Abstract
Background and aim
The liver is susceptible to ischemia-reperfusion injury (IRI) during hepatic surgery, when the vessels are compressed to control bleeding, or liver transplantation, when there is an obligate period of ischemia. The hallmark of IRI comprises mitochondrial dysfunction, which generates reactive oxygen species, and cell death through necrosis or apoptosis. Cyclosporine (CsA), which is a well-known immunosuppressive agent that inhibits calcineurin, has the additional effect of inhibiting the mitochondrial permeability transition pore (mPTP), thereby, preventing mitochondrial swelling and injury. NIM-811, which is the nonimmunosuppressive analog of CsA, has a similar effect on the mPTP. In this study, we tested the effect of both agents on mitigating warm hepatic IRI in a murine model.
Materials and methods
Before ischemic insult, the mice were administered with intraperitoneal normal saline (control); CsA at 2.5, 10, or 25 mg/kg; or NIM-811 at 10 mg/kg. Thereafter, the mice were subjected to partial warm hepatic ischemia by selective pedicle clamping for 60 min, followed by 6 h of recovery after reperfusion. Serum alanine transaminase (ALT) was measured, and the liver tissue was examined histologically for the presence of apoptosis and the levels of inflammatory cytokines.
Results
Compared with the control mice, the mice treated with 10 and 25 mg/kg of CsA and NIM-811 had significantly lower ALT levels (P < 0.001, 0.007, and 0.031, respectively). Moreover, the liver tissue showed reduced histological injury scores after treatment with CsA at 2.5, 10, and 25 mg/kg and NIM-811 (P = 0.041, <0.001, 0.003, and 0.043, respectively) and significant decrease in apoptosis after treatment with CsA at all doses (P = 0.012, 0.007, and <0.001, respectively). Levels of the pro-inflammatory cytokines, particularly interleukin (IL)-1β, IL-2, IL-4, IL-10, and keratinocyte chemoattractant/human growth-regulated oncogene significantly decreased in the mice treated with the highest dose of CsA (25 mg/kg) than those in the control mice.
Conclusions
Premedication with CsA or NIM-811 mitigated hepatic IRI in mice, as evidenced by the decreased ALT and reduced injury on histology. These results have potential implications on mitigating IRI during liver transplantation and resection.
Keywords: Cyclosporine (CsA), Cyclosporine analogue, Liver surgery, Ischemia-reperfusion injury (IRI), Animal model, NIM-811
1. Introduction
Ischemia-reperfusion injury (IRI) results from a transient loss of blood flow to an organ.1 The first phase is ischemia, which is marked by cell death from metabolic stress secondary to a lack of oxygen.2 During ischemia, a cascade of intersecting events occurs as a consequence of the rapid depletion of cellular energy stores, i.e., adenosine triphosphate (ATP).3 After immediate consumption of the pre-existing aerobically generated ATP, the liver resorts to anaerobic glycolysis to generate ATP; however, the by-product generated is lactic acid, which decreases tissue pH and further inhibits ATP production. Lack of ATP is a key feature of ischemia and causes functional loss of ATP-dependent electrolyte transporters, including Na+/K+-ATPase and Ca2+-ATPase, thereby, resulting in intracellular hyperosmolarity and hypercalcemia, cellular swelling from osmotic fluid shifts, and activation of Ca2+-dependent proteases and phosphorylases.4
The second reperfusion phase occurs after restoration of blood flow and is marked by activation of the innate immune system, leading to infiltration of peripheral immune cells.2 Ischemic injury to the necrotic cells are exacerbated by injury from reactive oxygen species (ROS), which are generated by the mitochondria after resumption of oxidative phosphorylation; this triggers the release of damage-associated molecular patterns,3 which are detected by local immune cells that release cytokines and other chemotactic factors to perpetuate the local innate immune response and recruit immune cells from the periphery. The influx of immune cells, mainly neutrophils in the early stages, initiates a pro-inflammatory cascade that leads to further cell death and is thought to be mainly responsible for the extent of injury.
Clinically, IRI in the liver may occur during hepatic resection, in cases that need intermittent compression of the arterial and portal venous inflow (i.e., Pringle maneuver) to control bleeding, or during liver transplantation, which entails an obligate period of ischemia during transport and implantation.5 Liver IRI during transplantation is mainly addressed by the use of preservation solutions during cold ischemia and, more recently, machine perfusion of grafts.6 Although several pharmacological strategies have demonstrated efficacy in preclinical studies, few have been proven to be clinically effective and none have been adopted universally.
