Abstract
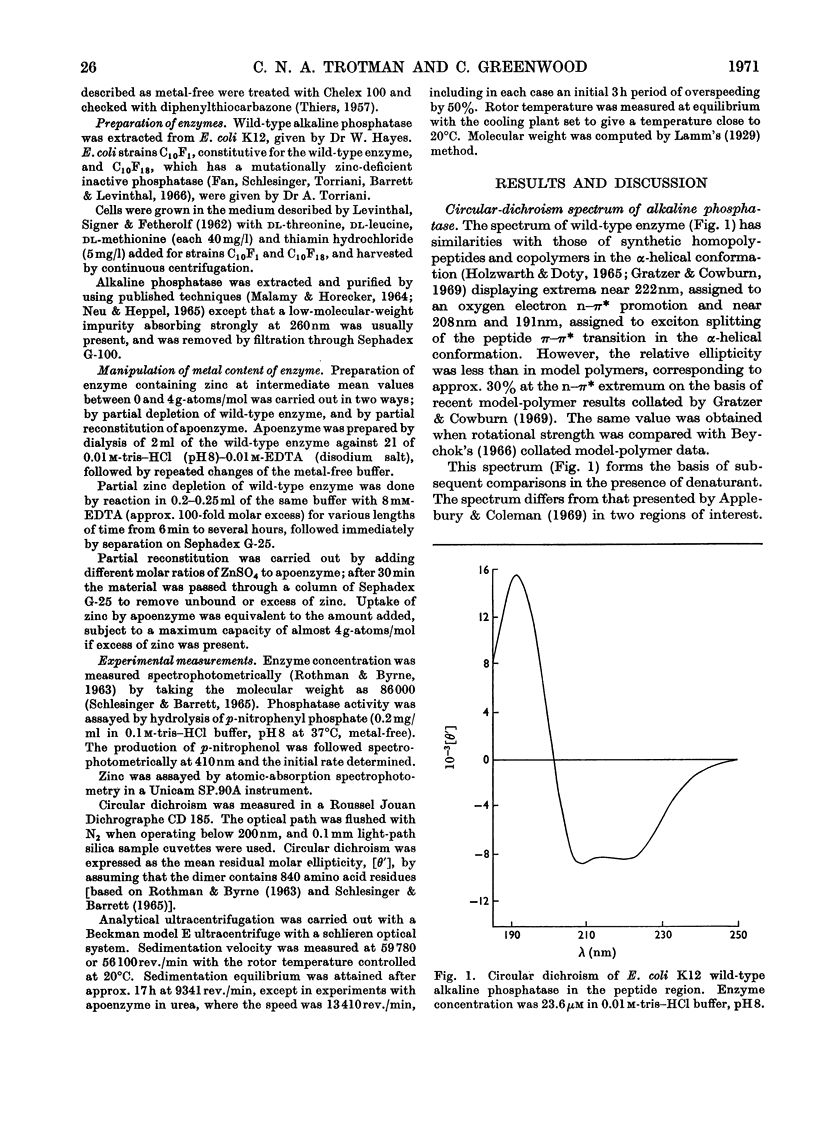
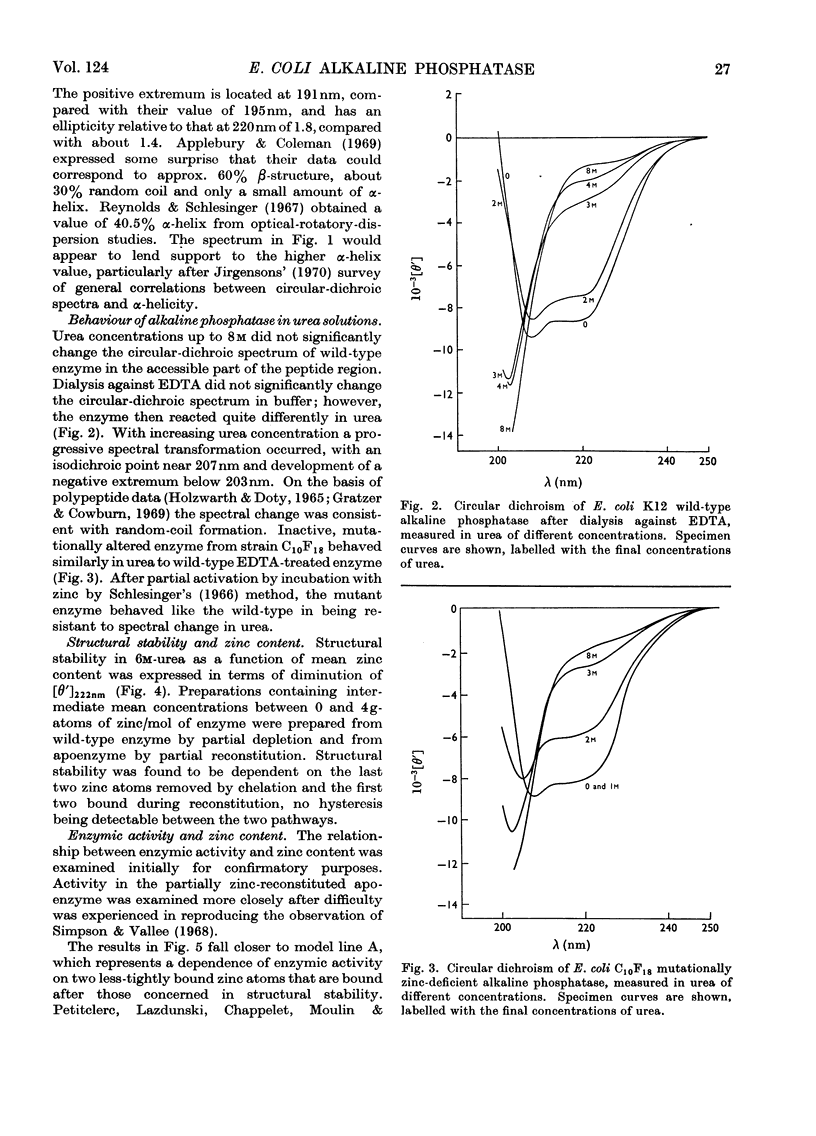
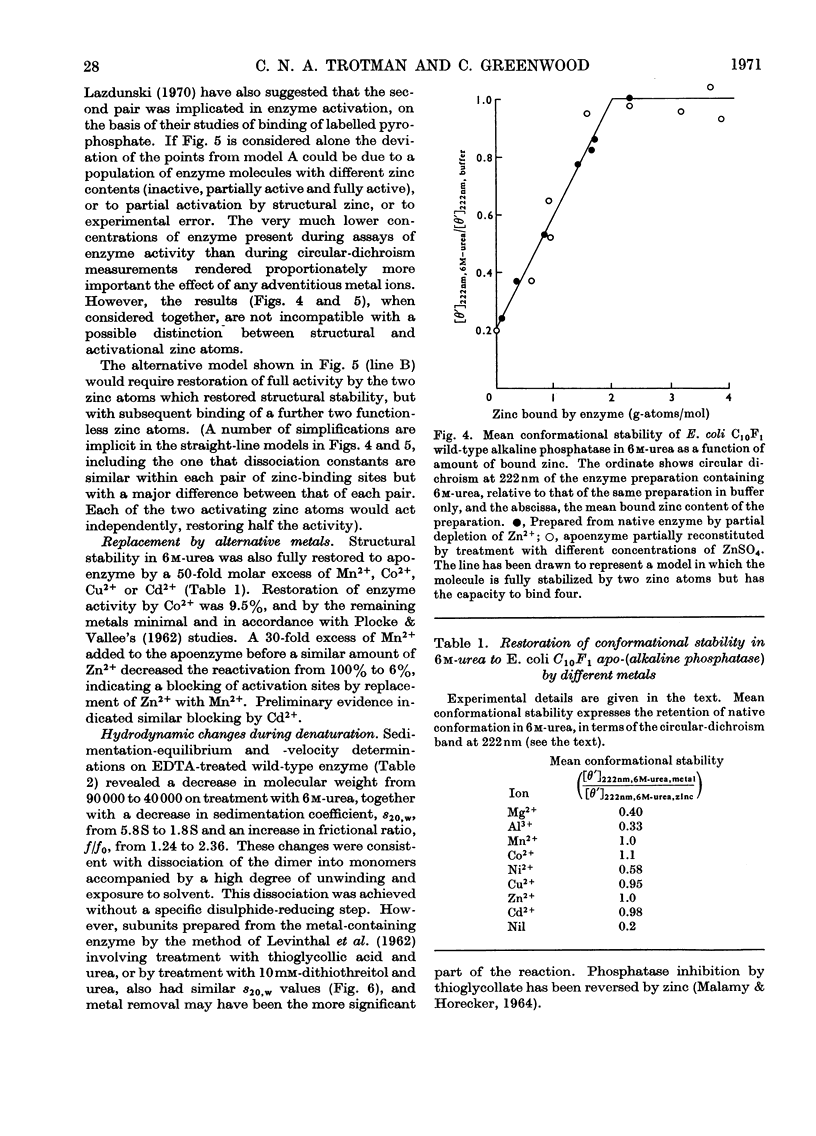
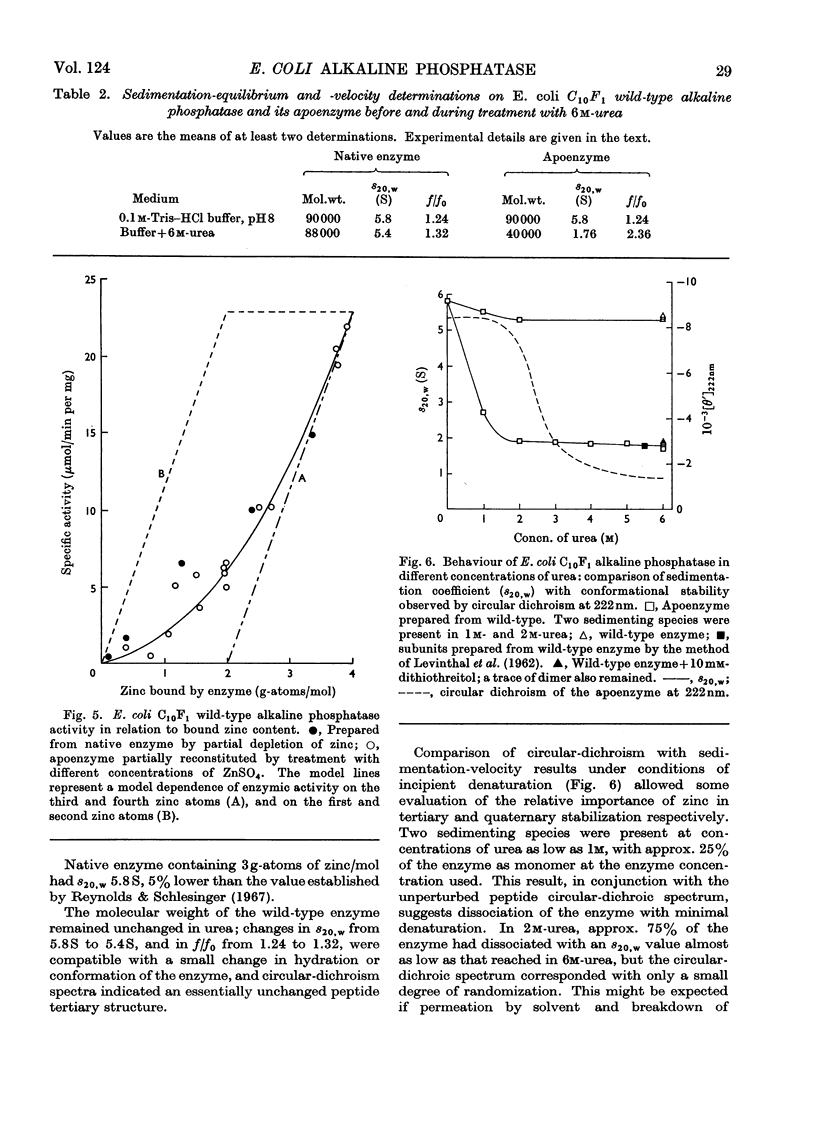
Measurement of the ultraviolet circular dichroism of apo-(alkaline phosphatase) in urea solutions showed substantial denaturation in 3m-urea. A zinc-deficient mutant alkaline phosphatase behaved similarly. The stability of the enzyme in 6m-urea was followed as a function of its zinc content and was found to be dependent on the first two of the four zinc atoms bound by apoenzyme. Phosphatase activity was mostly dependent on a second pair of zinc atoms. Mn2+, Co2+, Cu2+ or Cd2+ also restored structural stability. Sedimentation-velocity and -equilibrium experiments revealed that dissociation of the dimer accompanied apoenzyme denaturation in urea concentrations of 1m or higher, without treatment with disulphide-reducing agent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Coleman J. E. Escherichia coli alkaline phosphatase. Metal binding, protein conformation, and quaternary structure. J Biol Chem. 1969 Jan 25;244(2):308–318. [PubMed] [Google Scholar]
- Beychok S. Circular dichroism of biological macromolecules. Science. 1966 Dec 9;154(3754):1288–1299. doi: 10.1126/science.154.3754.1288. [DOI] [PubMed] [Google Scholar]
