Abstract
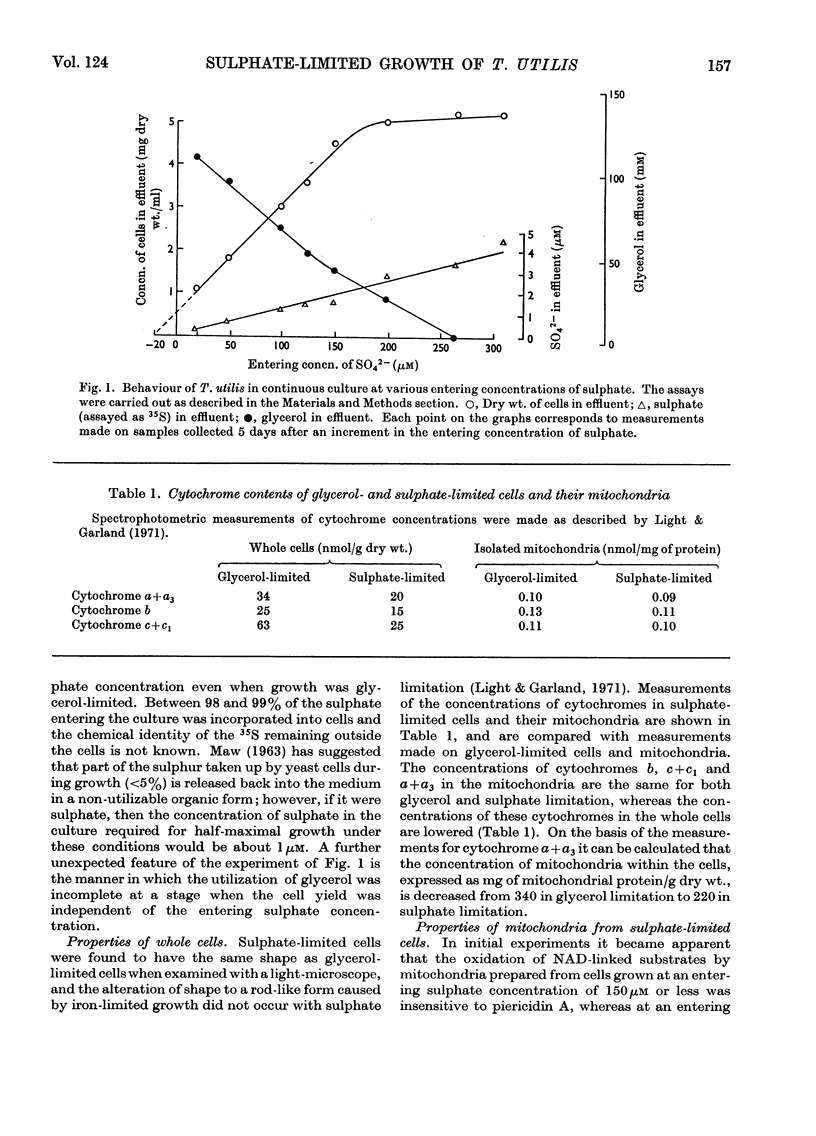
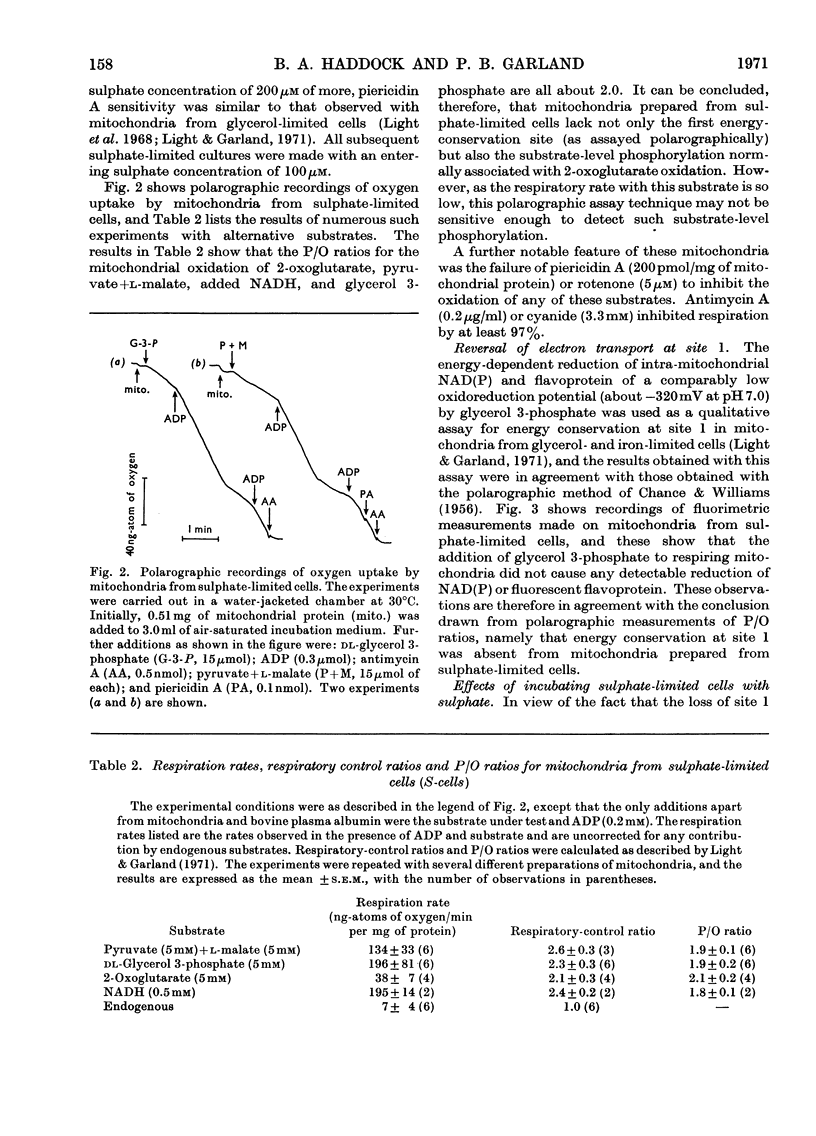
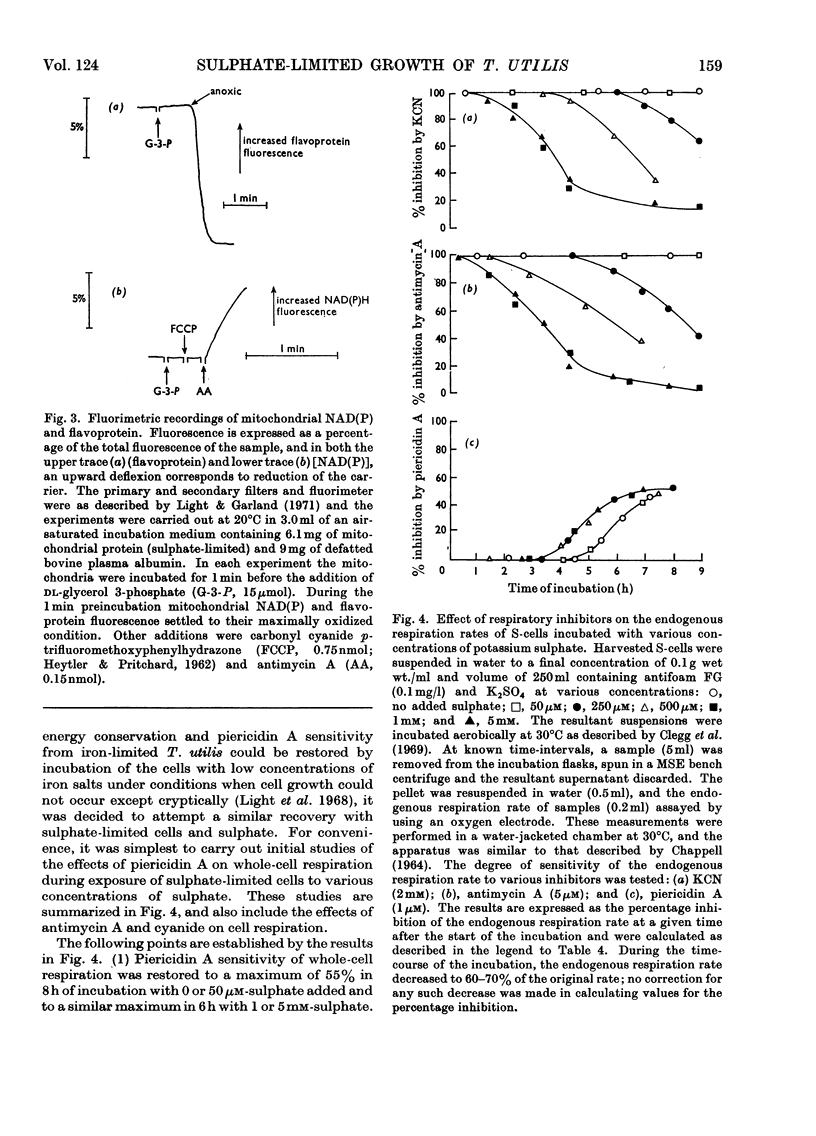
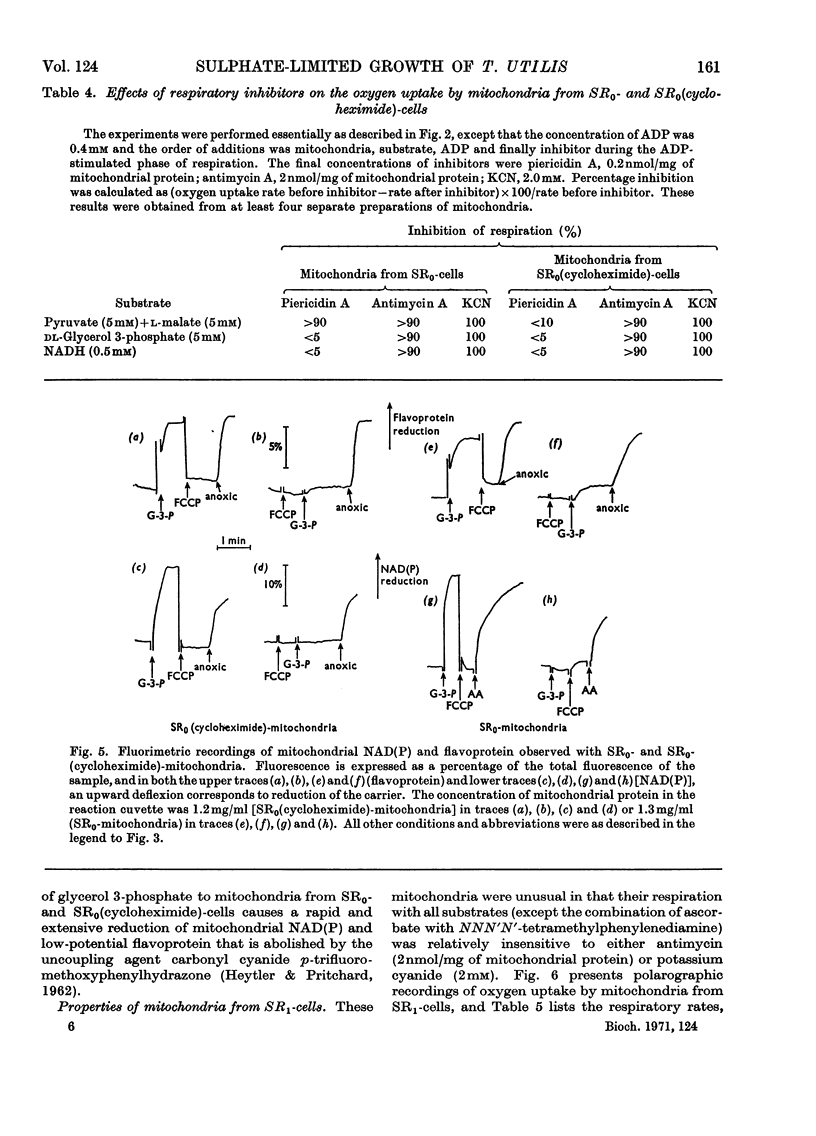
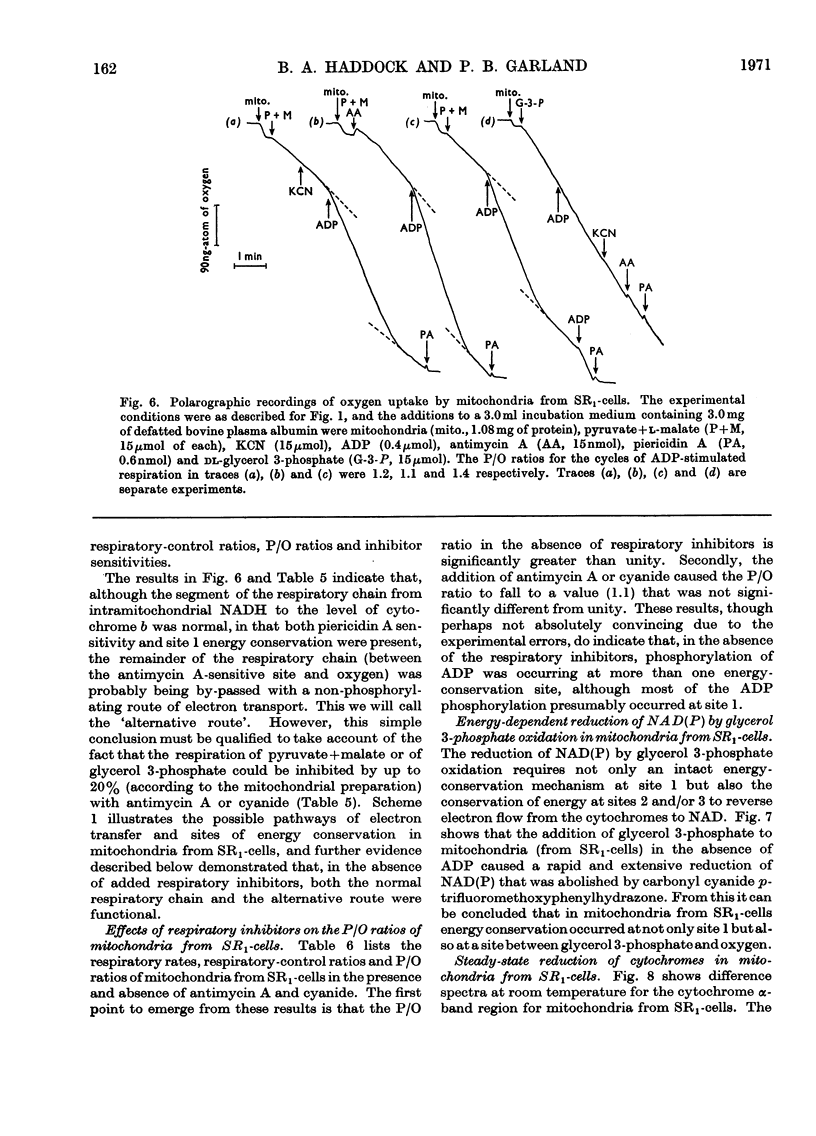
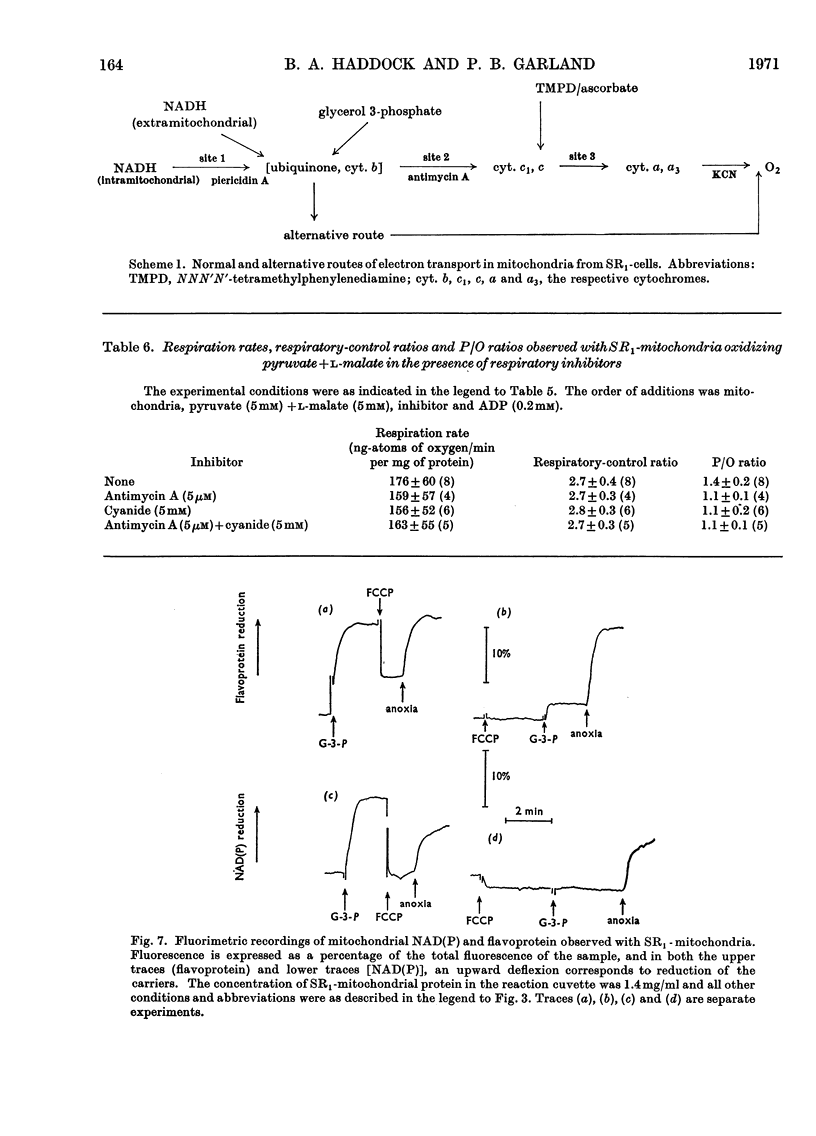
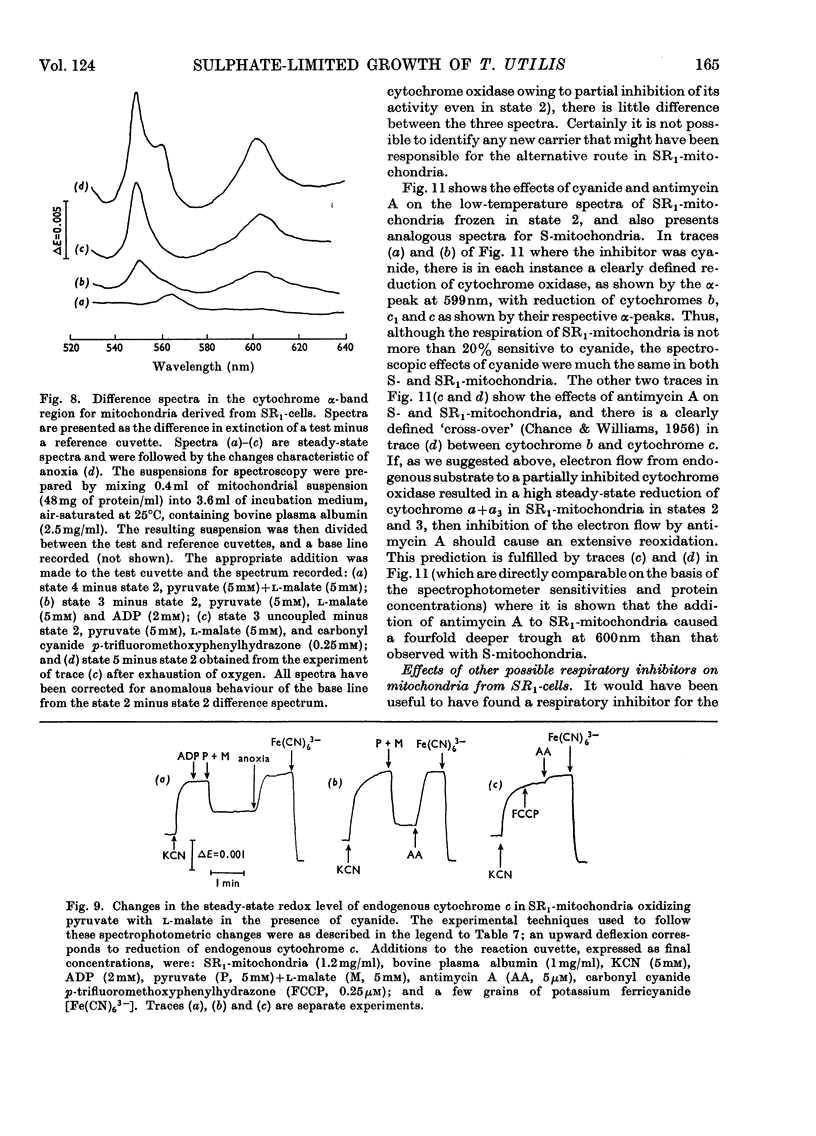
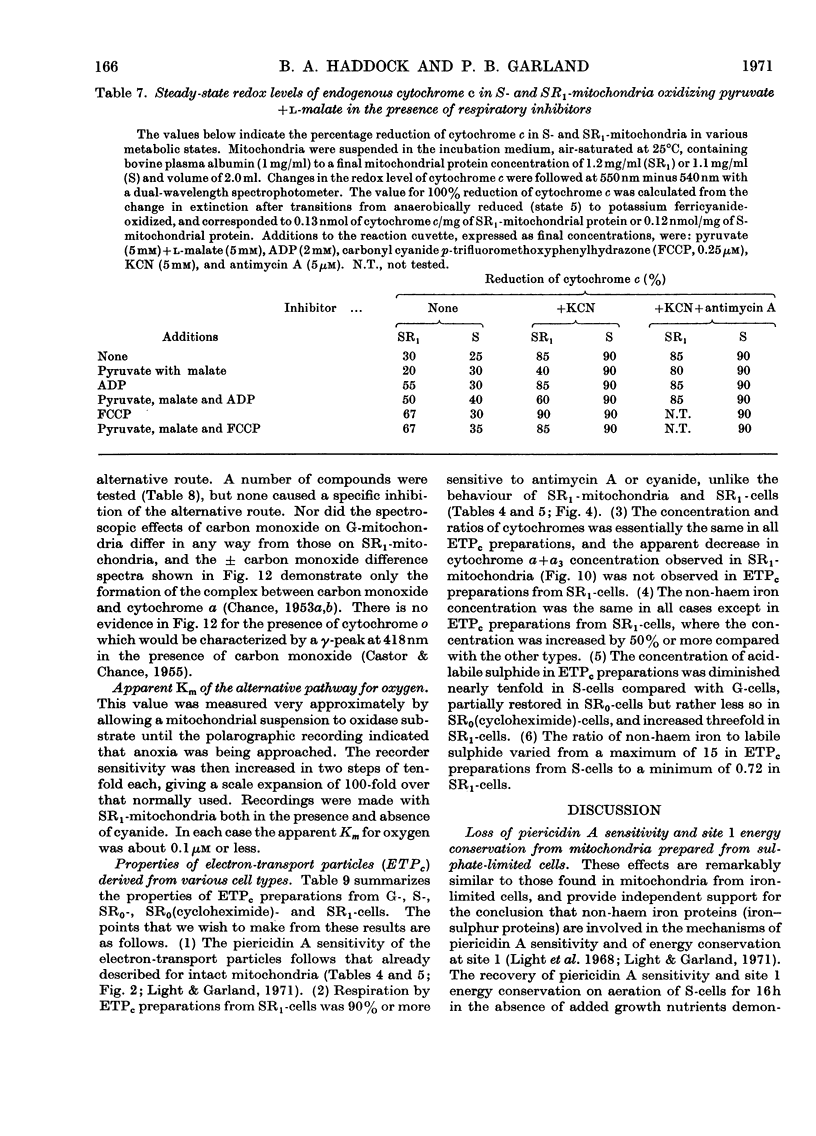
1. Conditions have been established for the sulphate-limited growth of Torulopsis utilis in continuous culture. 2. Mitochondria prepared from sulphate-limited cells lack both piericidin A sensitivity and the first energy-conservation site (site 1). Sensitivity to antimycin A or cyanide and the