Cyclosporine (CsA) is well known for its immunosuppressive effect and works by preventing T cell activation through inhibition of calcineurin.7 A secondary effect of CsA is inhibition of cyclophilin D, which is a key component of the mitochondrial permeability transition pore (mPTP).8 As alluded above, the mitochondria feature prominently in the pathophysiology of IRI because of their role in ATP production, ROS generation, and initiation of apoptosis and necrosis. Inhibition mPTP has the potential to prevent mitochondrial swelling during oxidative stress, which, if severe enough, leads to irreversible cell death. Similarly, the CsA analog NIM-811 inhibits cyclophilin D but lacks the immunosuppressive effect of CsA.9
The use of CsA for the prevention of IRI has been investigated in other clinical scenarios, including revascularization following thrombosis of the coronary and cerebral arteries in myocardial infarction and stroke, respectively.10,11 Despite the strong preclinical evidence from animal models of myocardial IRI, the randomized controlled CIRCUS trial, wherein patients received 2.5 mg/kg of CsA prior to coronary revascularization, failed to show improvements in clinically meaningful outcomes, including death at one year, worsening heart failure, rehospitalization, and adverse ventricular remodeling.12 One reason for that result might be the clinically observed longer ischemia time in myocardial IRI than in hepatic IRI.
Although CsA has been established as an immunosuppressive agent in solid organ transplantation, its potential to mitigate IRI in this setting has been reported by only few studies. In France, the first clinical randomized controlled trial (NCT02907554) on the value of donor administration of CsA in preventing IRI during kidney transplantation is currently underway.13 Contrary to the spontaneous onset of ischemia in myocardial infarction or stroke, the more controlled predictability of IRI in organ transplantation and hepatic surgery offers the potential for greater benefit and more controlled, timely delivery of CsA. Moreover, the CsA analog NIM-811 may have the same effect without inducing unnecessary immune suppression.
Before extending the clinical use of CsA to mitigate liver IRI, further evidence on its efficacy is required from preclinical studies. In this study, we described the application of CsA and its analog NIM-811 to ameliorate warm hepatic IRI in a murine model. NIM-811 served as an important control to identify a comparable effect with CsA, without the immunosuppressive action.
2. Materials and methods
2.1. Ethical approval
All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Alberta, Edmonton, AB, Canada (Ref. No. AUP00002033), following the guidelines of the Canadian Council of Animal Care.
2.2. Animal model
A similar model that was reported in our previous study was used for this present study.14 Male C57BL/6 mice aged 10–12 weeks were purchased from Charles River Laboratories (Saint-Constant, QC, Canada). Two hours prior to ischemia, the mice were injected intraperitoneally with either 100 μL of normal saline (NS, control) or 2.5, 10, or 25 mg/kg of CsA (Sandimmune IV; Novartis Pharmaceuticals Canada Inc., Dorval, QC, Canada) diluted in the same volume of NS. An additional group was treated in the same way with 10 mg/kg of NIM-811 (MedChemExpress, Monmouth Junction, NJ, USA).
To induce liver ischemia, the mice were anesthetized with isoflurane (Frensenius Kabi Canada, Toronto, ON, Canada) and underwent midline laparotomy. Under 10X magnification (MZ6, Leica Microsystems Inc., Concord, ON, Canada), two atraumatic microvascular clamps (Micro Serrefines; Fine Science Tools Inc., North Vancouver, BC, Canada) were placed across the arterial and portal venous branches that supplied the median and left lobes (i.e., approximately 70% of the liver mass). Ischemia was verified visually by immediate blanching of the lobes (Fig. 1). Notably, this model used partial hepatic ischemia to avoid venous congestion of the bowel secondary to occlusion of the entire portal vein. Thereafter, the mice were given intraperitoneal heparin (Frensenius Kabi Canada, Toronto, ON, Canada) at 5 IU diluted in 500 μL of NS; the abdominal skin was closed with running 4-0 polypropylene suture (Prolene; Ethicon, Cincinnati, OH, USA) to prevent heat loss. A heating pad was placed beneath the mice to maintain a temperature of 37 °C. The mice were maintained under an inhalational anesthetic (Isoflurane; Frensenius Kabi Canada, Toronto, ON, Canada) for the entire duration of ischemia.
Fig. 1.
Liver ischemia was verified visually by expected intraoperative findings. Blanching of the median lobe (ML) and left lateral lobe (LLL) is noted with the application of a vascular clamp. The unclamped right lateral lobe (RLL) appears slightly hyperemic.
