Abstract
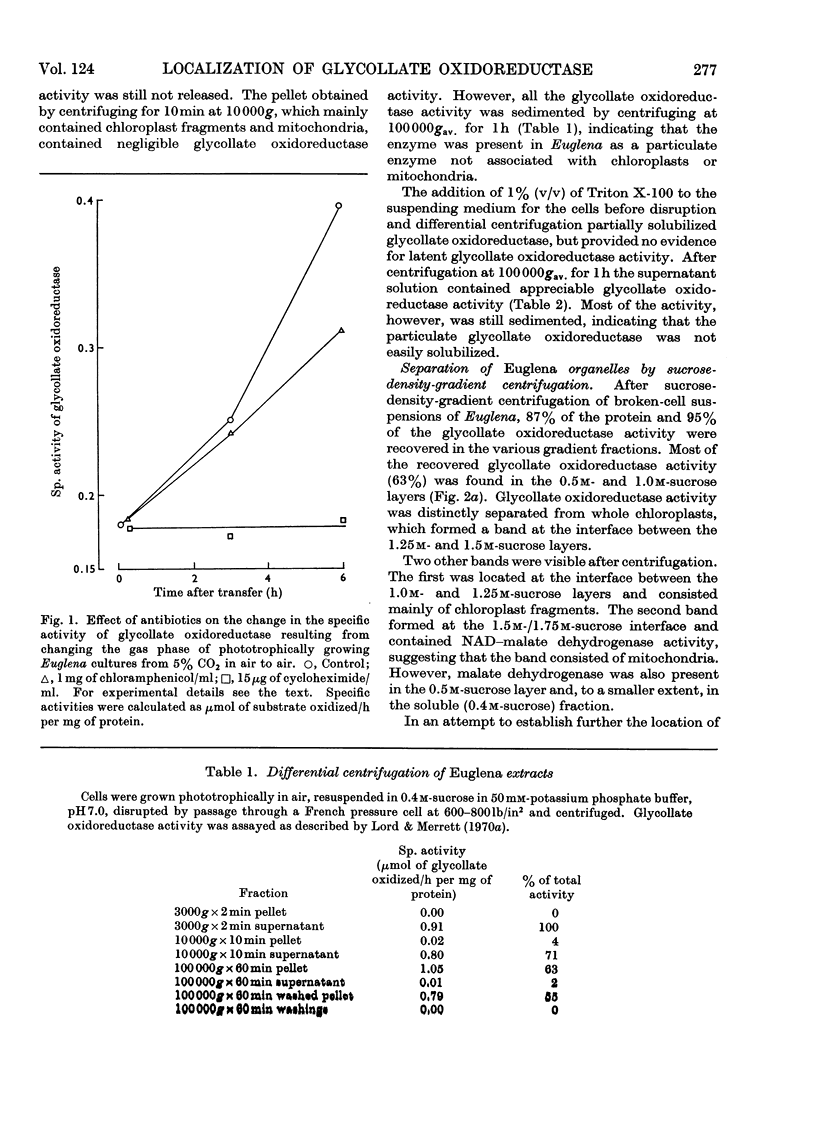
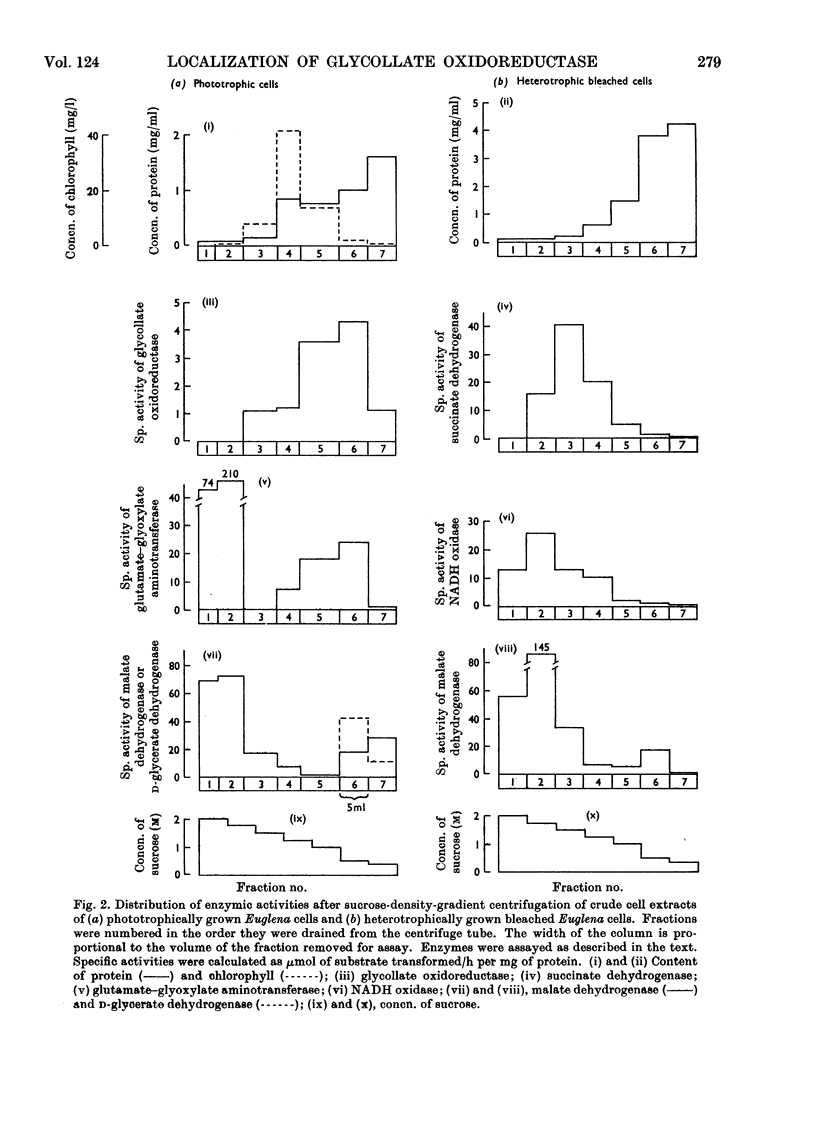
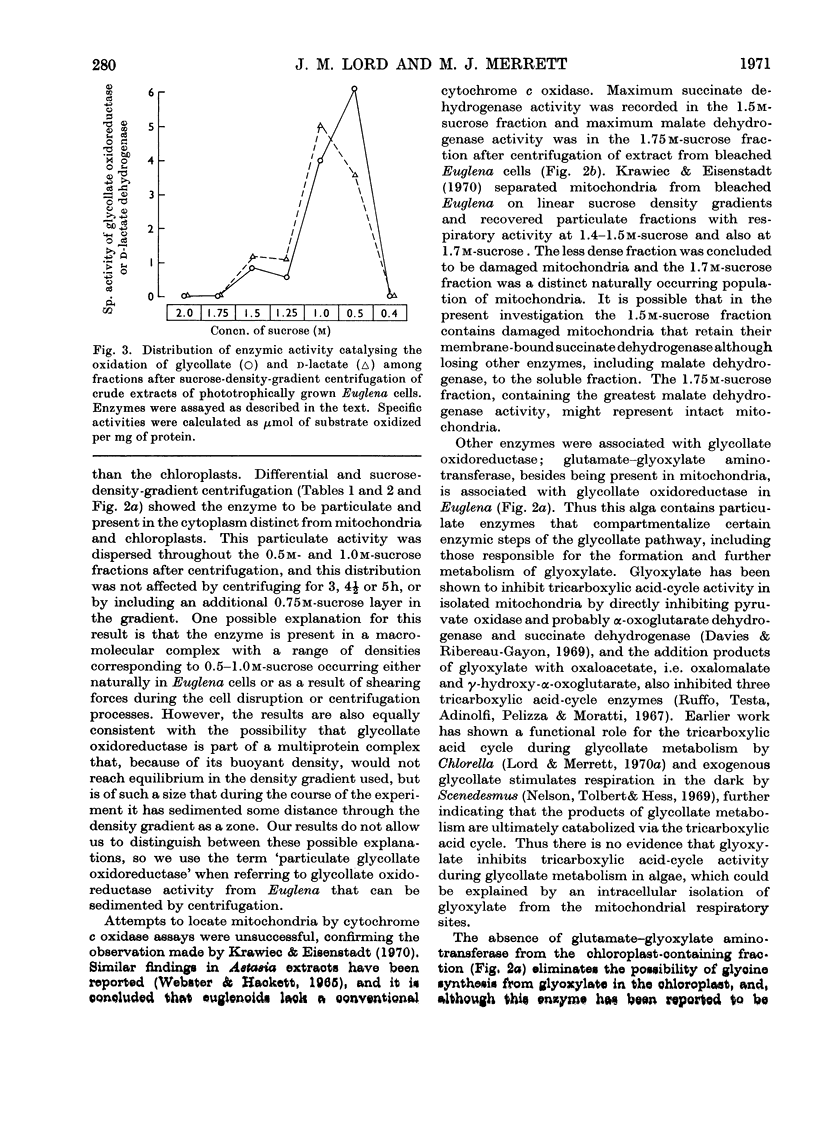
1. Lowering of the concentration of carbon dioxide in air available to phototrophically growing Euglena cultures from 5% to the normal value (0.03%) resulted in an increased specific activity of glycollate oxidoreductase. 2. The effects of chloramphenicol and cycloheximide suggested that this increase in activity was due to enzyme synthesis de novo on cytoplasmic ribosomes. 3. The Km for glycollate oxidation by the enzyme in crude cell extracts was 3.0×10−3m. 4. Differential centrifugation established that glycollate oxidoreductase present in phototrophically grown Euglena is a particulate enzyme. The enzyme was partially solubilized by the non-ionic detergent Triton X-100. 5. Sucrose-density-gradient centrifugation achieved the separation of the particulate glycollate oxidoreductase from chloroplasts and mitochondria. 6. Glutamate–glyoxylate aminotransferase was associated with particulate glycollate oxidoreductase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUETOW D. E., BUCHANAN P. J. ISOLATION OF MITOCHONDRIA FROM EUGLENA GRACILIS. Exp Cell Res. 1964 Oct;36:204–207. doi: 10.1016/0014-4827(64)90174-0. [DOI] [PubMed] [Google Scholar]
- Bruin W. J., Nelson E. B., Tolbert N. E. Glycolate pathway in green algae. Plant Physiol. 1970 Sep;46(3):386–391. doi: 10.1104/pp.46.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd G. A., Lord J. M., Merrett M. J. The glycollate oxidising enzyme of algae. FEBS Lett. 1969 Dec 30;5(5):341–342. doi: 10.1016/0014-5793(69)80352-2. [DOI] [PubMed] [Google Scholar]