second and third energy-conservation sites were apparently unaffected by sulphate-limited growth. 3. Aerobic incubation for 8h of sulphate-limited cells with a low concentration of sulphate (50μm or less) resulted in the recovery of mitochondrial piericidin A sensitivity and site 1. The use of higher concentrations of sulphate (250μm or more) still resulted in the recovery of mitochondrial piericidin A sensitivity and site 1, but also resulted in the appearance of a non-phosphorylating oxidase, which mediated oxidation of the respiratory chain at about the level of cytochrome b in an antimycin A- and cyanide-insensitive manner. Both this alternative route and the conventional normal route of respiration were shown to coexist and to intercommunicate at the level of cytochrome b. 4. Low-temperature spectroscopy failed to identify any new respiratory component to explain the alternative route. 5. The apparent affinity of the alternative route for oxygen was similar to that for the conventional route through cytochrome oxidase, namely half-maximal activity at 0.1μm-oxygen or less. 6. The non-haem iron concentration of submitochondrial particles was unaffected by sulphate limitation, whereas the acid-labile sulphide concentration was lowered tenfold. Marked increases (between four- and 30-fold) in the acid-labile sulphide concentration of submitochondrial particles were observed in sulphate-limited cells after aerobic incubation with various concentrations of sulphate. The lowest increase (fourfold) was observed without added sulphate, the highest (30-fold) with 1.0mm added sulphate. 7. The ratio of non-haem iron to acid-labile sulphide in submitochondrial particles varied with different growth conditions from a maximum of 15.0 to a minimum of 0.72. It is suggested that analytical measurements of non-haem iron are an inadequate guide to the concentration of iron–sulphur protein in complex systems. 8. The effects of sulphate-limited growth on site 1 and piericidin sensitivity are interpreted to indicate a role for iron–sulphur protein in these properties. 9. The aerobic incubation of sulphate-limited cells with cycloheximide resulted in the recovery by mitochondria of site 1 but not of piericidin sensitivity. 10. The appearance of the alternative route for cyanide- and antimycin-A (but not piericidin A-) insensitive respiration on incubating sulphate-limited cells with sulphate concentrations higher than 250μm indicates that the alternative route involves an iron–sulphur protein.