After 1 h, the clamps were removed, and return of blood flow was observed. A bolus of 500-μL NS was given intraperitoneally before closing the fascia with running 4-0 polyglactin suture (Vicryl; Ethicon, Cincinnati, OH, USA). The skin was secured with 9-mm wound clips (Autoclip; Becton Dickinson Canada Inc., Mississauga, ON, Canada). For postoperative pain control, approximately 200 μL of local anesthetic (0.25% bupivacaine, Sensorcaine; AstraZeneca Canada Inc., Mississauga, ON, Canada) was applied topically to the wound and 100 μL of 0.015 mg/kg buprenorphine (Temgesic; Schering-Plough Canada Inc., Pointe-Claire, QC, Canada) was subcutaneously injected. Recovery was facilitated by placing the mice under heat lamps and returning them to their cage, where they had free access to food and water.
At 6 h after ischemia, the mice were sacrificed humanely by exsanguination and thoracotomy under isoflurane (Frensenius Kabi Canada, Toronto, ON, Canada) anesthesia. A 6-h endpoint was chosen, based on our preliminary tests and reference sources for the time of peak serum alanine transaminase (ALT) elevation.14,15 The laparotomy incision was reopened. Using a 23G needle, blood was removed by cardiac puncture and collected in capillary blood collection tubes (Microvette 500; Sarstedt AG & Co., Nümbrecht, Germany) for subsequent centrifugation at 10,000 g for 5 min; the supernatant was frozen at −80 °C. Hepatectomy was also performed. A portion of the ischemic and nonischemic control lobes were immediately frozen at −80 °C, and another portion was fixed in 10% formalin. A schematic illustration of the model is shown in Fig. 2. We included seven sham surgical animals that underwent laparotomy and hepatic manipulation but were not subjected to hepatic ischemia.
Fig. 2.
Schematic illustration of the model. Timeline (in hours) of 2.5, 10, or 25 mg/kg of cyclosporine (CsA) or 10 mg/kg of NIM-811 treatment and induction of hepatic ischemia-reperfusion injury are shown. Abbreviations: IP, intraperitoneal; NS, normal saline.
2.3. Determination of serum ALT
After 6 h of ischemia, serum ALT was determined using a VetTest Chemistry Analyzer (IDEXX Laboratories Canada Corp., Markham, ON, Canada). NS was used to dilute the samples as required.
2.4. Histology
At 6 h after ischemia, tissues were collected, fixed in formalin and embedded in paraffin, sectioned, and stained with hematoxylin and eosin. The slides were reviewed by a trained pathologist, who was blinded to the treatment groups. Scores were assigned based on the assessment of necrosis and hemorrhage (0 for absent, 1 for pericentral, 2 for zonal, and 3 for panlobular); cholestasis (0 for absent and 1 for present); and sinusoidal dilatation (0 for none, 1 for mild, 2 for moderate, and 3 for severe), in accordance with a previously published scoring system.16
2.5. Terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling (TUNEL)
Deadend™ fluorometric TUNEL system (Promega, Madison, WI, USA) was used to identify apoptosis in the paraffin-embedded tissue sections. Briefly, the slides were washed in xylene, followed by rehydration with ethanol. The tissue on the slides was fixed using 4% paraformaldehyde and permeabilized with proteinase K. A nucleotide mixture containing fluorescein-12-deoxyuridine triphosphate was added to both tissue sections on the slide, whereas recombinant terminal deoxynucleotidyl transferase was added to only one tissue section, with the other serving as a negative control. After 1 h at 37 °C, the reaction was stopped, and the slides were washed and mounted with media containing 4′,6-diamidino-2-phenylindole (DAPI) for counterstaining. The slides were viewed and imaged at 20X (Axio Observer Z1; Carl Zeiss Canada Ltd., Toronto, ON, Canada). Quantification of the images was performed using Fiji software (General Public License).
2.6. Pro-inflammatory cytokines
Frozen tissue samples were thawed, and a 0.5 mg portion was placed in lysis buffer, including 50 nmol 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; MilliporeSigma Canada Ltd, Oakville, ON, Canada), 1 mmol ethylenediamine tetraacetic acid (EDTA; MilliporeSigma Canada Ltd, Oakville, ON, Canada), 150 nmol NaCl (MilliporeSigma Canada Ltd, Oakville, ON, Canada), and 1% (V/V) Triton X-100 (MilliporeSigma Canada Ltd, Oakville, ON, Canada) and homogenized. A pro-inflammatory panel mouse kit (Meso Sector S 600 plate reader; Meso Scale Diagnostics LLC, Rockville, MD, USA) was used to measure the following tissue cytokines: interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, keratinocyte chemoattractant/human growth-regulated oncogene (KC/GRO), tumor necrosis factor alpha (TNFα), and interferon-gamma (IFN-γ).
