Abstract
Objective
Neonatal sepsis, a severe infectious disease associated with high mortality rates, is characterized by metabolic disturbances that play a crucial role in its progression. The aim of this study is to develop a metabolism-related model for assessing 30-day mortality in neonatal sepsis.
Methods
The clinical data of neonatal sepsis at Ganzhou Women and Children’s Health Care Hospital from January 2019 to December 2022 were retrospectively analyzed. Neonatal sepsis cases were divided into survival and non-survival groups. Multivariate logistic regression analysis was used to identify the independent risk factors for 30-day mortality. A nomogram model was developed based on these risk factors. Internal validation of the model was performed using 10-fold cross-validation. The predictive performance was evaluated through receiver operating characteristic (ROC) curves and calibration curve analyses. Decision curve analysis (DCA) was conducted to evaluate the clinical applicability of the developed model.
Results
The study included a total of 156 cases of neonatal sepsis. Multivariate logistic regression analysis revealed that alanine(ALA), citrulline(CIT)), octadecanoylcarnitine(C18) and methionine(MET) were identified as independent risk factors for 30-day mortality of neonatal sepsis. The ROC curve showed an area under the curve of AUC = 0.866 (95% CI 0.796–0.936, P < 0.05). The calibration curve and DCA indicated excellent performance of the model.
Conclusion
This study establishes a predictive model for neonatal sepsis-associated 30-day mortality, effectively capturing the perturbations in amino acid metabolism and fatty acid oxidation, thereby demonstrating robust predictive capabilities.
Keywords: Amino acids, Acylcarnitines, Mortality, Neonatal sepsis, Prognosis
Background
Sepsis is a leading cause of neonatal mortality, and its early diagnosis poses challenges due to the lack of specificity in clinical manifestations and variable laboratory indicators. Although blood culture serves as the gold standard for neonatal sepsis diagnosis, it is not suitable for early detection due to its long culture time and low positive predictive value. Many researchers have explored reliable biomarkers such as antithrombin III [1], cytokine profiles [2], serum resistin [3], and prognostic nutritional index [4] to assess prognosis. Nonetheless, these biomarkers demonstrate limitations in terms of sensitivity and specificity. Therefore, achieving early detection of neonatal sepsis remains an ongoing challenge.
Amino acid and fatty acid metabolism play a pivotal role in the development and progression of neonatal sepsis [5]. As essential nutrients with unique physiological, biochemical, and immunological functions, amino acids have significant implications for the prognosis of neonatal sepsis. Disorders in amino acid metabolism may have a direct impact on disease prognosis [6]. Acylcarnitine serves as an intermediate product of fatty acid β-oxidation, closely associated with mitochondrial function and energy metabolism. Mitochondrial dysfunction and abnormal energy metabolism are important features of neonatal sepsis [7]. The documented alterations in amino acid and fatty acid metabolism during sepsis provide valuable insights into disease pathogenesis. Several amino acids, such as citrulline, arginine [8], and homocysteine [9], have demonstrated alterations in sepsis. Additionally, two separate studies have shown that alanine and acetylcarnitine are associated with the prognosis of sepsis [10, 11]. Consequently, we monitored variations in amino acids and acylcarnitines in neonatal sepsis to identify reliable biomarkers for disease assessment and mortality prediction.
However, the prediction of mortality risk in neonatal sepsis is limited by the low sensitivity and specificity of a single biomarker. To effectively assess the likelihood of adverse outcomes, clinical models are incorporating multiple independent risk factors associated with disease progression. An increasing array of models is currently being developed to aid in assessing sepsis mortality [12–14]. Nevertheless, there is a lack of available models that utilize metabolic markers such as amino acids and acylcarnitines to predict 30-day mortality risk in neonates. Therefore, this study aims to develop a model that leverages multiple amino acids and acylcarnitines to estimate 30-day mortality in neonatal sepsis.