Full text
PDF



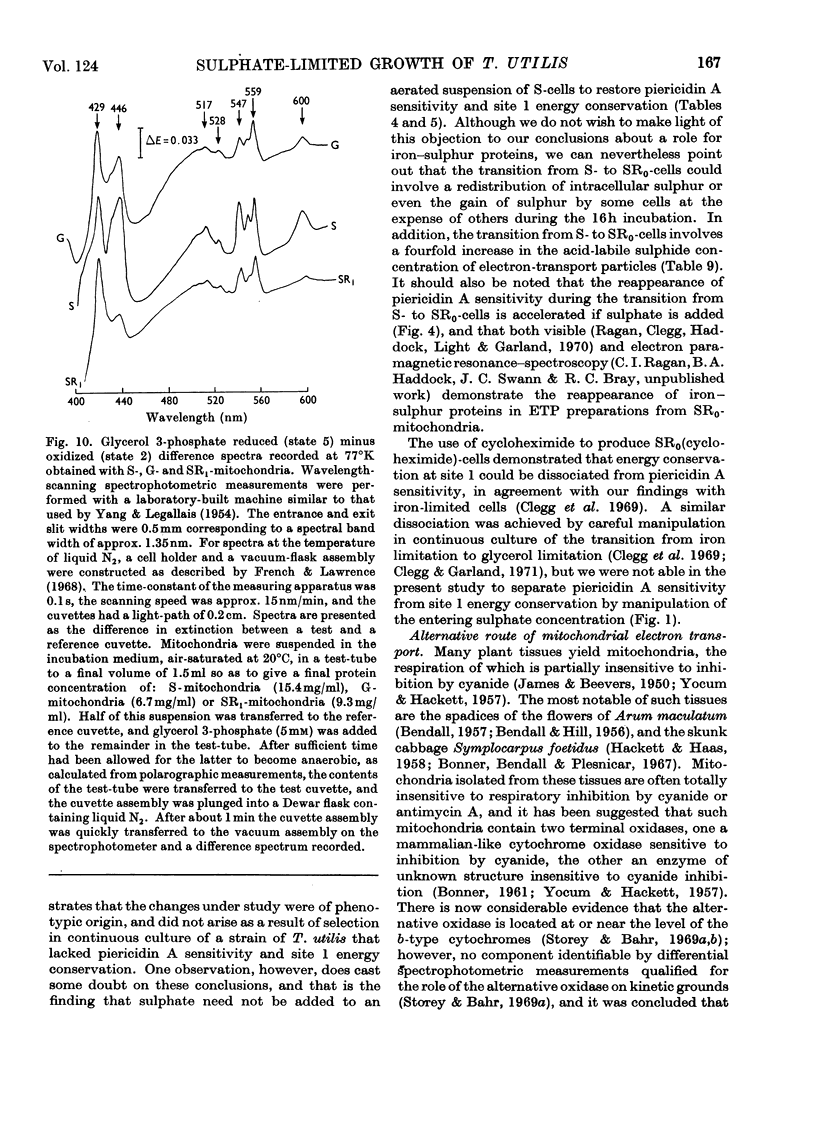
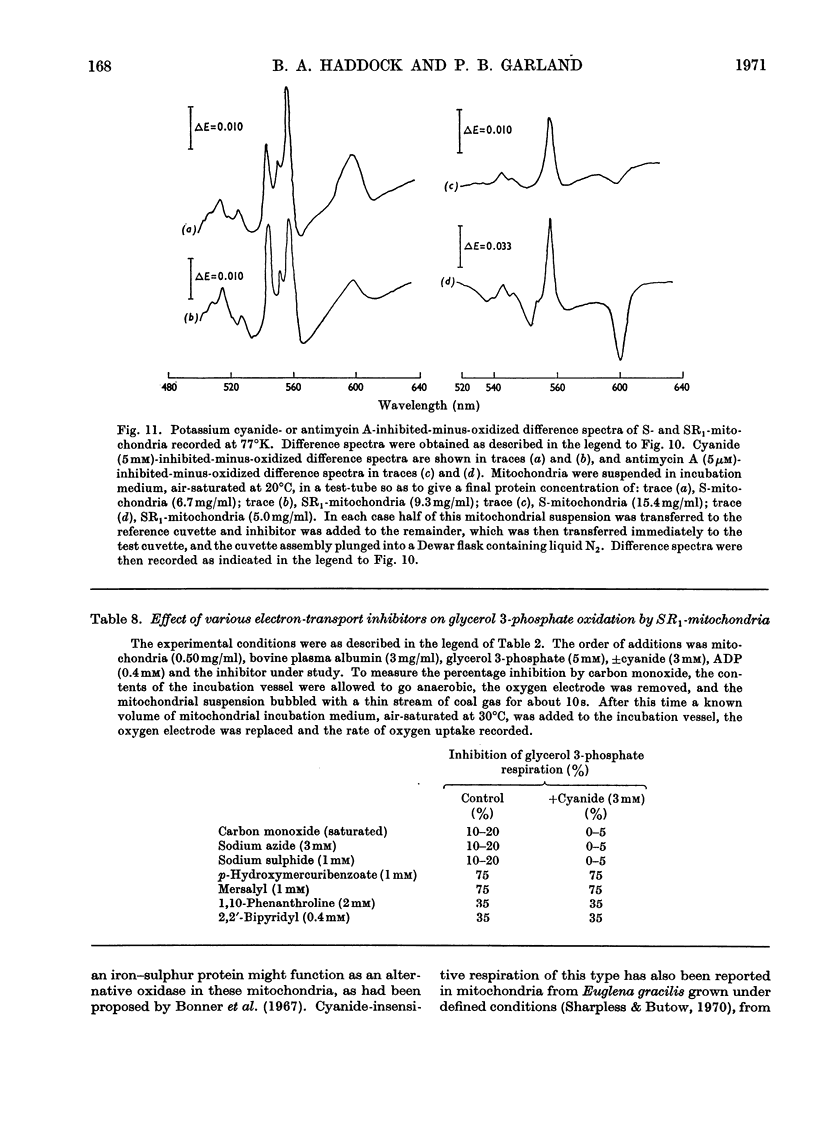
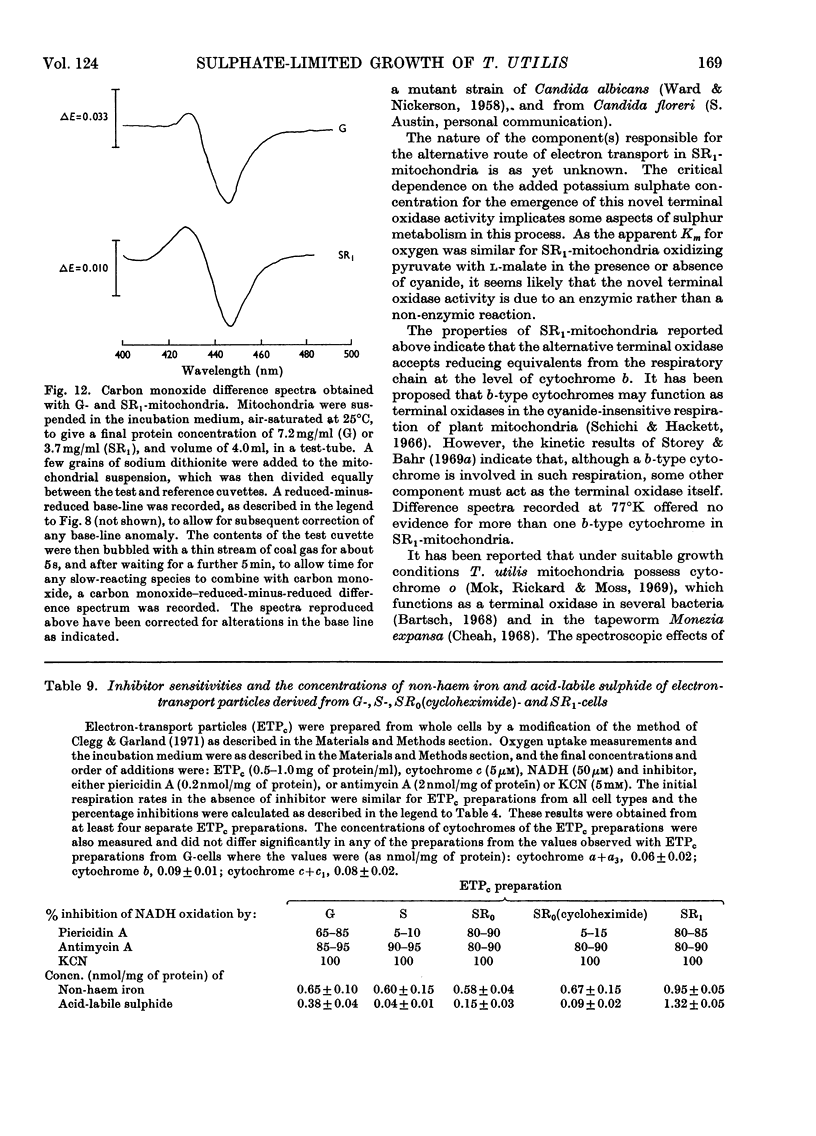

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDALL D. S. Cytochromes and some respiratory enzymes in mitochondria from the spadix of Arum maculatum. Biochem J. 1958 Nov;70(3):381–390. doi: 10.1042/bj0700381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch R. G. Bacterial cytochromes. Annu Rev Microbiol. 1968;22:181–200. doi: 10.1146/annurev.mi.22.100168.001145. [DOI] [PubMed] [Google Scholar]
- CASTOR L. N., CHANCE B. Photochemical action spectra of carbon monoxide-inhibited respiration. J Biol Chem. 1955 Nov;217(1):453–465. [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. I. Difference spectra. J Biol Chem. 1953 May;202(1):383–396. [PubMed] [Google Scholar]
- CHANCE B. The carbon monoxide compounds of the cytochrome oxidases. II. Photodissociation spectra. J Biol Chem. 1953 May;202(1):397–406. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S. The respiratory components of Moniezia expansa (Cestoda). Biochim Biophys Acta. 1968 Apr 2;153(3):718–720. doi: 10.1016/0005-2728(68)90200-4. [DOI] [PubMed] [Google Scholar]
- Clegg R. A., Garland P. B. Non-haem iron and the dissociation of piericidin A sensitivity from site 1 energy conservation in mitochondria from Torulopsis utilis. Biochem J. 1971 Aug;124(1):135–151. doi: 10.1042/bj1240135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg R. A., Ragan C. I., Haddock B. A., Light P. A., Garland P. B., Swann J. C., Bray R. C. Inter-relationships between mitochondrial energy conservation at site I, piericidin a sensitivity, and EPR spectra in torulopsis utilis. FEBS Lett. 1969 Nov 12;5(3):207–210. doi: 10.1016/0014-5793(69)80333-9. [DOI] [PubMed] [Google Scholar]