2.7. Statistical analysis
Statistical analysis was performed using SPSS version 18 (IBM Canada, Markham, ON, Canada). The data are presented as median and interquartile range and were tested for normality using the Shapiro–Wilk test and a histogram of distribution. Non-normal continuous data were transformed for normality using a two-step approach, as previously described.17 Student's t-test was used for comparison between two groups, and ordinary one-way analysis of variance test was used for multigroup comparisons. A P value < 0.05 was considered statistically significant.
3. Results
3.1. CsA reduced serum ALT following warm IRI
Figure 3 illustrates the differences in the serum ALT seen among the groups. The serum ALT level was significantly reduction in the mice treated with 10 or 25 mg/kg of CsA than in the control mice (1793.5 (1603.0–2157.0) U/L and 1823.0 (1668.0–2932.0) U/L, respectively vs. 3300.5 (2481.0–4911.0) U/L; P < 0.001 and 0.007, respectively) but was not significantly different between the mice treated with 2.5 mg/kg of CsA (4041.0 (2327.0–4871.0) U/L; P = 0.845) and the control mice. The serum ALT was significantly lower in the sham mice (383.0 (229.0–562.0) U/L; P < 0.001) than in all the treatment groups. Moreover, the mice treated with 10 mg/kg NIM-811 had significantly lower serum ALT (2375.0 (1963.0–2919.0) U/L; P = 0.031), compared with that in the control.
Fig. 3.
Effect of cyclosporine on serum alanine transaminase (ALT). The serum ALT levels at 6 h after partial liver ischemia in mice treated with normal saline (NS); 2.5, 10, or 25 mg/kg of cyclosporine (CsA 2.5, 10, 25); or 10 mg/kg of NIM-811 are shown (n = 7 for sham group, n = 9 to 14 for other groups). Results that were not significantly different are not shown.
The serum CsA levels were 414.50 (412.75–416.25) μg/L, 1145.00 (1102.50–1187.50) μg/L, and >2500.00 μg/L at 2 h after injection of CsA at 2.5, 10, and 25 mg/kg, respectively. All levels were within or above the range required for chronic immunosuppression.18 At this time point, treatment of the mice with CsA only (25 mg/kg) had no effect on the serum ALT (67 (61–87) U/L; normal range, 28–132 U/L).
3.2. CsA decreased the degree of necrosis on histological evaluation
Figure 4 shows the differences in histological scores among the treatment groups. Compared with the control mice, the mice treated with CsA at 2.5, 10, and 25 mg/kg had significantly lower total scores for histological injury (P = 0.041, <0.001, and 0.003, respectively) (Fig. 4A). This was driven mainly by the lower subscores for necrosis in the mice treated with all doses of CsA than in the control mice (P = 0.007, <0.001, and <0.001 for the 2.5, 10, and 25 mg/kg doses, respectively) (Fig. 4B). Moreover, the subscores for sinusoidal dilatation were lower in the CsA-treated mice than in the control mice (P = 0.018, <0.001, and <0.001 for the 2.5, 10, and 25 mg/kg doses, respectively), although only pericentral dilatation was seen in the control mice (Fig. 4C). Representative photomicrographs of livers from treated and untreated mice are shown in Fig. 4D. Significantly lower histological scores for injury were seen in the mice treated with NIM-811 than in the control mice (P = 0.043, Fig. 4A). Compared with the control, the NIM-811-treated mice had similar subscores for necrosis (P = 0.158) but significantly lower scores for sinusoidal dilation (P < 0.001, Fig. 4C).
Fig. 4.
Effect of cyclosporine (CsA) on tissue injury.(A–C) The (A) total histological scores and subscores for (B) necrosis and (C) sinusoidal dilatation of the liver tissue sections after 6 h of partial ischemia in the mice treated with normal saline (NS); 2.5, 10, or 25 mg/kg of CsA (CsA 2.5, 10, 25); or 10 mg/kg of NIM-811 are shown. Results that were not significantly different are not shown. (D) Representative photomicrographs of the liver sections from the control mice (top left) and the mice treated with 2.5 and 10 mg/kg of CsA (top right and bottom left, respectively) and 10 mg/kg of NIM-811 (bottom right) are shown. The yellow circles highlight the area of necrosis. n = 5 to 6 for each group.