- Codd G. A., Merrett M. J. The regulation of glycolate metabolism in division synchronized cultures of euglena. Plant Physiol. 1971 May;47(5):640–643. doi: 10.1104/pp.47.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- Ellyard P. W., San Pietro A. The Warburg effect in a chloroplast-free preparation from Euglena gracilis. Plant Physiol. 1969 Dec;44(12):1679–1683. doi: 10.1104/pp.44.12.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawiec S., Eisenstadt J. M. Ribonucleic acids from the mitochondria of bleached Euglena gracilis Z. I. Isolation of mitochondria and extraction of nucleic acids. Biochim Biophys Acta. 1970 Sep 17;217(1):120–131. doi: 10.1016/0005-2787(70)90128-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The pathway of glycollate utilization in Chlorella pyrenoidosa. Biochem J. 1970 May;117(5):929–937. doi: 10.1042/bj1170929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Merrett M. J. The regulation of glycollate oxidoreductase with photosynthetic capacity in Chlamydomonas mundana. Biochem J. 1970 Aug;119(1):125–127. doi: 10.1042/bj1190125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNER H. W., LAWRENCE N. S., FRENCH C. S. Colloidal dispersion of chloroplast material. Science. 1950 Jun 9;111(2893):633–634. doi: 10.1126/science.111.2893.633. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. Glycolate dehydrogenase in green algae. Arch Biochem Biophys. 