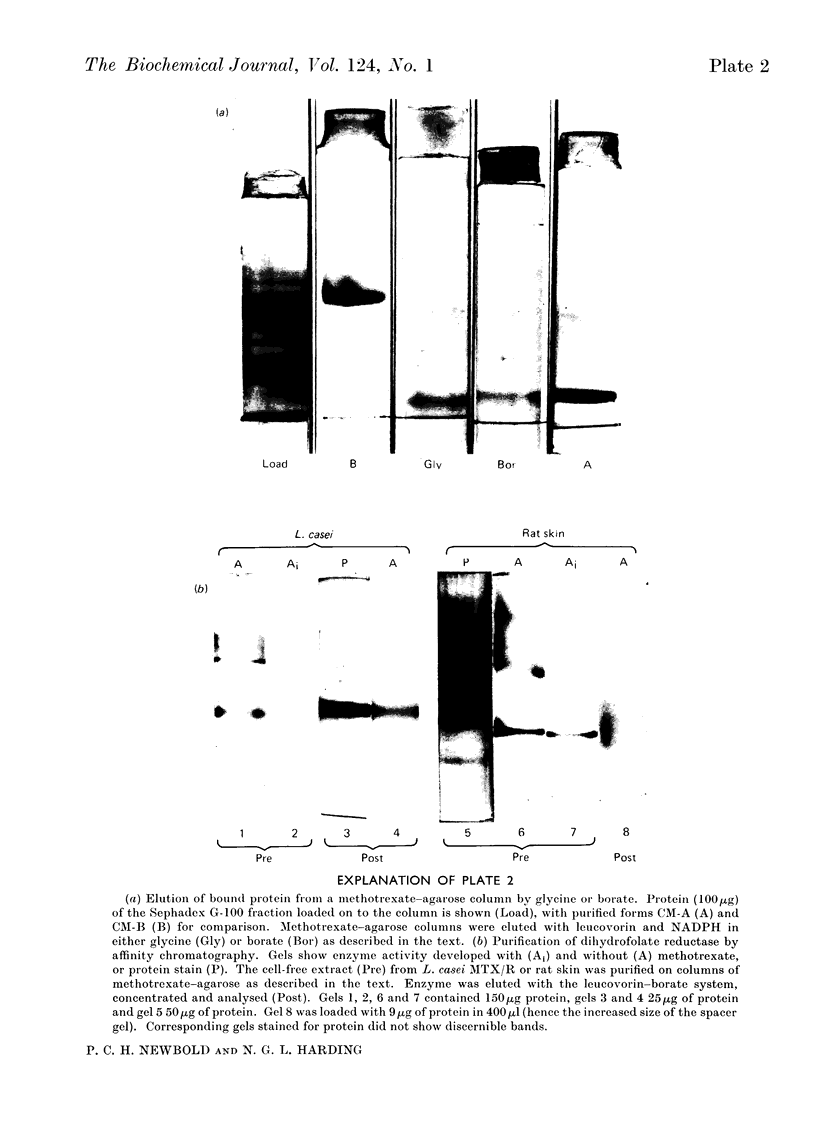
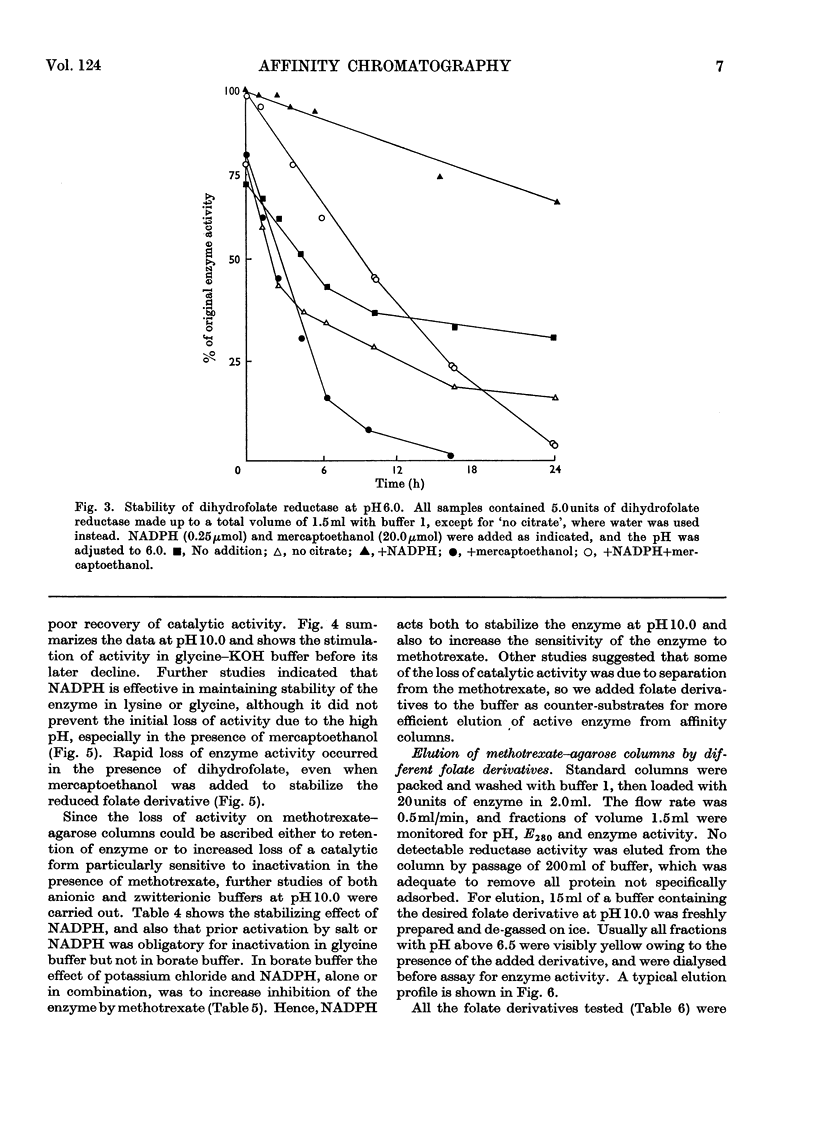
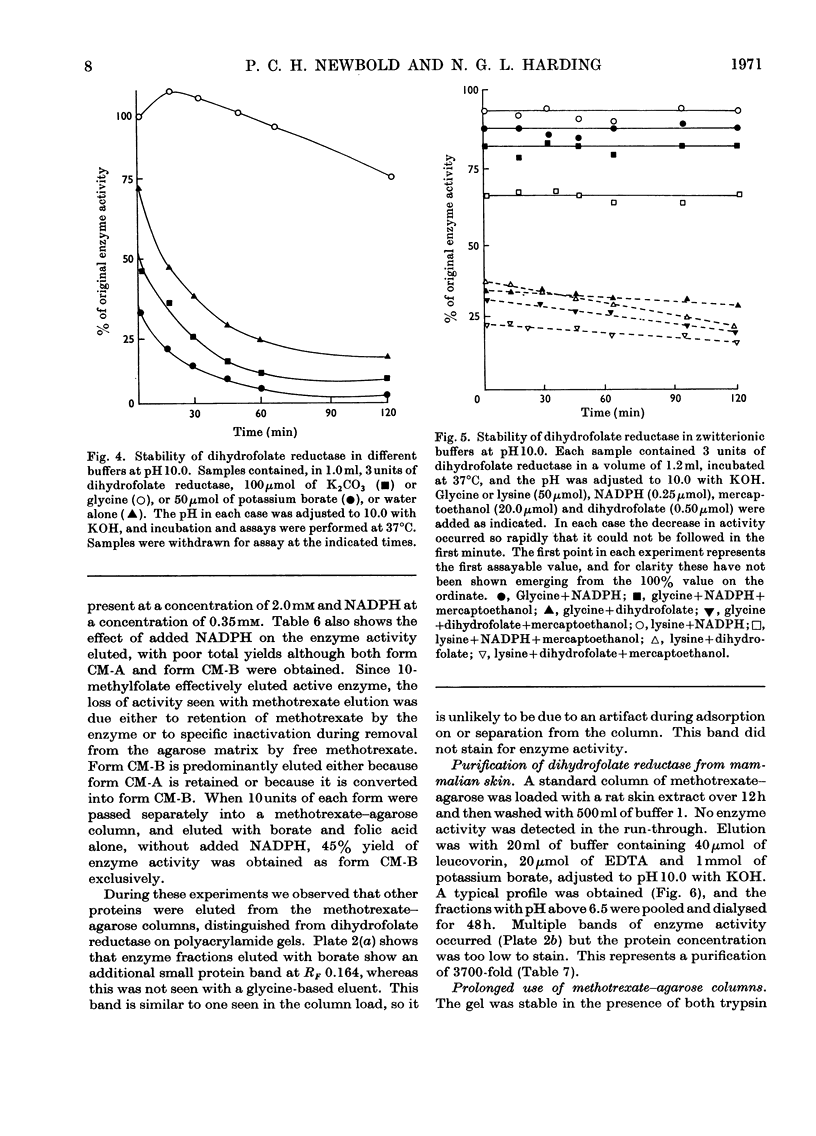
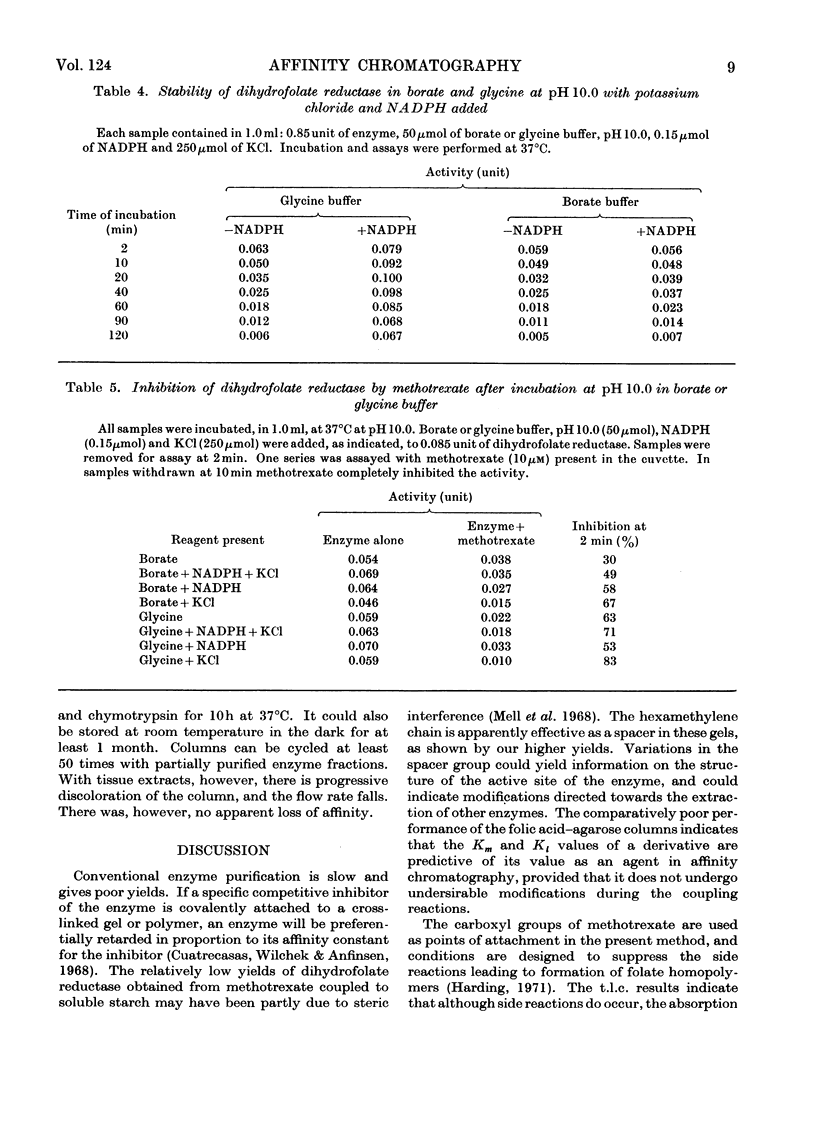
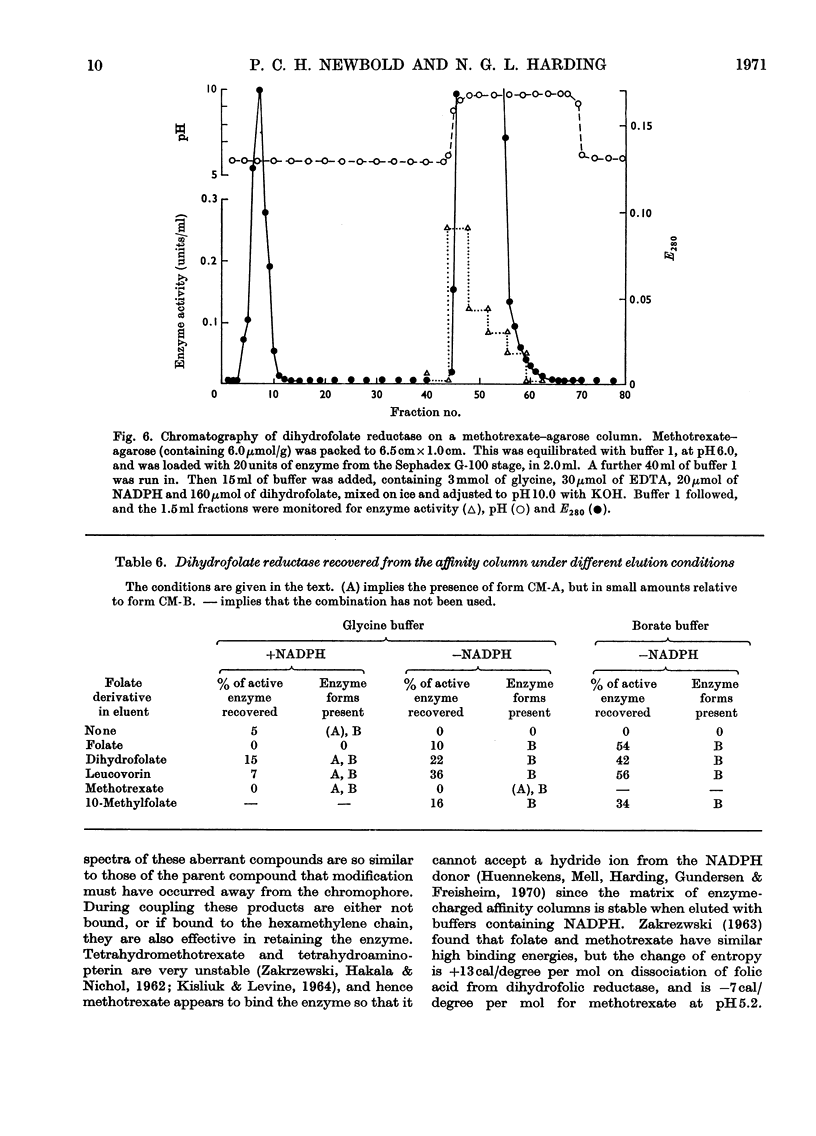
1. Dihydrofolate reductase was purified from Lactobacillus casei MTX/R, and studied on affinity columns containing folic acid and methotrexate. Two forms of the enzyme were interconverted by incubation with substrates. 2. Affinity columns were prepared from agarose activated with cyanogen bromide and coupled with 1,6-diaminohexane. Stable folate derivatives were covalently attached by using a carbodi-imide condensation. 3. Columns containing folic acid retarded but did not retain the enzyme. 4. Methotrexate at pH 6.0 was particularly effective for retention of the enzyme. 5. There is selective loss of one form of the enzyme during affinity chromatography in the absence of added NADPH. This loss is due to conversion into a single enzyme form on the column. 6. NADPH has a dual effect in stabilizing the enzyme and in sensitizing it to inactivation by methotrexate, particularly in the presence of glycine. 