Abstract
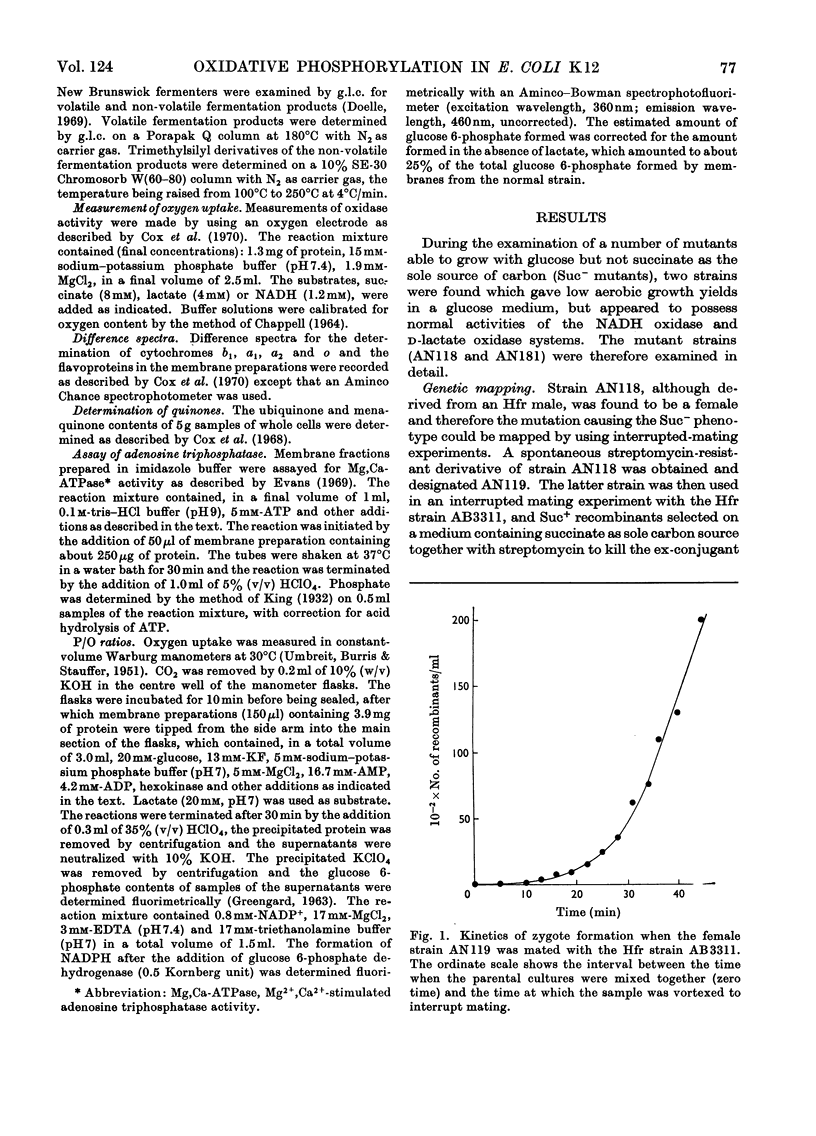
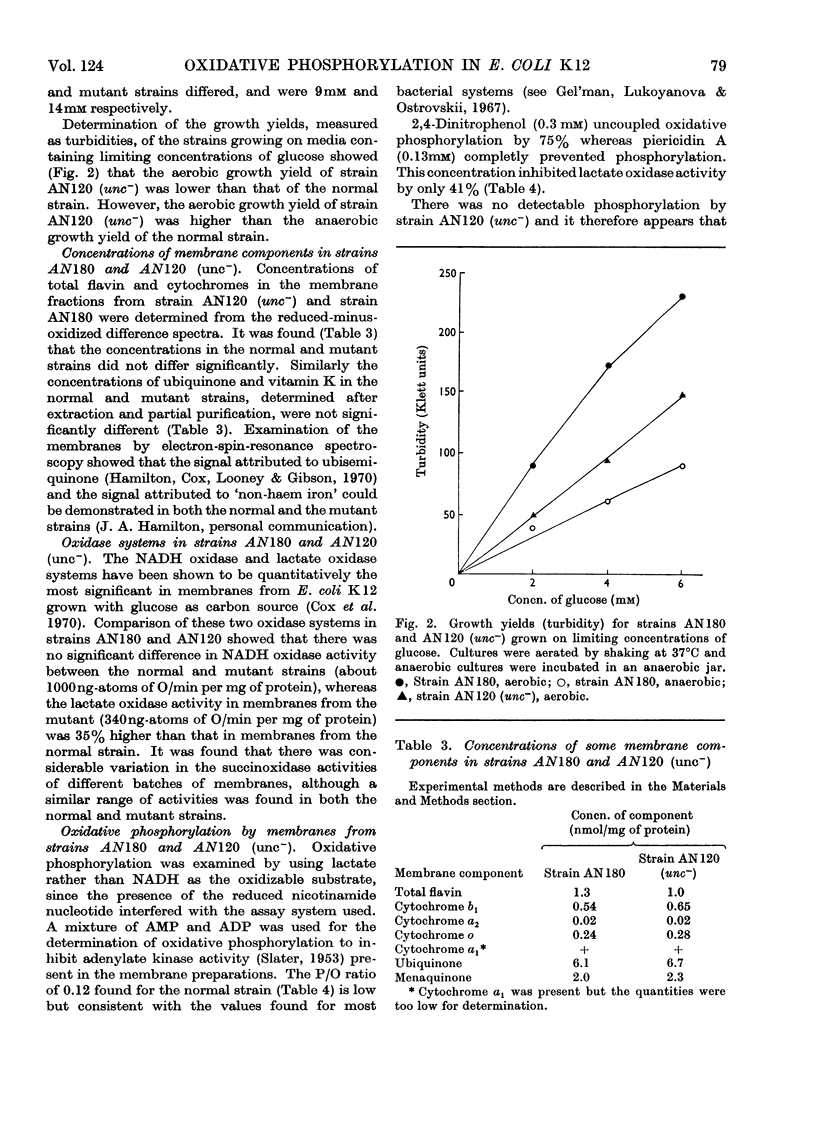
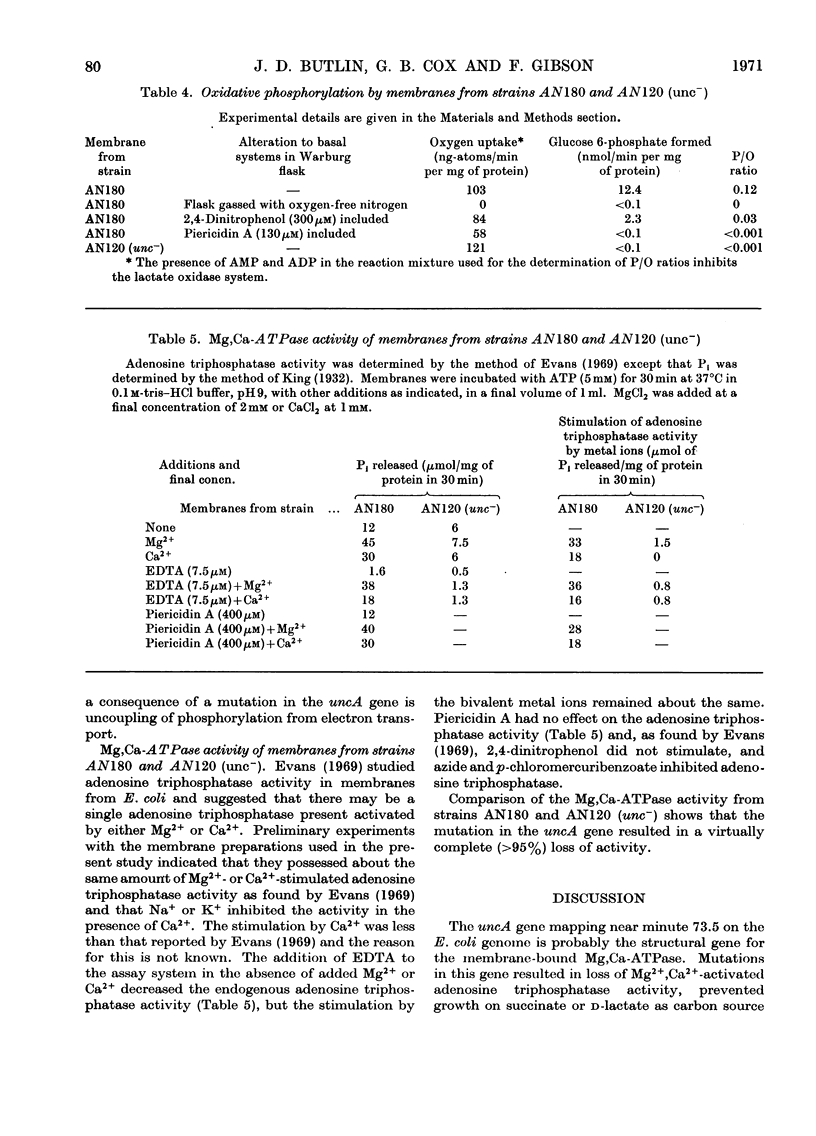
1. Two mutants of Escherichia coli K 12 were isolated which, although able to grow on glucose, are unable to grow with succinate or d-lactate as the sole source of carbon. 2. Genetic mapping of these mutants showed that they both contain a mutation in a gene (designated uncA) mapping at about minute 73.5 on the E. coli chromosome. 3. The uncA− alleles were transferred by bacteriophage-mediated transduction into another strain of E. coli and the transductants compared with the parent strain to determine the nature of the biochemical lesion in the mutants. 4. The mutants gave low aerobic growth yields when grown on limiting concentrations of glucose, but oxidase activities in membranes from both the mutants and the normal strain were similar. 5. Measurement of P/O ratios with d-lactate as substrate indicated that a mutation in the uncA gene causes uncoupling of phosphorylation associated with electron transport. 6. Determination of the Mg2+,Ca2+-stimulated adenosine triphosphatase activities in the mutant and normal strains indicated that the uncA gene is probably the structural gene for Mg2+,Ca2+-stimulated adenosine triphosphatase. 7. Mg2+,Ca2+-stimulated adenosine triphosphatase therefore appears to be essential for oxidative phosphorylation in E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Gibson F., Pittard J. Mutant strains of Escherichia coli K-12 unable to form ubiquinone. J Bacteriol. 1968 May;95(5):1591–1598. doi: 10.1128/jb.95.5.1591-1598.