- Cohen S. R., Wilson I. B. Measurement of the zinc dissociation constants of alkaline phosphatase from Escherichia coli by equilibration with zinc ion buffers. Biochemistry. 1966 Mar;5(3):904–909. doi: 10.1021/bi00867a014. [DOI] [PubMed] [Google Scholar]
- Csopak H., Falk K. E. The specific binding of copper(II) to alkaline phosphatase of E. coli. FEBS Lett. 1970 Apr 2;7(2):147–150. doi: 10.1016/0014-5793(70)80142-9. [DOI] [PubMed] [Google Scholar]
- Csopak H. The specific binding of zinc(II) to alkaline phosphatase of Escherichia coli. Eur J Biochem. 1969 Jan;7(2):186–192. doi: 10.1111/j.1432-1033.1969.tb19590.x. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., Cowburn D. A. Optical activity of biopolymers. Nature. 1969 May 3;222(5192):426–431. doi: 10.1038/222426a0. [DOI] [PubMed] [Google Scholar]
- HOLZWARTH G., DOTY P. THE ULTRAVIOLET CIRCULAR DICHROISM OF POLYPEPTIDES. J Am Chem Soc. 1965 Jan 20;87:218–228. doi: 10.1021/ja01080a015. [DOI] [PubMed] [Google Scholar]
- Harris M. I., Coleman J. E. The biosynthesis of apo- and metalloalkaline phosphatases of Escherichia coli. J Biol Chem. 1968 Oct 10;243(19):5063–5073. [PubMed] [Google Scholar]