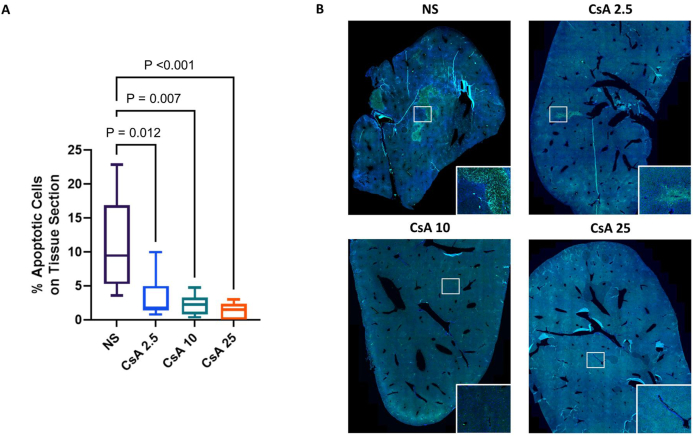
3.3. Apoptosis from IRI was decreased in the mice treated with CsA
Figure 5 shows the results of the TUNEL staining. Compared with the control mice, the mice treated with CsA at 2.5, 10, and 25 mg/kg had significantly lower percentage of apoptotic cells (P = 0.012, 0.007 and < 0.001, respectively) (Fig. 5A). Representative images of the TUNEL-stained tissue sections from the control mice and the mice treated with 2.5, 10, and 25 mg/kg of CsA are also shown in Fig. 5B (20X magnification). The bright green nuclei, which were seen most clearly in the magnified inset (50X magnification), indicated positive TUNEL staining.
Fig. 5.
Effect of cyclosporine (CsA) on cell death from apoptosis. (A) The results of the TUNEL staining for quantification of the percentage of apoptotic cells after 6 h of partial liver ischemia in the mice treated with normal saline (NS) and 2.5, 10, or 25 mg/kg of CsA (CsA 2.5, 10, 25) are shown. (B) Representative images from the control (NS) and the mice treated with 2.5, 10, or 25 mg/kg of CsA (CsA 2.5, CsA 10, and CsA 25, respectively) are shown (DAPI counterstain, 20X magnification, the inset shows a portion of the image at 50X magnification). n = 5 to 6 for each group. Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick-end labeling.
3.4. Pro-inflammatory cytokine profiles after ischemia in the mice treated with CsA
As shown in Fig. 6, the liver tissue levels of IL-1β, IL-2, IL-4, IL-10, and KC/GRO were lower in the mice treated with the highest dose of CsA (25 mg/kg) than in the control mice (P = 0.044, 0.021, 0.045, 0.038, and 0.046, respectively).
Fig. 6.
Effect of cyclosporine (CsA) on tissue cytokines. The pro-inflammatory cytokine levels in the liver tissue at 6 h postischemia in the mice treated with normal saline (NS) and 2.5, 10, or 25 mg/kg of CsA (CsA 2.5, 10, 25) are shown. n = 5 to 14 for each group. Abbreviations: IFN-γ, interferon-gamma; IL, interleukin; KC/GRO, keratinocyte chemoattractant/human growth-regulated oncogene; TNFα, tumor necrosis factor alpha.
4. Discussion
Our study provided evidence that administration of escalating doses of CsA prior to warm liver IRI was beneficial in reducing hepatic injury. We found a greater than 50% reduction in serum ALT in the mice treated with 10 or 25 mg/kg of CsA than in the mice treated with only NS. These findings corresponded with the lower degree of histological scores for necrosis. Furthermore, we observed that CsA administration decreased the level of apoptosis following warm liver IRI. The CsA analog NIM-811, which lacks immunosuppressive properties, showed similar reductions in the serum ALT and histologic liver injury, suggesting that the protective effect of CsA may be unrelated to its immunosuppressive effect.
CsA has been previously shown to mitigate IRI in preclinical studies and has been extensively explored, particularly in myocardial IRI.19 However, these findings have not been reliably translated into clinical practice, which is perhaps best exemplified by the CIRCUS trial.12 Difficulties in translating the findings of preclinical studies on myocardial and cerebral infarction models, in particular, reflect the fact that these events occur unexpectedly and patients present after the onset of ischemia. In these settings, CsA cannot be given before ischemia, and the duration of ischemia cannot be controlled. Both of these variables have been reported by preclinical studies to affect the efficacy of CsA, which seemed to work best if given prior to ischemia and has diminished efficacy beyond a critical window of ischemia.20, 21, 22 In contrast, hepatic ischemia can often be anticipated, particularly in the setting of liver transplantation, and can be controlled within certain limits. In addition, achieving the same effect with the use of NIM-811 suggested that unnecessary immunosuppression can be avoided. However, the fact that immunosuppression is required in liver transplantation makes this point somewhat moot.
