Abstract
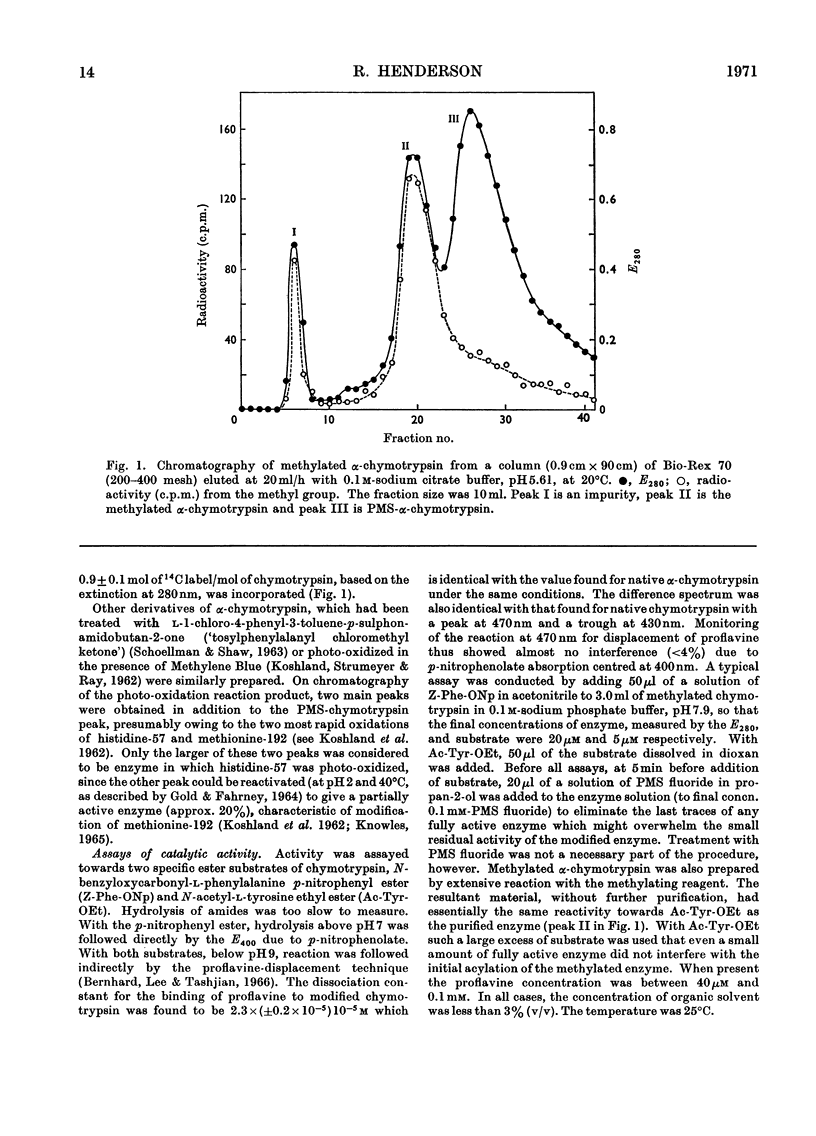
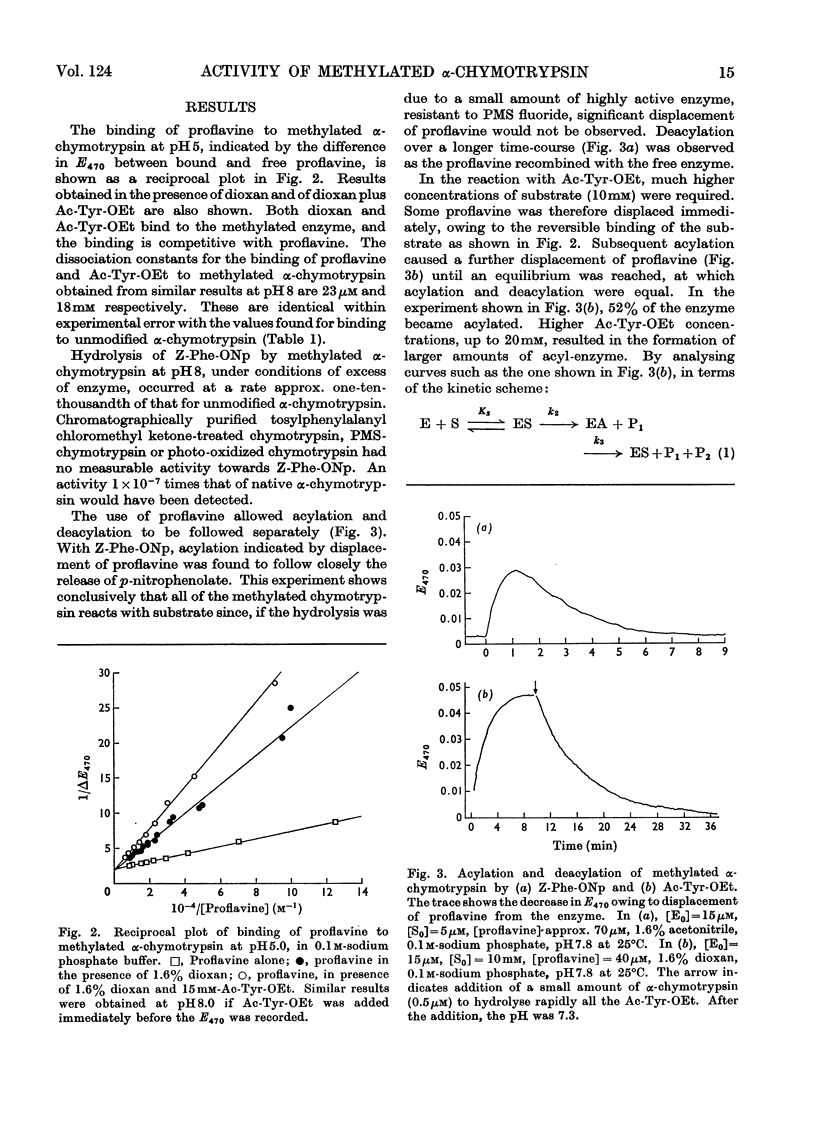
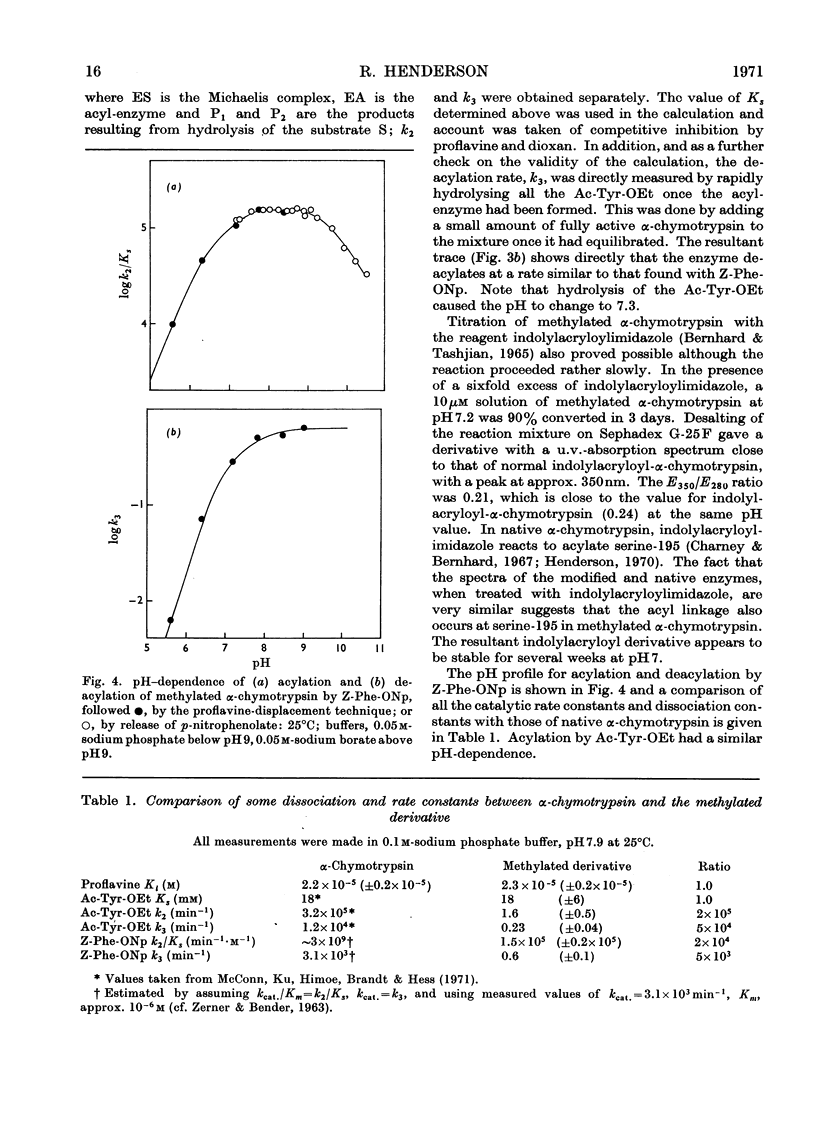
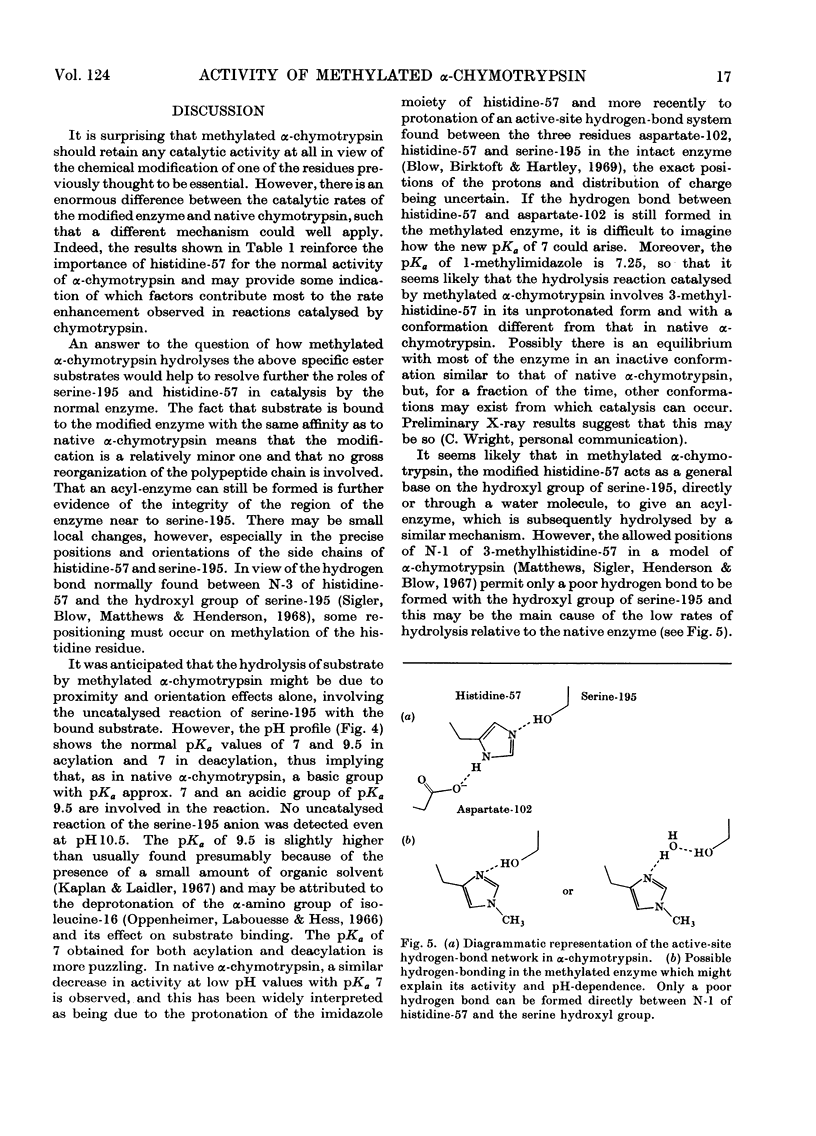
The properties of a derivative of α-chymotrypsin in which histidine-57 has been methylated have been examined. Although the modified enzyme binds substrate with the same affinity as does native α-chymotrypsin, acylation and deacylation occur at much decreased rates. As for native α-chymotrypsin, a basic group of pKa approx. 7 is involved in both acylation and deacylation. The significance of these results is considered in relation to the normal function of histidine-57.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERHARD S. A., TASHJIAN Z. H. ACYL INTERMEDIATES IN THE ALPHA-CHYMOTRYPSIN-CATALYZED