- Garland P. B. Biochemical applications of continuous culture: energy-conservation mechanisms in Torulopsis utilis. Biochem J. 1970 Jul;118(3):329–339. doi: 10.1042/bj1180329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEYTLER P. G., PRICHARD W. W. A new class of uncoupling agents--carbonyl cyanide phenylhydrazones. Biochem Biophys Res Commun. 1962 May 4;7:272–275. doi: 10.1016/0006-291x(62)90189-4. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Evans M. C. Iron-sulphur proteins. Nature. 1969 Sep 27;223(5213):1342–1348. doi: 10.1038/2231342a0. [DOI] [PubMed] [Google Scholar]
- Light P. A., Garland P. B. A comparison of mitochondria from Torulopsis utilis grown in continuous culture with glycerol, iron, ammonium, magnesium or phosphate as the growth-limiting nutrient. Biochem J. 1971 Aug;124(1):123–134. doi: 10.1042/bj1240123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light P. A., Ragan C. I., Clegg R. A., Garland P. B. Iron-limited growth of torulopsis utilis, and the reversible loss of mitochondrial energy conservation at site 1 and of sensitivity to rotenone and piericidin A. FEBS Lett. 1968 Jul;1(1):4–8. doi: 10.1016/0014-5793(68)80004-3. [DOI] [PubMed] [Google Scholar]
- Mok T. C., Rickard P. A., Moss F. J. The carbon monoxide-reactive haemoproteins of yeast. Biochim Biophys Acta. 1969 Apr 8;172(3):438–449. doi: 10.1016/0005-2728(69)90140-6. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Kawaguchi K., Hagihara B. Preparation and some properties of yeast mitochondria. J Biol Chem. 1966 Apr 25;241(8):1797–1806. [PubMed] [Google Scholar]