In this study, the ability of CsA to prevent both necrotic and apoptotic cell death may be attributed its action at the mitochondrial level. This was supported by the similar effect of the nonimmunosuppressive NIM-811 on reducing hepatic injury. In cases of severe insult, mitochondrial swelling and rupture can precipitate necrotic cell death.23 A less severe damage might induce an apoptotic response. The effect of CsA on other programmed cell death responses, such as necroptosis and ferroptosis, is less clear and was not investigated in this study. It is worth noting that our study found some benefits of reduced histological injury and apoptosis with even the lowest tested dose of CsA (2.5 mg/kg), although this was not reflected by the serum ALT. The similar benefits of the 10 and 25 mg/kg CsA doses suggested that increasing the dose beyond 10 mg/kg will not improve protection. After an extensive review of the studies on myocardial IRI, the most commonly used CsA dose of 10 mg/kg was used for this study, and doses lower and higher than 10 mg/kg were chosen to identify a possible dose-dependent effect. We chose a single dose of NIM-811, because the group that received this mainly served as a nonimmunosuppressive control to illustrate the importance of mPTP inhibition. We thought that the addition of further doses of NIM-811 would complicate the interpretation of the results and would be better left to a separate study. For this present study, our intention was to use this secondary agent as a positive control, without a direct intent to use it as a therapeutic protective agent clinically. Notably, the serum concentrations of CsA in this study were in the range that was similar to the usual CsA peak after 2 h of an oral dose for immunosuppressive indications but different from the 12-h trough levels that are normally used in clinical practice. We simply wanted to show that the peak CsA level that can achieve a hepatoprotective effect was somewhat similar to that used in chronic dosing for immunosuppression. Expectedly, using a single dose without steady state kinetics would make the risk of overimmunosuppression in the recipient irrelevant.
The role of pro-inflammatory cytokines in inducing IRI through the recruitment of immune cells is well documented.3 In this study, we found small but significant differences in the levels of pro-inflammatory cytokines. Those that achieved statistical significance (i.e., IL-2 and IL-4) are associated with T cell activation, which tends to occur later in the course of IRI when the adaptive immune system becomes involved.24 This could also be a reflection of calcineurin inhibition by CsA.7 The absence of significant differences in the other pro-inflammatory cytokine levels in this study could be attributed to the later time point that was chosen to detect maximal tissue damage or may reflect the fact that immunosuppression might not be the main mechanism of protection from IRI by CsA. However, our study was not specifically designed to detect differences at this level. We would expect more pronounced differences in tissue cytokine levels earlier in the course of IRI. Other studies on liver IRI have implicated a variety of different cytokines, including TNFα, IL-1β, and IL-6.25 Of these, with the highest dose of CsA, only IL-1β was found to be slightly decreased after 6 h of ischemia. In this study, the liver enzymes at the 6-h time point could have differed. Further examination at multiple time points could identify the possibly different peak levels of the cytokines. In choosing a later time point, our goal was to identify clinically relevant effects, but we acknowledge that this may be at the cost of mechanistic insight, which could further be explored in future studies.
Previous studies have well described the challenges in modeling liver IRI in mice, particularly the variability in the level of injury among the animals, which can be difficult to control even within the same study.26 Differences in the sex, strain, and even substrain of mice are known to affect outcomes. Our choice of male mice was in line with the majority of other studies, because female sex had been thought to be protective.27 In our experience, we found several factors that decreased the variation in serum transaminases in the model. Applying the clamp under magnification was important, because it allowed consistent application and verification that the clamp crossed the entire portal venous branch and captured the smaller arterial branches. We also observed that application of a second clamp reduced variability. In some cases, the portal branches to the upper lobes were abnormally short or the branches to the right lobe were higher than usual, resulting in ischemia to the right lobe upon clamping. This often resulted in venous congestion of the bowels and precipitated demise of the animal.