- Jirgensons B. Circular dichroism of proteins of known and unknown conformations. Biochim Biophys Acta. 1970 Jan 20;200(1):9–17. doi: 10.1016/0005-2795(70)90037-1. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C., SIGNER E. R., FETHEROLF K. Reactivation and hybridization of reduced alkaline phosphatase. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1230–1237. doi: 10.1073/pnas.48.7.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazdunski C., Chappelet D., Petitclerc C., Leterrier F., Douzou P., Lazdunski M. The Cu2 plus-alkaline phosphatase of Escherichia coli. Eur J Biochem. 1970 Dec;17(2):239–245. doi: 10.1111/j.1432-1033.1970.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Lazdunski C., Petitclerc C., Lazdunski M. Structure-function relationships for some metalloalkaline phosphatases of E. coli. Eur J Biochem. 1969 Apr;8(4):510–517. doi: 10.1111/j.1432-1033.1969.tb00556.x. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. PURIFICATION AND CRYSTALLIZATION OF THE ALKALINE PHOSPHATASE OF ESCHERICHIA COLI. Biochemistry. 1964 Dec;3:1893–1897. doi: 10.1021/bi00900a018. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- PLOCKE D. J., LEVINTHAL C., VALLEE B. L. Alkaline phosphatase of Escherichia coli: a zinc metalloenzyme. Biochemistry. 1962 May 25;1:373–378. doi: 10.1021/bi00909a001. [DOI] [PubMed] [Google Scholar]