- Ohnishi T., Schleyer H., Chance B. Studies on non-heme iron proteins and the piericidin A binding site of submitochondrial particles from Candida utilis cells grown in media of varying iron concentrations. Biochem Biophys Res Commun. 1969 Aug 7;36(3):487–493. doi: 10.1016/0006-291x(69)90591-9. [DOI] [PubMed] [Google Scholar]
- Ragan C. I., Clegg R. A., Haddock B. A., Light P. A., Garland P. B. Spectroscopic resolution of the "iron-flavoprotein trough" of the respiratory chain of submitochondrial particles from Torulopsis utilis. FEBS Lett. 1970 Jul 3;8(6):333–336. doi: 10.1016/0014-5793(90)80007-6. [DOI] [PubMed] [Google Scholar]
- Sharpless T. K., Butow R. A. An inducible alternate terminal oxidase in Euglena gracilis mitochondria. J Biol Chem. 1970 Jan 10;245(1):58–70. [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. I. Electron transport between succinate and oxygen in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):115–125. doi: 10.1104/pp.44.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey B. T., Bahr J. T. The respiratory chain of plant mitochondria. II. Oxidative phosphorylation in skunk cabbage mitochondria. Plant Physiol. 1969 Jan;44(1):126–134. doi: 10.1104/pp.44.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD J. M., NICKERSON W. J. Respiratory metabolism of normal and divisionless strains of Candida albicans. J Gen Physiol. 1958 Mar 20;41(4):703–724. doi: 10.1085/jgp.41.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum C. S., Hackett D. P. Participation of Cytochromes in the Respiration of the Aroid Spadix. Plant Physiol. 1957 May;32(3):186–191. doi: 10.1104/pp.32.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]