Method
Study population
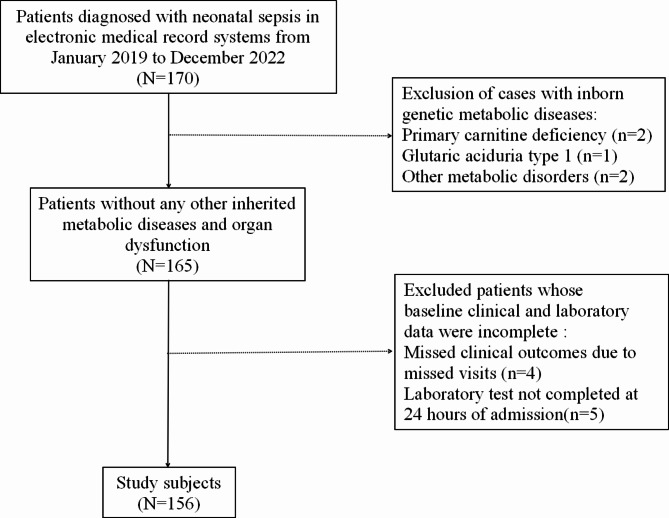
Clinical data and laboratory indicators of neonatal sepsis patients admitted between January 2019 and December 2022 were collected. Admission information was obtained from the medical records. Patient information was complete and within 28 days of age. A total of 156 patients with neonatal sepsis were included in the study, (Fig. 1). The diagnosis of neonatal sepsis was based on the International Consensus on Pediatric Sepsis [15]. Neonatal sepsis was defined as the presence of clinical signs suggestive of sepsis together with one of the following criteria: positive blood culture or detection of pathogenic DNA in blood; two or more positive nonspecific blood tests ( CRP, total white blood cell count, platelet count, immature neutrophil/total neutrophil ratio > 0.2); and cerebrospinal fluid examination for purulent meningitis changes or positive cerebrospinal fluid culture. The exclusion criteria encompassed cases with incomplete clinical data, including but not limited to birth weight, gestational age, laboratory test results, as well as those with other inherited metabolic diseases or organ dysfunction. The study protocol followed the principles set forth in the Declaration of Helsinki, and the study was approved by the Medical Ethics Committee of the Ganzhou Women and Children’s Health Care Hospital(2023-007).
Fig. 1.
The flowchart for selection procedure of neonatal sepsis
Data collection
The following clinical data of neonatal sepsis patients were obtained from the electronic medical records system: sex, age, gestational age, weight, Apgar scores at 5 min and 10 min, and blood culture. Within 24 h after admission, neonatal plantar blood drops were collected and spotted onto dry filter paper blood cards. Blood spots were punched and placed in the holes of microtitre plates using an automated punching machine (Panthera-Puncher™ 9). After nonderivative pretreatment, amino acids and acylcarnitines in the blood spots were detected via tandem mass spectrometry (Waters TQD). A NeoBase Nonderivatized MS/MS Kit manufactured by PerkinElmer was used as a reagent. The analytes included 11 amino acids and 10 acylcarnitines: the 11 amino acids were alanine (Ala), arginine (Arg), citrulline (Cit), glycine (Gly), leucine (Leu), methionine (Met), ornithine (Orn), phenylalanine (Phe), proline (Pro), tyrosine (Tyr), and valine (Val); the 10 acylcarnitines were free carnitine (C0), acetylcarnitine (C2), propionylcarnitine (C3), butyrylcarnitine (C4), isovalerylcarnitine (C5), hexanoylcarnitine (C6), octanoylcarnitine (C8), decanoylcarnitine (C10), tetradecanoylcarnitine (C14), and octadecanoylcarnitine (C18). The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), urea nitrogen (UREA), creatinine (CREA), albumin (ALB) and other biochemical indicators were determined via an automated biochemical analyser (Roche Cobas8000).
Statistical methods
Statistical analysis of the data was conducted using SPSS(Version 25; IBM, Armonk, NY, USA) software. The normality of the data was assessed via the Shapiro-Wilk test, normally distrbuted quantitative variables are expressed as the means ± standard deviations, and nonnormal distrbuted quantitative variables are expressed as medians. Categorical variables are reported in numbers and percentages. Comparisons between the two groups were analysed using t-tests and Mann-Whitney U tests. Univariate and multivariate logistic regression were used to identify independent risk factors for 30-day mortality due to neonatal sepsis. The predictive accuracy of the model was evaluated using receiver operating characteristic (ROC) curves. Model calibration and DCA curves were generated via R software(version 4.4.0). 10-fold cross-validation was used to assess the model’s performance. A p-value of less than 0.05 was considered indicative of a statistically significant difference.
Results
Clinical characteristics of the study population
At the time of enrolment, 156 patients with neonatal sepsis were evaluated. These patients fulfilled all the inclusion criteria. Among these patients, 131 patients were classified as surviving and 25 patients died after 30 days of follow-up, all of whom were did not surviving. The demographic and clinical characteristics of the patients are shown in Table 1. There was no difference in weight, gestational age and Apgar score between the two groups (all p > 0.05). Comparison of amino acid metabolic indices between the two groups revealed that ALA was greater in the nonsurvival group than in the survival group (p < 0.05), and ARG, CIT, GLY and MET were lower in the nonsurvival group than in the survival group (all p < 0.05); C4, C5 and C18 in the non-survival group were lower than those in the survival group (all p < 0.05); Comparing the liver and kidney function indices of the two groups, TBIL and ALT were greater in the nonsurvival group than in the survival group (all p < 0.05), and CHE was lower in the nonsurvival group than in the survival group (p < 0.05).
Table 1.