- PLOCKE D. J., VALLEE B. L. Interaction of alkaline phosphatase of E. coli with metal ions and chelating agents. Biochemistry. 1962 Nov;1:1039–1043. doi: 10.1021/bi00912a014. [DOI] [PubMed] [Google Scholar]
- Petitclerc C., Lazdunski C., Chappelet D., Moulin A., Lazdunski M. The functional properties of the Zn2(plus)-and Co2(plus)-alkaline phosphatases of Escherichia coli. Labelling of the active site with pyrophosphate, complex formation with arsenate, and reinvestigation of the role of the zinc atoms. Eur J Biochem. 1970 Jun;14(2):301–308. doi: 10.1111/j.1432-1033.1970.tb00290.x. [DOI] [PubMed] [Google Scholar]
- ROTHMAN F., BYRNE R. Fingerprint analysis of alkaline phosphatase of Escherichia coli K12. J Mol Biol. 1963 Apr;6:330–340. doi: 10.1016/s0022-2836(63)80092-3. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Conformational states of the subunit of Escherichia coli alkaline phosphatase. Biochemistry. 1967 Nov;6(11):3552–3559. doi: 10.1021/bi00863a029. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Schlesinger M. J. Hydrogen ion equilibria of conformational states of Escherichia coli alkaline phosphatase. Biochemistry. 1968 Jun;7(6):2080–2085. doi: 10.1021/bi00846a009. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J. Activation of a mutationally altered form of the Escherichia coli alkaline phosphatase by zinc. J Biol Chem. 1966 Jul 10;241(13):3181–3188. [PubMed] [Google Scholar]