HYDROLYSIS OF INDOLEACRYLOYLIMIDAZOLE. J Am Chem Soc. 1965 Apr 20;87:1806–1807. doi: 10.1021/ja01086a042. [DOI] [PubMed] [Google Scholar]
- Bernhard S. A., Lee B. F., Tashjian Z. H. On the interaction of the active side of alpha-chymotrypsin with chromophores: proflavin binding and enzyme conformation during catalysis. J Mol Biol. 1966 Jul;18(3):405–420. doi: 10.1016/s0022-2836(66)80033-5. [DOI] [PubMed] [Google Scholar]
- Blow D. M., Birktoft J. J., Hartley B. S. Role of a buried acid group in the mechanism of action of chymotrypsin. Nature. 1969 Jan 25;221(5178):337–340. doi: 10.1038/221337a0. [DOI] [PubMed] [Google Scholar]
- Charney E., Bernhard S. A. Optical properties and the chemical nature of acyl-chymotrypsin linkages. J Am Chem Soc. 1967 May 24;89(11):2726–2733. doi: 10.1021/ja00987a040. [DOI] [PubMed] [Google Scholar]
- GOLD A. M., FAHRNEY D. SULFONYL FLUORIDES AS INHIBITORS OF ESTERASES. II. FORMATION AND REACTIONS OF PHENYLMETHANESULFONYL ALPHA-CHYMOTRYPSIN. Biochemistry. 1964 Jun;3:783–791. doi: 10.1021/bi00894a009. [DOI] [PubMed] [Google Scholar]
- KNOWLES J. R. THE ROLE OF METHIONINE IN ALPHA-CHYMOTRYPSIN-CATALYSED REACTIONS. Biochem J. 1965 Apr;95:180–190. doi: 10.1042/bj0950180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, STRUMEYER D. H., RAY W. J., Jr Amino acids involved in the action of chymotrypsin. Brookhaven Symp Biol. 1962 Dec;15:101–133. [PubMed] [Google Scholar]
- Matthews B. W., Sigler P. B., Henderson R., Blow D. M. Three-dimensional structure of tosyl-alpha-chymotrypsin. Nature. 1967 May 13;214(5089):652–656. doi: 10.1038/214652a0. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Bender M. L. Methylation of histidine-57 in alpha-chymotrypsin by methyl p-nitrobenzenesulfonate. A new approach to enzyme modification. Biochemistry. 1970 Jan 20;9(2):259–267. doi: 10.1021/bi00804a011. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Bender M. L. Modification of alpha-chymotrypsin by methyl p-nitrobenzenesulfonate. J Am Chem Soc. 1969 Mar 12;91(6):1566–1567. doi: 10.1021/ja01034a068. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H. L., Labouesse B., Hess G. P. Implication of an ionizing group in the control of conformation and activity of chymotrypsin. J Biol Chem. 1966 Jun 10;241(11):2720–2730. [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]