Characteristics of patients
| Variables | Survivors (n = 131) | Non-survivors (n = 25) | p-value |
|---|---|---|---|
| Gender (male/female), n | 90/41 | 14/11 | 0.217 |
| Median age (range), day | 3 (1, 27) | 5 (1, 28) | 0.407 |
| Weight (g) | 2700.00 (1920.00, 3350.00) | 3100.00 (2700.00, 3400.00) | 0.075 |
| Gestational age at birth (weeks) | 36.00 (32.00, 39.00) | 38.00 (37.00, 39.00) | 0.074 |
| Apgar score (1 min) | 9.00 (8.00, 10.00) | 9.00 (8.00, 10.00) | 0.947 |
| Apgar score (5 min) | 10.00 (9.00, 10.00) | 10.00 (9.00, 10.00) | 0.627 |
| Respiratory distress syndrome, n(%) | 34(25.95) | 7 (28.00) | 0.831 |
| Hyperbilirubinemia, n(%) | 31 (23.66) | 6 (24.00) | 0.971 |
| Purulent meningitis, n(%) | 41 (31.30) | 6 (24.00) | 0.466 |
| Pneumonia, n(%) | 49 (37.40) | 10 (40.00) | 0.806 |
| Culture positive, n (%) | 24(18.32) | 6(24.00) | 0.701 |
| ALA(µmol/L) | 201.67 (172.84, 239.88) | 237.96 (205.94, 303.26) | 0.006 |
| ARG(µmol/L) | 9.16 (4.77, 18.56) | 5.64 (3.26, 8.76) | 0.003 |
| CIT(µmol/L) | 12.46 ± 4.53 | 10.51 ± 3.59 | 0.044 |
| GLY(µmol/L) | 348.32 (288.76, 411.51) | 279.45 (257.14, 356.53) | 0.026 |
| LEU(µmol/L) | 162.29 (125.55, 199.34) | 154.97 (143.90, 179.26) | 0.714 |
| MET(µmol/L) | 23.26 (19.17, 30.61) | 18.78 (17.67, 25.06) | 0.049 |
| ORN(µmol/L) | 94.24 (70.60, 129.98) | 88.79 (82.58, 123.47) | 0.961 |
| PHE(µmol/L) | 56.05 (43.66, 69.88) | 53.81 (42.29, 64.29) | 0.300 |
| PRO(µmol/L) | 146.29 (119.34, 192.79) | 136.28 (123.63, 174.15) | 0.411 |
| TYR(µmol/L) | 70.37 (55.54, 95.75) | 59.78 (49.69, 77.80) | 0.104 |
| VAL(µmol/L) | 123.02 (95.44, 155.47) | 125.14 (100.70, 142.96) | 0.790 |
| C0(µmol/L) | 18.48 (14.36, 23.15) | 18.14 (14.82, 23.95) | 0.958 |
| C2(µmol/L) | 10.22 (6.18, 13.29) | 9.73 (7.15, 12.76) | 0.967 |
| C3(µmol/L) | 0.93 (0.61, 1.48) | 0.82 (0.76, 1.03) | 0.309 |
| C4(µmol/L) | 0.19 (0.14, 0.24) | 0.16 (0.14, 0.19) | 0.049 |
| C5(µmol/L) | 0.11 (0.08, 0.15) | 0.07 (0.06, 0.10) | 0.004 |
| C6(µmol/L) | 0.03 (0.02, 0.05) | 0.03 (0.03, 0.05) | 0.466 |
| C8(µmol/L) | 0.05 (0.04, 0.06) | 0.04 (0.03, 0.06) | 0.249 |
| C10(µmol/L) | 0.04 (0.03, 0.06) | 0.05 (0.03, 0.06) | 0.891 |
| C14(µmol/L) | 0.08 (0.06, 0.11) | 0.08 (0.06, 0.11) | 0.985 |
| C18(µmol/L) | 0.47 ± 0.22 | 0.38 ± 0.11 | 0.002 |
| TBIL(µmol/L) | 52.70 (37.40, 111.50) | 87.80 (45.93, 170.53) | 0.024 |
| ALB(U/L) | 31.82 ± 4.55 | 31.37 ± 3.81 | 0.648 |
| ALT(U/L) | 7.00 (4.00, 11.00) | 10.00 (7.00, 17.75) | 0.003 |
| AST(U/L) | 38.00 (23.00, 59.00) | 43.00 (30.25, 65.75) | 0.483 |
| CHE(U/L) | 4977.58 ± 1526.80 | 4276.50 ± 1223.61 | 0.044 |
| UREA(mmol/L) | 3.44 (2.80, 5.30) | 3.60 (2.58, 5.80) | 0.713 |
| CREA(µmol/L) | 53.00 (43.00, 66.00) | 55.00 (37.50, 70.50) | 0.750 |
ALA, alanine; ALB, albumin; ALT, alanine aminotransferase; ARG, arginine; AST, aspartate transaminase; CHE, cholinesterase; CIT, citrulline; CREA, creatinine; C0, carnitine; C2, acetylcarnitine; C3, propionylcarnitine; C4, butyrylcarnitine; C5, isovalerylcarnitine; C6, hexanoylcarnitine; C8, octanoylcarnitine; C10, decanoylcarnitine; C14, tetradecanoylcarnitine; C18, octadecanoylcarnitine; GLY, glycine; LEU, leucine; MET, methionine; ORN, ornithine; PHE, phenylalanine; PRO, proline; TBIL, total bilirubin; TYR, tyrosine; VAL, valine; UREA, urea
Variables in the cohort
Variables with p < 0.1 in Table 1 were selected and converted to categorical variables. Univariate analysis was first performed on selected variables with p < 0.1, and the results indicated that the levels of ALA, ARG, CIT, GLY, MET, C5, C18, TBIL, ALT, and CHE were significantly associated with the differences observed between the two groups (all p < 0.05), Table 2. The inclusion of the above variables in the multivariate logistic regression analysis revealed that high ALA and low levels of CIT, C18, and MET at admission were independently associated with increased 30-day mortality from neonatal sepsis(Table 2).