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Gibson F., Snoswell A. M., Hamilton J. A. The function of ubiquinone in Escherichia coli. Biochem J. 1970 Apr;117(3):551–562. doi: 10.1042/bj1170551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Young I. G., McCann L. M., Gibson F. Biosynthesis of ubiquinone in Escherichia coli K-12: location of genes affecting the metabolism of 3-octaprenyl-4-hydroxybenzoic acid and 2-octaprenylphenol. J Bacteriol. 1969 Aug;99(2):450–458. doi: 10.1128/jb.99.2.450-458.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelle H. W. Gas chromatographic separation and determination of micro quantities of C1-C7 branched and straight-chain saturated fatty acids. J Chromatogr. 1969 Feb 25;39(4):398–406. doi: 10.1016/s0021-9673(01)98028-8. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr Membrane adenosine triphosphatase of Escherichia coli: activation by calcium ion and inhibition by monovalent cations. J Bacteriol. 1969 Nov;100(2):914–922. doi: 10.1128/jb.100.2.914-922.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Cox G. B., Looney F. D., Gibson F. Ubisemiquinone in membranes from Escherichia coli. Biochem J. 1970 Jan;116(2):319–320. doi: 10.1042/bj1160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovác L., Lachowicz T. M., Slonimski P. P. Biochemical genetics of oxidative phosphorylation. Science. 1967 Dec 22;158(3808):1564–1567. doi: 10.1126/science.158.3808.1564. [DOI] [PubMed] [Google Scholar]
- LINNANE A. W., TITCHENER E. B. Studies on the mechanism of oxidative phosphorylation. VI. A factor for coupled oxidation in the electron transport particle. Biochim Biophys Acta. 1960 Apr 22;39:469–478. doi: 10.1016/0006-3002(60)90200-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LURIA S. E., BURROUS J. W. Hybridization between Escherichia coli and Shigella. J Bacteriol. 1957 Oct;74(4):461–476. doi: 10.1128/jb.74.4.461-476.