Abstract
1. A binding site (site 1) is present in mitochondria with affinity for trimethyltin and triethyltin adequate for a site to which they could be attached when the processes of energy conservation are inhibited. 2. The quantitative relationships between the binding of trimethyltin and triethyltin to site 1 and their effects on various mitochondrial functions have been examined. 3. ATP synthesis linked to the oxidation of pyruvate, succinate and intramitochondrial substrate, ATP synthesis and oxygen uptake (succinate or pyruvate as substrate) stimulated by uncoupling agents are all inhibited by trimethyltin and triethyltin; when inhibition is less than 50% the ratio (percentage inhibition)/(percentage of binding site 1 complexed) is approx. 10:1. 4. ATP synthesis linked to the oxidation of reduced cytochrome c (ascorbate+NNN′N′-tetramethyl-p-phenylenediamine), ATP hydrolysis and oxygen uptake in the presence of low concentrations of trimethyltin and triethyltin approach zero activity as the proportion of binding site 1 complexed approaches 100%. 5. Possible interpretations of these findings are discussed with reference to published arrangements for coupling of electron transport to ATP synthesis and also to our present knowledge of the chemical and biological specificity of trialkyltin compounds.
Full text
PDF


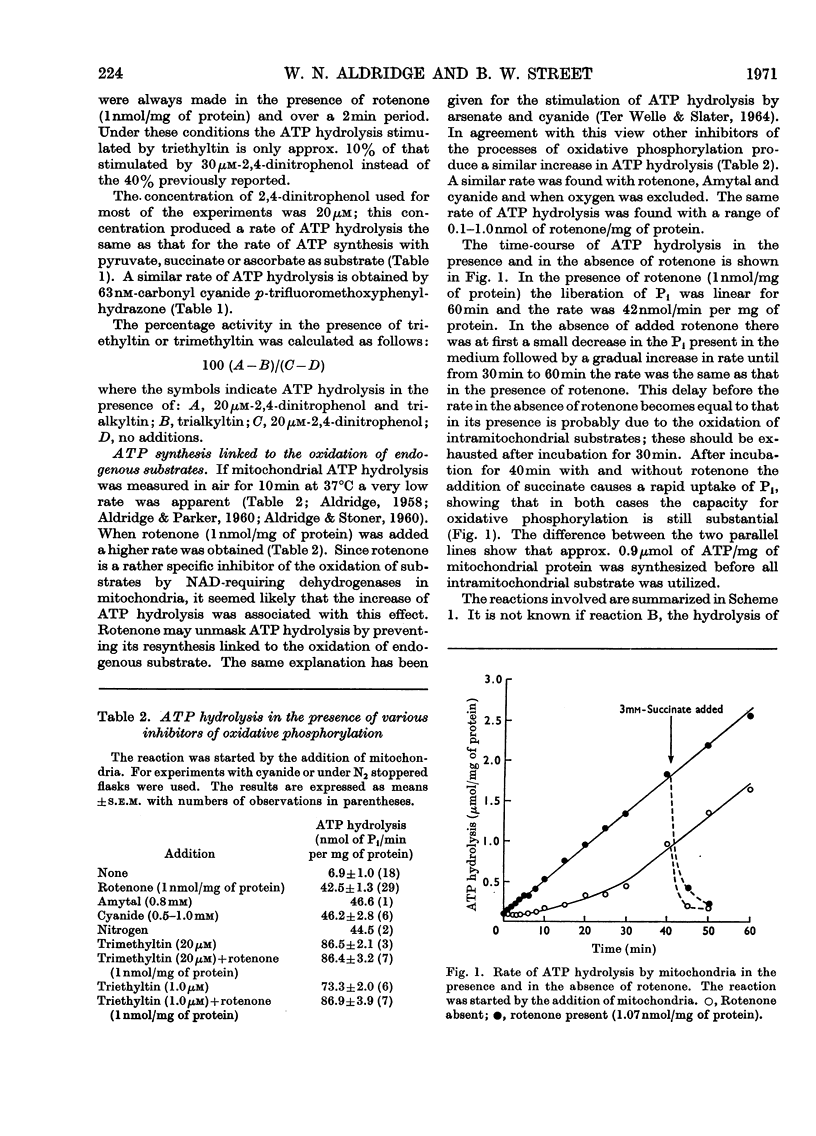
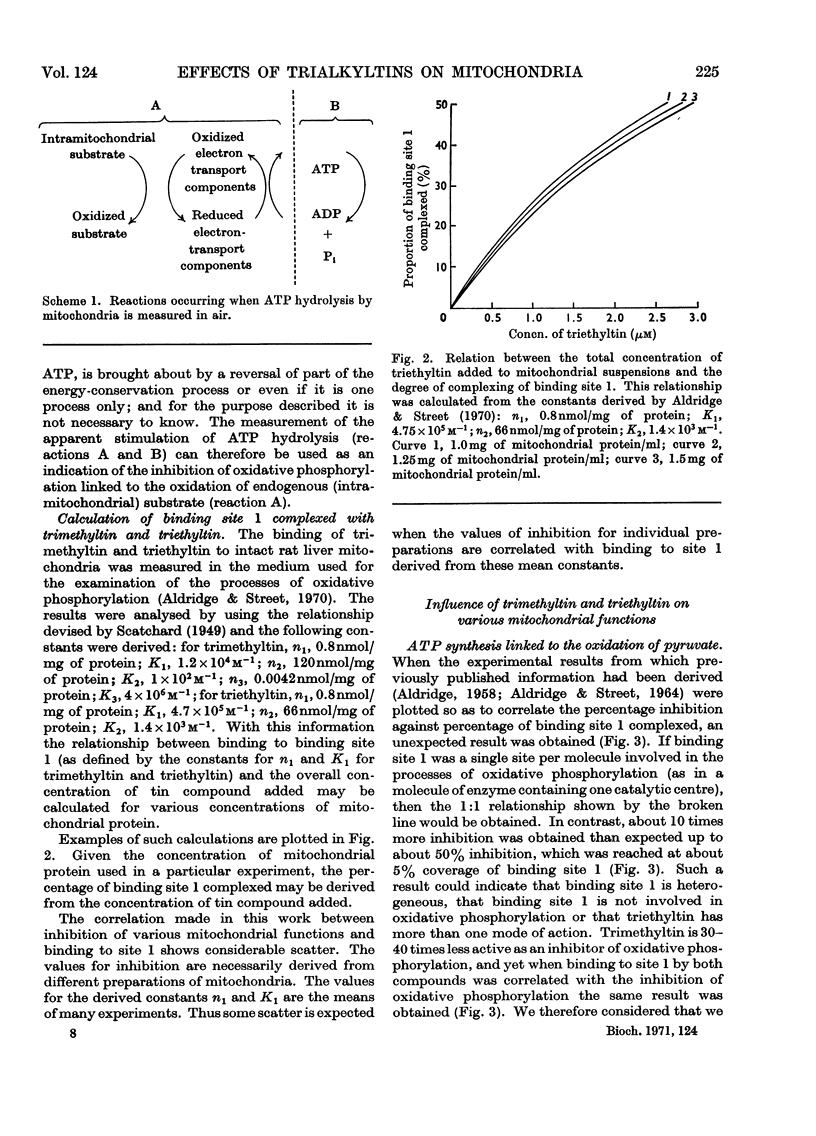
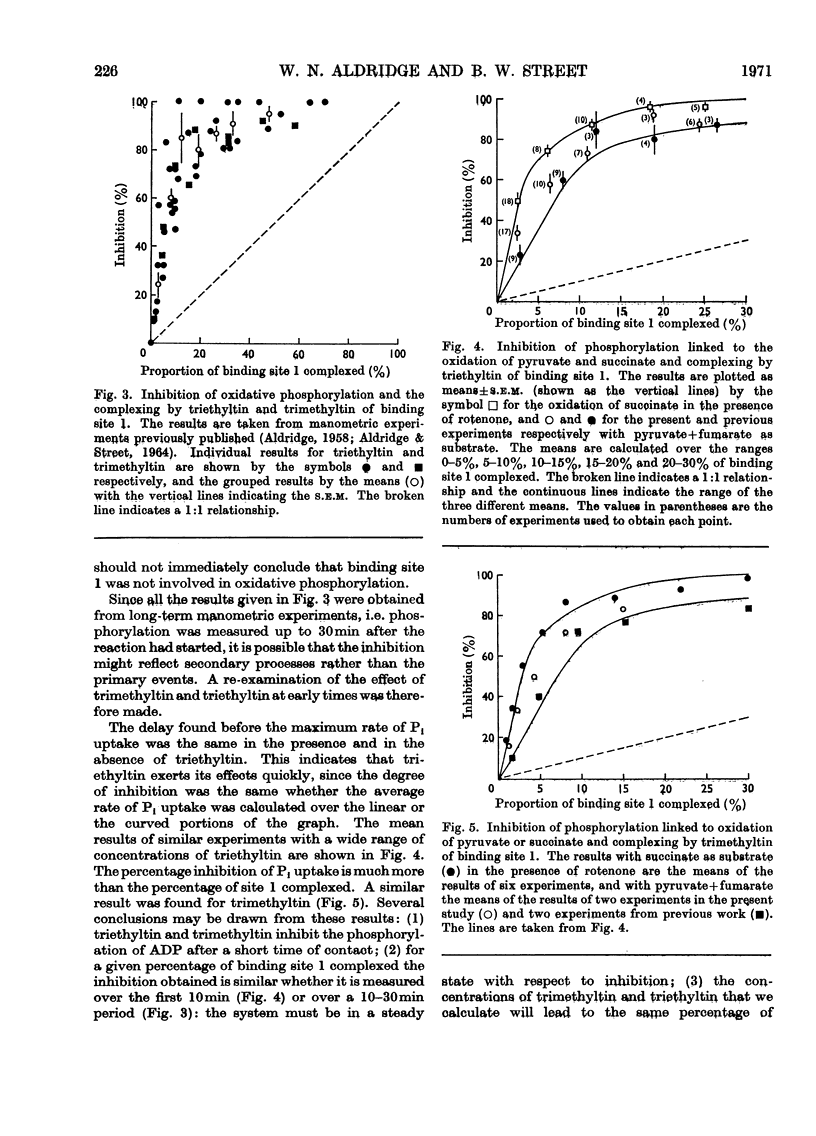
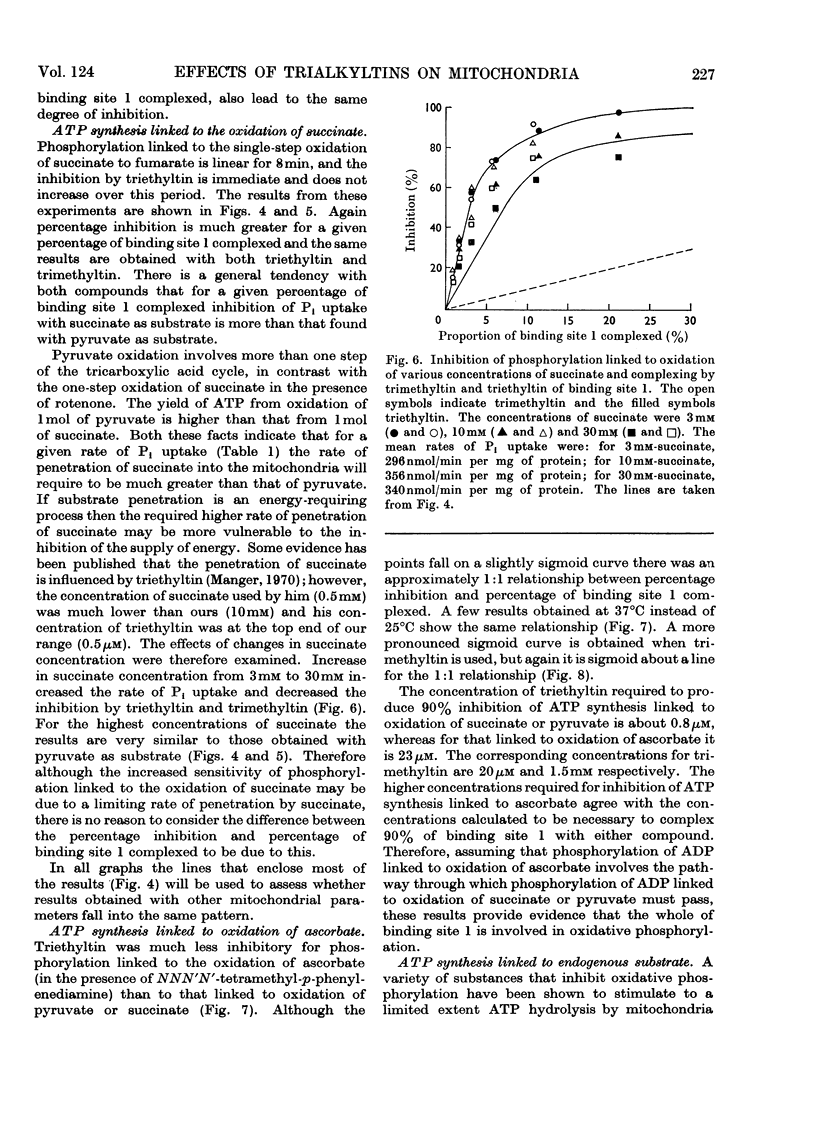
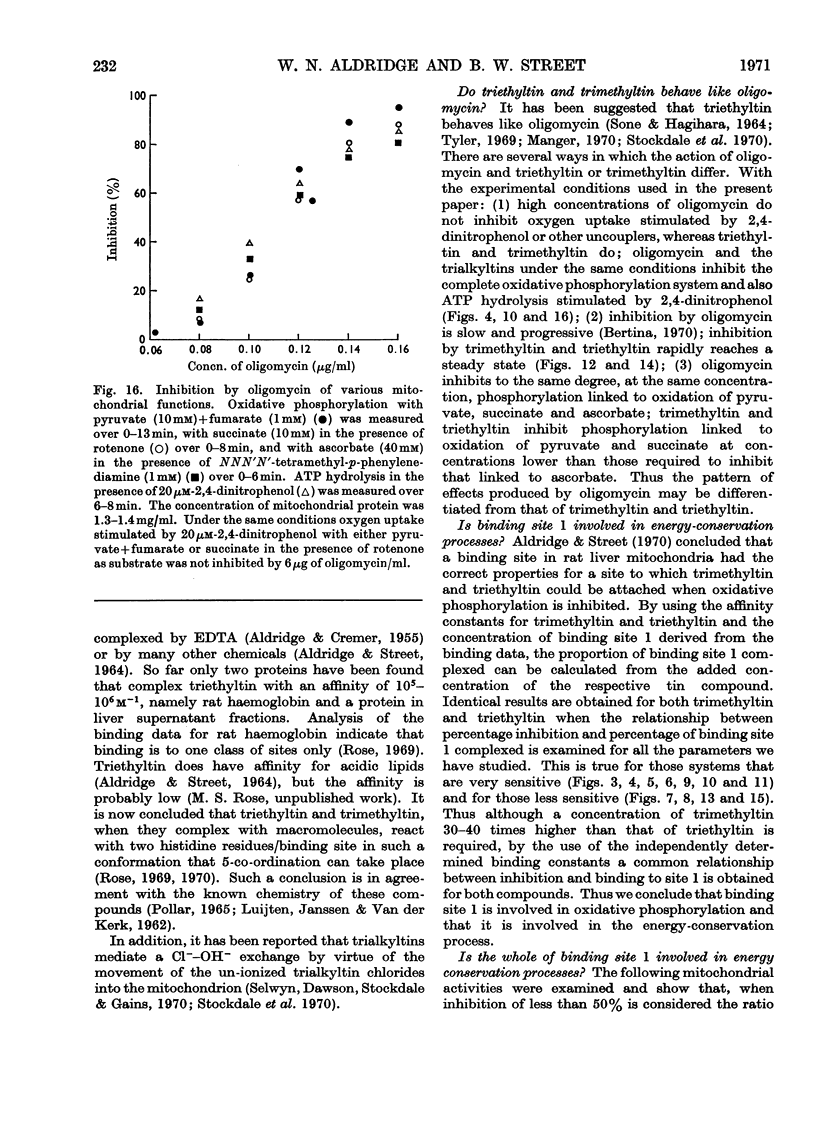


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., EMERY R. C., STREET B. W. A tissue homogenizer. Biochem J. 1960 Nov;77:326–327. doi: 10.1042/bj0770326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge W. N., Rose M. S. The mechanism of oxidative phosphorylation A hypothesis derived from studies of trimethyltin and triethyltin compounds. FEBS Lett. 1969 Jul;4(2):61–68. doi: 10.1016/0014-5793(69)80197-3. [DOI] [PubMed] [Google Scholar]