Table 2.
Analysis of factors for mortality in neonatal sepsis
| Variables | Univariate analysis | Multivariate analyses | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95%CI | P | β | OR | 95%CI | P | |||
| Weight<2706.68 g | 2.65 | 1.04–6.78 | 0.042 | ||||||
| Gestational age<35.69 weeks | 4.86 | 1.58–14.95 | 0.006 | ||||||
| ALA>233.86µmol/L | 4.35 | 1.77–10.70 | 0.001 | 2.46 | 11.74 | 3.05–45.12 | < 0.001 | ||
| ARG<6.35µmol/L | 0.25 | 0.10–0.61 | 0.002 | ||||||
| CIT<10.36µmol/L | 0.31 | 0.13–0.77 | 0.011 | -1.76 | 0.17 | 0.05–0.59 | 0.005 | ||
| GLY<285.06µmol/L | 0.24 | 0.10–0.59 | 0.002 | ||||||
| MET<20.05µmol/L | 0.26 | 0.11–0.64 | 0.003 | -1.60 | 0.20 | 0.06–0.66 | 0.008 | ||
| C4<0.18µmol/L | 0.42 | 0.17–1.05 | 0.063 | ||||||
| C5<0.075µmol/L | 0.23 | 0.10–0.57 | 0.001 | ||||||
| C18<0.52µmol/L | 0.16 | 0.04–0.69 | 0.014 | -2.59 | 0.08 | 0.01–0.41 | 0.003 | ||
| TBIL>43.85µmol/L | 3.08 | 1.09–8.71 | 0.034 | ||||||
| ALT>4.50U/L | 6.22 | 1.40–27.57 | 0.016 | ||||||
| CHE<3602.50U/L | 0.39 | 0.16–0.96 | 0.041 | ||||||
ALA, alanine; ALT, alanine aminotransferase; ARG, arginine; CHE, cholinesterase; CIT, citrulline; C4, butyrylcarnitine; C5, isovalerylcarnitine; C18, octadecanoylcarnitine; GLY, glycine; MET, methionine; TBIL, total bilirubin
Establishment of a clinical prediction model with a nomogram
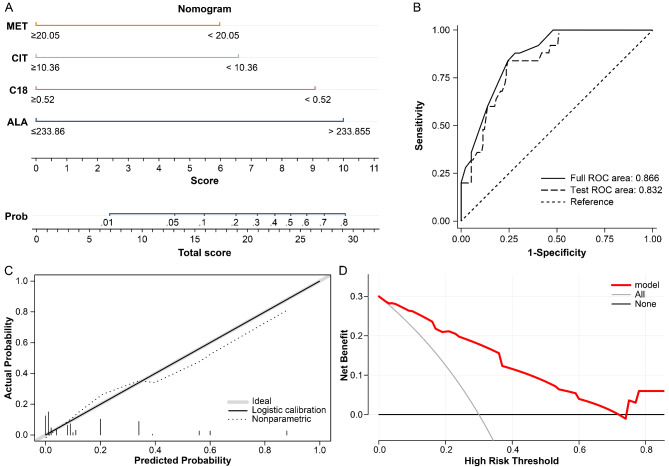
The variables ALA, CIT, MET and C18 were included in the final model after multivariate logistic regression analysis. On the basis of the independent risk factors and their regression coefficients, multivariate regression equations were constructed for the prediction model. The prediction formula is as follows: LogitP = 2.46×ALA-1.76×CIT—1.60×MET—2.59×C18—8.21. On the basis of the logistic regression model, we constructed a nomogram to predict 30-day mortality of neonatal sepsis patients (Fig. 2A).
Fig. 2.
Model Nomogram and Performance Assessment. (A) predictive nomogram for 30-day mortality in neonatal sepsis. ALA, alanine; CIT, citrulline; C18, octadecanoylcarnitine; MET, methionine; (B) ROC curve for the clinical model and 10-fold cross-validation; (C) Calibration curve of predictive model; (D) Decision curve analysis (DCA) of predictive model
Performance of the clinical prediction model
The ROC curve was used to assess the predictive performance, as shown in Fig. 2B, and the AUC of this model was 0.866 (95% CI 0.796–0.936, P < 0.05). After cross-validation, the AUC was 0.832, Fig. 2B. This finding indicates that the current model is stable; The calibration curve of the clinical model was good, as shown in Fig. 2C. The Hosmer-Lemeshow test showed that χ2 = 6.249, P = 0.715, and the model had a large differential predictive value. In addition, decision curve analysis (DCA) shows that using the model to make decisions can lead to better results than alternative strategies or not using the model at all (Fig. 2D).