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K. W., Warshaw J. B., Sanadi D. R. The mechanism of oxidative phosphorylation. XIV. Purification and properties of a second energy-transfer factor. Arch Biochem Biophys. 1967 Mar;119(1):477–484. doi: 10.1016/0003-9861(67)90479-1. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- PINCHOT G. B. Phosphorylation coupled to electron transport in cell-free extracts of Alcaligenes faecalis. J Biol Chem. 1953 Nov;205(1):65–74. [PubMed] [Google Scholar]
- PITTARD J. EFFECT OF INTEGRATED SEX FACTOR ON TRANSDUCTION OF CHROMOSOMAL GENES IN ESCHERICHIA COLI. J Bacteriol. 1965 Mar;89:680–686. doi: 10.1128/jb.89.3.680-686.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penefsky H. S., Warner R. C. Partial resolution of the enzymes catalyzing oxidative phosphorylation. VI. Studies on the mechanism of cold inactivation of mitochondrial adenosine triphosphatase. J Biol Chem. 1965 Dec;240(12):4694–4702. [PubMed] [Google Scholar]
- SALTER E. C. A method of measuring the yield of oxidative phosphorylation. Biochem J. 1953 Mar;53(4):521–530. doi: 10.1042/bj0530521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNOSWELL A. M. The reduction of diphosphopyridine nucleotide of rabbit-heart sarcosomes by succinate. Biochim Biophys Acta. 1962 Jun 18;60:143–157. doi: 10.1016/0006-3002(62)90381-5. [DOI] [PubMed] [Google Scholar]
- Somlo M. Respiration-linked ATP formation by an "oxidative phosphorylation mutant" of yeast. Arch Biochem Biophys. 1970 Jan;136(1):122–133. doi: 10.1016/0003-9861(70)90334-6. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallin I., Löw H. The effect of piericidin A on energy-linked processes in submitochondrial particles. Eur J Biochem. 1968 Aug;5(3):402–408. [PubMed] [Google Scholar]