7. Protein with affinity for methotrexate, but without dihydrofolate reductase activity, may also be eluted from the columns. 8. In a single-step procedure the enzyme was purified nearly 4000-fold from mammalian skin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumenthal G., Greenberg D. M. Evidence for two molecular species of dihydrofolate reductase in amethopterin resistant and sensitive cells of the mouse leukemia L4946. Oncology. 1970;24(3):223–229. doi: 10.1159/000224522. [DOI] [PubMed] [Google Scholar]
- Borsa J., Whitmore G. F. Studies relating to the mode of action of methotrexate. II. Studies on sites of action in L-cells in vitro. Mol Pharmacol. 1969 Jul;5(4):303–317. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal R. Graphic methods for determination of Km and Ki values for dihydrofolate reductase. Anal Biochem. 1970 Jun;35(2):346–350. doi: 10.1016/0003-2697(70)90194-6. [DOI] [PubMed] [Google Scholar]
- Grignani F., Martelli M. F., Tonato M., Finzi A. F. Folate-dependent enzymes in human epidermis. Tetrahydrofolate-dependent enzymes and relationship between dihydrofolic reductase and amethopterin. Arch Dermatol. 1967 Nov;96(5):577–585. [PubMed] [Google Scholar]
- Harding N. G., Martelli M. F., Huennekens F. M. Amethopterin-induced changes in the multiple forms of dihydrofolate reductase from L1210 cells. Arch Biochem Biophys. 1970 Mar;137(1):295–296. doi: 10.1016/0003-9861(70)90440-6. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Murphy J. B., Hartman F. C. Isoenzymes of Drosophila alcohol dehydrogenase. I. Isolation and interconversion of different forms. J Biol Chem. 1970 Mar 10;245(5):1075–1083. [PubMed] [Google Scholar]
- KISLIUK R. L., LEVINE M. D. PROPERTIES OF REDUCED DERIVATIVES OF AMINOPTERIN. J Biol Chem. 1964 Jun;239:1900–1904. [PubMed] [Google Scholar]
- Kaufman B. T., Gardiner R. C. Studies on dihydrofolic reductase. I. Purification and properties of dihydrofolic reductase from chicken liver. J Biol Chem. 1966 Mar 25;241(6):1319–1328. [PubMed] [Google Scholar]
- Kessel D., Roberts D. Dihydrofolate reductase from Lactobacillus leichmannii. I. Purification and characterization. Biochemistry. 1965 Dec;4(12):2631–2636. doi: 10.1021/bi00888a012. [DOI] [PubMed] [Google Scholar]