Clinical model statistics and independent markers
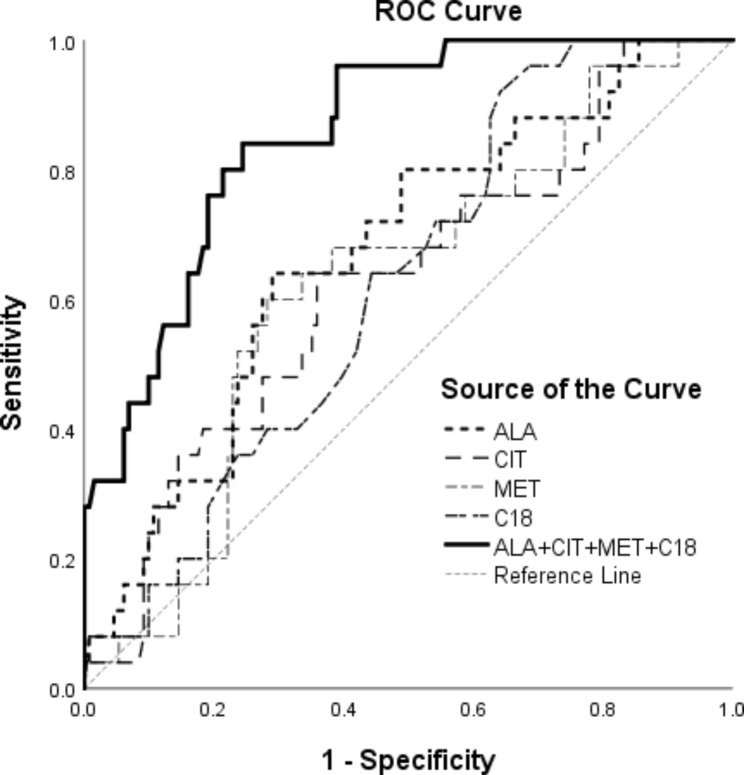
Other statistics for the clinical model and independent risk factor markers are shown in Table 3, and the ROC curves for the clinical model and independent risk factor markers are shown in Fig. 3. Clinical model has good predictive power.
Table 3.
Diagnostic statistics for independent markers
| Variables | AUC (95%CI) | Yuden | Sensitivity | Specificity | PPV | NPV | Cut off |
|---|---|---|---|---|---|---|---|
| ALA | 0.675 (0.561–0.790) | 0.35 | 0.71 | 0.64 | 0.91 | 0.30 | 233.86 |
| C18 | 0.626 (0.521–0.731) | 0.28 | 0.36 | 0.92 | 0.96 | 0.21 | 0.52 |
| CIT | 0.631 (0.511–0.751) | 0.28 | 0.64 | 0.64 | 0.88 | 0.23 | 10.36 |
| MET | 0.626 (0.511–0.741) | 0.32 | 0.60 | 0.72 | 0.90 | 0.29 | 20.05 |
| Model | 0.866 (0.796–0.936) | 0.60 | 0.76 | 0.84 | 0.96 | 0.40 | 0.173 |
ALA, alanine; CIT, citrulline; C18, octadecanoylcarnitine; MET, methionine; NPV, negative predictive value; PPV, positive predictive value; Model: ALA + CIT + MET + C18
Fig. 3.
Area under the curves (AUC) of factors for predicting mortality
ALA, alanine; CIT, citrulline; C18, octadecanoylcarnitine; MET, methionine; Clinical Model: ALA + CIT + MET + C18
Discussion
Neonatal sepsis is a systemic inflammatory response syndrome triggered by infection, and due to the immaturity of neonates’ immune systems, sepsis progresses rapidly and carries a high mortality rate [16]. The excessive release of inflammatory mediators and stress hormones leads to hemodynamic disturbances and metabolic alterations in neonatal sepsis, ultimately resulting in multi-organ failure [17]. Monitoring changes in metabolites during the early stages of neonatal sepsis and identifying metabolic markers for early diagnosis will help clinicians to improve individual treatment strategies and clinical decision-making. In this study, we examined 11 amino acids and 10 acylcarnitine markers within 24 h of admission for neonatal sepsis patients, unveiling that elevated ALA levels along with decreased CIT, MET, and C18 levels independently contribute as risk factors for mortality in neonatal sepsis.