For this study, we followed the attestation of other authors to the importance of heparin administration to prevent the formation of blood clots, which can lead to additional hepatic injury.15 The effects of fasting state, body temperature, and diurnal variations in serum transaminases were not examined in this study, although we did maintain consistency in the timing of the experiments and the use of warming devices during and after surgery. Although fasting had been employed in models of toxic liver injury, such as those with acetaminophen, to reduce glutathione levels and increase susceptibility to injury, fasting in IRI liver models is not routinely used, and prolonged fasting was even found to be protective against liver IRI.28
A murine model provided a relatively straightforward means of screening novel compounds for the potential to protect against liver IRI. However, several important differences from clinical scenarios limit its direct translation. Liver transplantation is the most common clinical setting wherein liver IRI occurs. In this murine model, the lacking features of IRI in liver transplantation were complete vascular isolation (i.e., no possibility for hepatic venous backflow), a period of cold ischemia, increased use of normothermic machine or regional oxygenated perfusion technologies, and the use of immunosuppression to prevent tissue rejection. This model fit more closely with the IRI encountered during hepatic resection. However, clinically, this type of ischemia usually occurs for a much shorter period (ideally <15 min) or in multiple brief periods interspersed with reperfusion, which may be less injurious to the liver. An extended duration of vascular inflow occlusion is rarely required to control bleeding. Therefore, in the clinical setting of hepatic resection, the effect of CsA or NIM-811 may be less pronounced. Lack of mechanistic findings is an additional limitation of this study. As discussed above, the endpoint was chosen to identify an effect on clinically relevant parameters. However, we acknowledge that it may not be suitable for detecting earlier changes in the course IRI that would explain why CsA is effective. The evidence for the effect of CsA on IRI via the mPTP is bolstered by its dose dependent effect and the efficacy of its non-immunosuppressive analog. However, we cannot say conclusively that the clinically relevant effect observed is due to the inhibition of mPTP. Further mechanistic studies, particularly with the more novel compound, NIM-811, would be required.
5. Conclusions
Our findings provided evidence that CsA can reduce hepatic warm IRI in a murine model. The similar findings with the use of its nonimmunosuppressive analog NIM-811 suggested that the protection was unlikely mediated by immunosuppressive pathways. However, we did not find evidence of this on a mechanistic level. Still, these results have potential implications for liver transplantation and resection. Prior to the initiation of a clinical trial, a study on CsA or NIM-811 using a large animal model of hepatic IRI, preferably simulating liver transplantation, would be beneficial.
Data availability statement
The data presented in this study are available from the authors upon request to the corresponding author. The study protocols and standard operating procedures are also available upon request. The data must not be processed for purposes other than statistical and scientific studies.
Authors’ contributions
Joshua Hefler contributed to research design, performance of the research, data analysis, and writing of the manuscript. Rena Pawlick, Braulio A. Marfil-Garza, Nerea Cuesta-Gomez, and Sanaz Hatami contributed to performance of the research, data analysis, and editing of the manuscript. Aducio Thiesen contributed to data analysis and editing of the manuscript. Darren H. Freed, Constantine Karvellas, and David L. Bigam contributed to research design and editing of the manuscript. A.M. James Shapiro contributed to research design, supervision, and editing of the manuscript. All authors read and approved the final version of this manuscript.
Declaration of competing interest
A.M. James Shapiro reports a consulting or advisory role with ViaCyte Inc., Hemostemix Inc., and Aspect Biosystems Ltd. All remaining authors declare no conflicts of interest. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Edited by Peiling Zhu
References
- 1.Dar W.A., Sullivan E., Bynon J.S., Eltzschig H., Ju C. Ischaemia reperfusion injury in liver transplantation: cellular and molecular mechanisms. Liver Int. 2019;39:788–801. doi: 10.1111/liv.14091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Y., Petrowsky H., Hong J.C., Busuttil R.W., Kupiec-Weglinski J.W. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nat Rev Gastroenterol Hepatol. 2013;10:79–89. doi: 10.1038/nrgastro.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalogeris T., Baines C.P., Krenz M., Korthuis R.J. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierce G.N., Czubryt M.P. The contribution of ionic imbalance to ischemia/reperfusion-induced injury. J Mol Cell Cardiol. 1995;27:53–63. doi: 10.1016/s0022-2828(08)80007-7. [DOI] [PubMed] [Google Scholar]