- Aldridge W. N., Street B. W. Oxidative phosphorylation. The specific binding of trimethyltin and triethyltin to rat liver mitochondria. Biochem J. 1970 Jun;118(1):171–179. doi: 10.1042/bj1180171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMER J. E. The action of triethyl tin, triethyl lead, ethyl mercury and other inhibitors on the metabolism of brain and kidney slices in vitro using substrates labelled with 14C. J Neurochem. 1962 May-Jun;9:289–298. doi: 10.1111/j.1471-4159.1962.tb09451.x. [DOI] [PubMed] [Google Scholar]
- Chamalaun R. A., Tager J. M. Stoicheiometry of oxidative phosphorylation with tetramethyl-p-phenylenediamine in rat-liver mitochondria. Biochim Biophys Acta. 1969 May;180(1):204–206. doi: 10.1016/0005-2728(69)90211-4. [DOI] [PubMed] [Google Scholar]
- Kahn J. S. Absence of a common intermediate pool among individual enzyme chains of the energy-conservation pathway in chloroplasts of Euglena gracilis. Biochem J. 1970 Jan;116(1):55–60. doi: 10.1042/bj1160055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S. Chlorotri-n-butylin. An inhibitor of photophosphorylation in isolated chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):203–210. doi: 10.1016/0005-2728(68)90161-8. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., JOHNSON D., McMURRAY W. C. Antibiotics as tools for metabolic studies. I. A survey of toxic antibiotics in respiratory, phosphorylative and glycolytic systems. Arch Biochem Biophys. 1958 Dec;78(2):587–597. doi: 10.1016/0003-9861(58)90383-7. [DOI] [PubMed] [Google Scholar]
- PARKER V. H. Effect of nitrophenols and halogenophenols on the enzymic activity of rat-liver mitochondria. Biochem J. 1958 Jun;69(2):306–311. doi: 10.1042/bj0690306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker V. H. Uncouplers of rat-liver mitochondrial oxidative phosphorylation. Biochem J. 1965 Dec;97(3):658–662. doi: 10.1042/bj0970658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S. Evidence for histidine in the triethyltin-binding site of rat haemoglobin. Biochem J. 1969 Jan;111(2):129–137. doi: 10.1042/bj1110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. S., Lock E. A. The interaction of triethyltin with a component of guinea-pig liver supernatant. Evidence for histidine in the binding sites. Biochem J. 1970 Nov;120(1):151–157. doi: 10.1042/bj1200151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- TER WELLEH, SLATER E. C. UNCOUPLING OF RESPIRATORY-CHAIN PHOSPHORYLATION BY ARSENATE AND EVIDENCE FOR THE EXISTENCE OF A STABLE X-P INTERMEDIATE OF OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1964 Aug 26;89:385–388. doi: 10.1016/0926-6569(64)90238-x. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]