Alanine is a non-essential amino acid that undergoes hepatic metabolism in the human body [18]. A systematic review study shows that during neonatal sepsis, glucose metabolism is altered [19]. Alanine, as an important precursor for gluconeogenesis, plays a significant role in maintaining stable blood glucose levels in neonates [20]. Therefore, monitoring the level of alanine is crucial for the management of neonatal sepsis. Previous studies have identified elevated levels of ALA in cases of sepsis-related brain injury and acute kidney injury [10]. In the present study, higher concentrations of ALA were observed in the non-survival group compared to the survival group. This potentially indicates impaired liver function induced by sepsis and an associated nitrogen imbalance that impairs brain and kidney function [21, 22]. The multi-organ damage caused by elevated ALA may act as a substantial risk factor for neonatal sepsis mortality. Citrulline, a non-protein amino acid, plays important roles in cellular metabolism and organ function regulation [23]. It is mainly involved in the synthesis of urea, nitric oxide, and arginine. CIT has been shown to alleviate sepsis-induced immunosuppression by restoring T-cell function through increasing effective arginine levels [24]. Existing research has confirmed that during neonatal sepsis, there is an abnormality in glutamine metabolism [25]. Glutamine serves as a precursor for citrulline, suggesting that during neonatal sepsis, the synthesis of citrulline may be weakened. In the present study, CIT levels were found to be lower in the non-survival group compared to the survival group, which could be attributed to reduced plasma synthesis of citrulline and increased consumption during neonatal sepsis [26]. Whereas low levels of CIT weaken autoregulation, the present study identified reduced CIT as a risk factor for mortality in neonatal sepsis. Methionine, an amino acid containing sulfur, can be converted to glutathione and plays a role in reducing sepsis-induced oxidative stress [27]. Animal studies have shown an elevation in methionine transsulphurisation during sepsis in rats [28]. An investigation into metabolic biomarkers in neonatal sepsis uncovered a disruption in the neonatal glutathione metabolic pathway during the course of the condition [29]. Since methionine, as a sulfur-containing amino acid, can be transformed into glutathione, this research supports our perspective. The decreased levels of methionine observed in this study were identified as a risk factor for mortality. This may be due to inadequate basal nutritional vitamin B12 deficiency in neonatal sepsis and the subsequent increase in methionine transsulphurisation, resulting in elevated homocysteine levels [30]. Decreased methionine may function as a potential prognostic marker for neonatal sepsis.
Mitochondrial dysfunction is another prominent feature of sepsis [31]. A study revealed a significant reduction in biopsy ATP/ADP ratios in the skeletal muscle of patients with fatal sepsis [32]. Another study showed that higher plasma short- and medium-chain acylcarnitines levels are linked to sepsis prognosis, with acetylcarnitine significantly associated with 28-day mortality [11]. Significant changes in lipid metabolism have been reported in infants with sepsis [33]. Acylcarnitines play a crucial role as transporters in fatty acid β-oxidation, and their levels can serve as biomarkers to assess fatty acid oxidation status, reflecting early imbalance and mitochondrial stress [34]. In the present study, several acylcarnitines were found to be lower in the non-survival group compared to the survival group, specifically C4, C5, and C18 (all p < 0.05). Multivariate analysis revealed an independent association between mortality and C18, indicating a relative carnitine deficiency in neonatal sepsis among non-survivors. Our findings suggest that carnitine deficiency may serve as a predictor for impaired fatty acid oxidation and abnormal energy metabolism associated with multiorgan dysfunction in neonatal sepsis, thus identifying C18 as a risk factor for 30-day mortality.
In this study, a model incorporating multiple amino acid and acylcarnitine indicators was successfully developed to effectively assess 30-day mortality in neonatal sepsis. This comprehensive model provides an early reflection of the metabolic status associated with neonatal sepsis, demonstrating its significant predictive value. Previous research on metabolic alterations in sepsis aligns with the findings of this study; however, investigations into metabolic indicators specific to neonatal sepsis remain scarce. As a distinct demographic, neonates exhibit underdeveloped metabolic functions, necessitating further exploration into the variations of their metabolic markers. Existing studies have substantiated disruptions in energy metabolism associated with neonatal sepsis [35], enhancing our comprehension of amino acid and acylcarnitine dynamics in this condition. In summary, our research provides valuable insights into the metabolism of amino acids and acylcarnitines in neonatal sepsis, enabling clinicians to monitor early changes in metabolic indicators. This, in turn, facilitates the development of personalized treatment strategies. For example, by optimizing nutritional support and adjusting antibiotic therapy based on dynamic metabolic profiling, clinicians can make more informed decisions tailored to the individual needs of neonates with sepsis.
The study, however, has certain limitations that should be acknowledged. For example, since it was conducted as a single-centre study with a limited sample size, there is a possibility of data bias. Henceforth, it is essential to validate these findings by using a larger patient cohort and multicentre data. Conversely, the analysis did not incorporate additional metabolic indicators due to insufficient available data. Future clinical work will focus on optimizing and validating the proposed model.
Conclusion
In the present study, a model incorporating multiple amino acids and acylcarnitines was developed to assess the prognosis of neonatal sepsis and predict 30-day mortality. This model can effectively capture alterations in amino acid and fatty acid metabolism during the early stages of neonatal sepsis, facilitating prompt assessment, personalized treatment, and informed clinical decision-making.