- MATHEWS C. K., HUENNEKENS F. M. FURTHER STUDIES ON DIHYDROFOLIC REDUCTASE. J Biol Chem. 1963 Oct;238:3436–3442. [PubMed] [Google Scholar]
- Mathews C. K. Evidence that bacteriophage-induced dihydrofolate reductase in a viral gene product. J Biol Chem. 1967 Sep 25;242(18):4083–4086. [PubMed] [Google Scholar]
- Mell G. P., Whiteley J. M., Huennekens F. M. Purification of dihydrofolate reductase via amethopterin-aminoethyl starch. J Biol Chem. 1968 Nov 25;243(22):6074–6075. [PubMed] [Google Scholar]
- Nixon P. F., Blakley R. L. Dihydrofolate reductase of Streptococcus faicium. II. Purification and some properties of two dihydrofolate reductases from the amethopterin-resistant mutant Streptococcus faecium var. Durans strain A. J Biol Chem. 1968 Sep 25;243(18):4722–4731. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Perkins J. P., Hillcoat B. L., Bertino J. R. Dihydrofolate reductase from a resistant subline of the L1210 lymphoma. Purification and properties. J Biol Chem. 1967 Oct 25;242(20):4771–4776. [PubMed] [Google Scholar]
- Porath J., Axen R., Ernback S. Chemical coupling of proteins to agarose. Nature. 1967 Sep 30;215(5109):1491–1492. doi: 10.1038/2151491a0. [DOI] [PubMed] [Google Scholar]
- Rees R. B., Bennett J. H., Maibach H. I., Arnold H. L. Methotrexate for psoriasis. Arch Dermatol. 1967 Jan;95(1):2–11. [PubMed] [Google Scholar]
- Roberts D., Wodinsky I. On the poor correlation between the inhibition by methotrexate of dihydrofolate reductase and of deoxynucleoside incorporation into DNA. Cancer Res. 1968 Oct;28(10):1955–1962. [PubMed] [Google Scholar]
- SCHRECKER A. W., HUENNEKENS F. M. FURTHER OBSERVATIONS OF THE BINDING AND INHIBITION OF DIHYDROFOLIC REDUCTASE BY FOLIC ACID ANTAGONISTS. Biochem Pharmacol. 1964 May;13:731–742. doi: 10.1016/0006-2952(64)90009-7. [DOI] [PubMed] [Google Scholar]
- WERKHEISER W. C., ZAKRZEWSKI S. F., NICHOL C. A. Assay for 4-amino folic acid analogues by inhibition of folic acid reductase. J Pharmacol Exp Ther. 1962 Aug;137:162–166. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- ZAKRZEWSKI S. F. The mechanism of binding of folate analogues by folate reductase. J Biol Chem. 1963 Apr;238:1485–1490. [PubMed] [Google Scholar]