- Schlesinger M. J., Barrett K. The reversible dissociation of the alkaline phosphatase of Escherichia coli. I. Formation and reactivation of subunits. J Biol Chem. 1965 Nov;240(11):4284–4292. [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L., Tait G. H. Alkaline phosphatase of Escherichia coli. Composition. Biochemistry. 1968 Dec;7(12):4336–4342. doi: 10.1021/bi00852a028. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Vallee B. L. Two differentiable classes of metal atoms in alkaline phosphatase of Escherichia coli. Biochemistry. 1968 Dec;7(12):4343–4350. doi: 10.1021/bi00852a029. [DOI] [PubMed] [Google Scholar]
- THIERS R. E. Contamination in trace element analysis and its control. Methods Biochem Anal. 1957;5:273–335. doi: 10.1002/9780470110218.ch6. [DOI] [PubMed] [Google Scholar]
- Trotman C. N., Greenwood C. Structural and activational zinc in Escherichia coli alkaline phosphatase. Biochem J. 1971 Jan;121(1):12P–12P. doi: 10.1042/bj1210012pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotman C. N., Greenwood C. Zinc-dependent conformational stability in the alkaline phosphatase of Escherichia coli. Biochem J. 1969 Oct;114(4):82P–83P. doi: 10.1042/bj1140082pb. [DOI] [PMC free article] [PubMed] [Google Scholar]