Acknowledgements
Not applicable.
Author contributions
J-K C and W L designed the study. All the authors contributed to the generation, collection, assembly, analysis and/or interpretation of data. X-W T wrote the manuscript, J-K C prepared Fig. 1 L prepared Figs. 2 and 3. All the authors revised the manuscript. All the authors have read manuscript and approved the final manuscript.
Funding
Not applicable.
Data availability
The datasets are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study protocol followed the principles set forth in the Declaration of Helsinki, and the study was approved by the Medical Ethics Committee of the Ganzhou Women and Children’s Health Care Hospital(2023-007), Jiangxi Province, China, and informed consent was waived by this ethics committee due to the retrospective design of the study. The ethics committee waived the need for individual informed consent because personally identifiable information was not used in this analysis.
Consent for publication
Not applicable.
Clinical trial
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xiangwen Tu and Junkun Chen contributed equally to this work.
References
- 1.Samra N, Alghwass M, Elgawhary S, et al. Serum level of Antithrombin III (ATIII) could serve as a prognostic biomarker in neonatal Sepsis. Fetal Pediatr Pathol. 2019;38(4):290–8. 10.1080/15513815.2019.1587118. [DOI] [PubMed] [Google Scholar]
- 2.Leal YA, Alvarez-Nemegyei J, Lavadores-May AI, Giron-Carrillo JL, Cedillo-Rivera R, Velazquez JR. Cytokine profile as diagnostic and prognostic factor in neonatal sepsis. J Matern-Fetal Neo M. 2019;32(17):2830–6. 10.1080/14767058.2018.1449828. [DOI] [PubMed] [Google Scholar]
- 3.Jain R, Acharya R, Kumud, et al. Serum Resistin as a potential mortality predictor in neonatal Sepsis. Cureus J Med Sci. 2024;16(2):e55289. 10.7759/cureus.55289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li T, Qi M, Dong G, et al. Clinical value of Prognostic Nutritional Index in Prediction of the Presence and Severity of neonatal Sepsis. J Inflamm Res. 2021;14:7181–90. 10.2147/JIR.S343992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su L, Huang Y, Zhu Y, et al. Discrimination of sepsis stage metabolic profiles with an LC/MS-MS-based metabolomics approach. Bmj Open Respir Res. 2014;1(1):e000056. 10.1136/bmjresp-2014-000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Q, Liang X, Wu T, et al. Integrative analysis of metabolomics and proteomics reveals amino acid metabolism disorder in sepsis. J Transl Med. 2022;20(1):123. 10.1186/s12967-022-03320-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu D, Sheeja PH, Zhang YY, Luo G, He W, Liou YC. Mitochondrial dysfunction in sepsis: mechanisms and therapeutic perspectives. Crit Care. 2024;28(1):292. 10.1186/s13054-024-05069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang P, Wang Z, Qiu H, Zhou W, Wang M, Cheng G. Machine learning applied to serum and cerebrospinal fluid metabolomes revealed altered arginine metabolism in neonatal sepsis with meningoencephalitis. Comput Struct Biotec. 2021;19:3284–92. 10.1016/j.csbj.2021.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wexler O, Gough MS, Morgan M, et al. Methionine metabolites in patients with Sepsis. J Intensive Care Med. 2018;33(1):37–47. 10.1177/0885066616666002. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Xu Y, Hu S, et al. Alanine, a potential amino acid biomarker of pediatric sepsis: a pilot study in PICU. Amino Acids. 2024;56(1):48. 10.1007/s00726-024-03408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung KP, Chen GY, Chuang TY, et al. Increased plasma acetylcarnitine in Sepsis is Associated with multiple organ dysfunction and mortality: a Multicenter Cohort Study. Crit Care Med. 2019;47(2):210–8. 10.1097/CCM.0000000000003517. [DOI] [PubMed] [Google Scholar]
- 12.Al GF, Lahni P, Alder MN, Wong HR. Biomarkers estimating baseline mortality risk for neonatal sepsis: nPERSEVERE: neonate-specific sepsis biomarker risk model. Pediatr Res. 2023;94(4):1451–6. 10.1038/s41390-022-02414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman MS, Islam KR, Prithula J, et al. Machine learning-based prognostic model for 30-day mortality prediction in Sepsis-3. Bmc Med Inf Decis. 2024;24(1):249. 10.1186/s12911-024-02655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koozi H, Lidestam A, Lengquist M, Johnsson P, Frigyesi A. A simple mortality prediction model for sepsis patients in intensive care. J Intensive Care Soc. 2023;24(4):372–8. 10.1177/17511437221149572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. [Consensus Development Conference; Journal Article; Research Support, Non-U.S. Gov’t; Review]. 2005 2005-01-01;6(1):2–8. [DOI] [PubMed]