- 5.Rampes S., Ma D. Hepatic ischemia-reperfusion injury in liver transplant setting: mechanisms and protective strategies. J Biomed Res. 2019;33:221–234. doi: 10.7555/JBR.32.20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrowsky H., Clavien P.A. In: Transplantation of the Liver. Busuttil R.W., Klintmalm G.B.G., editors. 3rd ed. Elsevier Saunders; 2015. Principles of liver preservation; pp. 582–599. [Google Scholar]
- 7.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 8.Halestrap A.P., Connern C.P., Griffiths E.J., Kerr P.M. Cyclosporin A binding to mitochondrial cyclophilin inhibits the permeability transition pore and protects hearts from ischaemia/reperfusion injury. Mol Cell Biochem. 1997;174:167–172. [PubMed] [Google Scholar]
- 9.El Baradie K.B.Y., Khan M.B., Mendhe B., Waller J., O’Brien F 3rd, Hamrick M.W. The cyclophilin inhibitor NIM-811 increases muscle cell survival with hypoxia in vitro and improves gait performance following ischemia-reperfusion in vivo. Sci Rep. 2021;11:6152. doi: 10.1038/s41598-021-85753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piot C., Croisille P., Staat P., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 11.Nighoghossian N., Berthezène Y., Mechtouff L., et al. Cyclosporine in acute ischemic stroke. Neurology. 2015;84:2216–2223. doi: 10.1212/WNL.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 12.Cung T.T., Morel O., Cayla G., et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 13.Orban J.C., Fontaine E., Cassuto E., et al. Effects of cyclosporine A pretreatment of deceased organ donors on kidney graft function (Cis-A-rein): study protocol for a randomized controlled trial. Trials. 2018;19:231. doi: 10.1186/s13063-018-2597-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bral M., Pawlick R., Marfil-Garza B., et al. Pan-caspase inhibitor F573 mitigates liver ischemia reperfusion injury in a murine model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0224567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe Y., Hines I.N., Zibari G., et al. Mouse model of liver ischemia and reperfusion injury: method for studying reactive oxygen and nitrogen metabolites in vivo. Free Radic Biol Med. 2009;46:1–7. doi: 10.1016/j.freeradbiomed.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brockmann J., Reddy S., Coussios C., et al. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 17.Templeton G.F. A two-step approach for transforming continuous variables to normal: implications and recommendations for IS research. Commun Assoc Inf Syst. 2011;28:41–58. doi: 10.17705/1CAIS.02804. [DOI] [Google Scholar]
- 18.Xu F., Chen Z.L., Jin W.J., Xie Q.D., Shi X.H. Ideal therapeutic range of cyclosporine in whole blood in kidney-transplanted patients. Int J Clin Pharmacol Res. 1993;13:221–224. [PubMed] [Google Scholar]
- 19.Rahman F.A., Abdullah S.S., Manan W.Z.W.A., et al. Efficacy and safety of cyclosporine in acute myocardial infarction: a systematic review and meta-analysis. Front Pharmacol. 2018;9:238. doi: 10.3389/fphar.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Paulis D., Chiari P., Teixeira G., et al. Cyclosporine A at reperfusion fails to reduce infarct size in the in vivo rat heart. Basic Res Cardiol. 2013;108:379. doi: 10.1007/s00395-013-0379-4. [DOI] [PubMed] [Google Scholar]
- 21.Kloner R.A., Hale S.L., Gorman R.C., et al. Abstract 9581: bendavia, a novel mitochondrial-targeted cytoprotective compound reduces ischemia/reperfusion injury: experience in 3 independent laboratories. Circulation. 2011;124:A9581. doi: 10.1161/circ.124.suppl_21.a9581. [DOI] [Google Scholar]
- 22.Hefler J., Marfil-Garza B.A., Campbell S., Freed D.H., Shapiro A.M.J. Preclinical systematic review & meta-analysis of cyclosporine for the treatment of myocardial ischemia-reperfusion injury. Ann Transl Med. 2022;10:954. doi: 10.21037/atm-22-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tait S.W., Green D.R. Mitochondrial regulation of cell death. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a008706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rao J., Lu L., Zhai Y. T cells in organ ischemia reperfusion injury. Curr Opin Organ Transplant. 2014;19:115–120. doi: 10.1097/MOT.0000000000000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olthof P.B., van Golen R.F., Meijer B., et al. Warm ischemia time-dependent variation in liver damage, inflammation, and function in hepatic ischemia/reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2017;1863:375–385. doi: 10.1016/j.bbadis.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 26.van Golen R.F., Reiniers M.J., Heger M., Verheij J. Solutions to the discrepancies in the extent of liver damage following ischemia/reperfusion in standard mouse models. J Hepatol. 2015;62:975–977. doi: 10.1016/j.jhep.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Harada H., Pavlick K.P., Hines I.N., et al. Sexual dimorphism in reduced-size liver ischemia and reperfusion injury in mice: role of endothelial cell nitric oxide synthase. Proc Natl Acad Sci U S A. 2003;100:739–744. doi: 10.1073/pnas.0235680100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verweij M., van Ginhoven T.M., Mitchell J.R., et al. Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transpl. 2011;17:695–704. doi: 10.1002/lt.22243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available from the authors upon request to the corresponding author. The study protocols and standard operating procedures are also available upon request. The data must not be processed for purposes other than statistical and scientific studies.