- 16.Fleischmann C, Reichert F, Cassini A, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child. 2021;106(8):745–52. 10.1136/archdischild-2020-320217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arina P, Singer M. Pathophysiology of sepsis. Curr Opin Anesthesio. 2021;34(2):77–84. 10.1097/ACO.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 18.Hou Y, Hu S, Li X, He W, Wu G. Amino acid metabolism in the liver: nutritional and physiological significance. Adv Exp Med Biol. 2020;1265:21–37. 10.1007/978-3-030-45328-2_2. [DOI] [PubMed] [Google Scholar]
- 19.Bjerkhaug AU, Granslo HN, Klingenberg C. Metabolic responses in neonatal sepsis-A systematic review of human metabolomic studies. Acta Paediatr. 2021;110(8):2316–25. 10.1111/apa.15874. [DOI] [PubMed] [Google Scholar]
- 20.Holeček M. Origin and roles of Alanine and glutamine in Gluconeogenesis in the liver, kidneys, and small intestine under physiological and pathological conditions. Int J Mol Sci. 2024;25(13). 10.3390/ijms25137037. [DOI] [PMC free article] [PubMed]
- 21.Coqueiro AY, Raizel R, Bonvini A, et al. Effects of glutamine and alanine supplementation on central fatigue markers in rats submitted to Resistance Training. Nutrients. 2018;10(2). 10.3390/nu10020119. [DOI] [PMC free article] [PubMed]
- 22.Nielsen PM, Mariager CO, Molmer M, et al. Hyperpolarized [1-(13) C] alanine production: a novel imaging biomarker of renal fibrosis. Magn Reson Med. 2020;84(4):2063–73. 10.1002/mrm.28326. [DOI] [PubMed] [Google Scholar]
- 23.Asgeirsson T, Zhang S, Nunoo R, et al. Citrulline: a potential immunomodulator in sepsis. Surgery. 2011;150(4):744–51. 10.1016/j.surg.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 24.Reizine F, Gregoire M, Lesouhaitier M, et al. Beneficial effects of citrulline enteral administration on sepsis-induced T cell mitochondrial dysfunction. P Natl Acad Sci Usa. 2022;119(8). 10.1073/pnas.2115139119. [DOI] [PMC free article] [PubMed]
- 25.Eaton S. Impaired energy metabolism during neonatal sepsis: the effects of glutamine. Proc Nutr Soc. 2003;62(3):745–51. 10.1079/PNS2003284. [DOI] [PubMed] [Google Scholar]
- 26.Druml W, Heinzel G, Kleinberger G. Amino acid kinetics in patients with sepsis. Am J Clin Nutr. 2001;73(5):908–13. 10.1093/ajcn/73.5.908. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Feng S, Du Q, et al. The Protective effects of Methionine on Nickel-Induced oxidative stress via NF-κB pathway in the kidneys of mice. Biol Trace Elem Res. 2024. 10.1007/s12011-024-04408-w. [DOI] [PubMed] [Google Scholar]
- 28.Malmezat T, Breuille D, Pouyet C, et al. Methionine transsulfuration is increased during sepsis in rats. Am J Physiol-Endoc M. 2000;279(6):E1391–7. 10.1152/ajpendo.2000.279.6.E1391. [DOI] [PubMed] [Google Scholar]
- 29.Mardegan V, Giordano G, Stocchero M, et al. Untargeted and targeted metabolomic profiling of Preterm newborns with EarlyOnset Sepsis: a case-control study. Metabolites. 2021;11(2):null. 10.3390/metabo11020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pregernig A, Held U,Schläpfer M, et al. Vitamin B12 status and the risk of developing sepsis in patients with bacterial infection: a prospective observational cohort study. BMC Med. 2024;22(1):330. 10.1186/s12916-024-03552-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar S, Srivastava VK, Kaushik S, et al. Free radicals, mitochondrial dysfunction and Sepsis-induced organ dysfunction: a mechanistic insight. Curr Pharm Des. 2024;30(3):161–8. 10.2174/0113816128279655231228055842. [DOI] [PubMed] [Google Scholar]
- 32.Lee I, Huttemann M. Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim Biophys Acta. 2014;1842(9):1579–86. 10.1016/j.bbadis.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, Cha X, Zhang Z, et al. Discrimination of serum metabolomics profiles in infants with sepsis, based on liquid chromatography-mass spectrometer. BMC Infect Dis. 2023;23(1):46. 10.1186/s12879-023-07983-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dambrova M, Makrecka-Kuka M, Kuka J, et al. Acylcarnitines: nomenclature, biomarkers, therapeutic potential, drug targets, and clinical trials. Pharmacol Rev. 2022;74(3):506–51. 10.1124/pharmrev.121.000408. [DOI] [PubMed] [Google Scholar]
- 35.Dessì A, Corsello G, Stronati M, et al. New diagnostic possibilities in systemic neonatal infections: metabolomics. Early Hum Dev. 2014;90(Suppl 1):S19–21. 10.1016/S0378-3782(14)70007-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.