1970 Nov;141(1):102–110. doi: 10.1016/0003-9861(70)90112-8. [DOI] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Ruffo A., Testa E., Adinolfi A., Pelizza G., Moratti R. Control of the citric acid cycle by glyoxylate. Mechanism of the inhibition by oxalomalate and gamma-hydroxy-alpha-oxoglutarate. Biochem J. 1967 Apr;103(1):19–23. doi: 10.1042/bj1030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMON E. W. The effect of digitonin on the cytochrome c oxidase activity of plant mitochondria. Biochem J. 1958 May;69(1):67–74. doi: 10.1042/bj0690067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smillie R. M., Graham D., Dwyer M. R., Grieve A., Tobin N. F. Evidence for the synthesis in vivo of proteins of the Calvin cycle and of the photosynthetic electron-transfer pathway on chloroplast ribosomes. Biochem Biophys Res Commun. 1967 Aug 23;28(4):604–610. doi: 10.1016/0006-291x(67)90356-7. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Kisaki T., Hageman R. H., Yamazaki R. K. Peroxisomes from spinach leaves containing enzymes related to glycolate metabolism. J Biol Chem. 1968 Oct 10;243(19):5179–5184. [PubMed] [Google Scholar]
- WOLKEN J. J., PALADE G. E. An electron microscope study of two flagellates, chloroplast structure and variation. Ann N Y Acad Sci. 1953 Oct 14;56(5):873–889. doi: 10.1111/j.1749-6632.1953.tb30266.x. [DOI] [PubMed] [Google Scholar]
- Webster D. A., Hackett D. P. Respiratory Chain of Colorless Algae I. Chlorophyta and Euglenophyta. Plant Physiol. 1965 Nov;40(6):1091–1100. doi: 10.1104/pp.40.6.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki R. K., Tolbert N. E. Enzymic characterization of leaf peroxisomes. J Biol Chem. 1970 Oct 10;245(19):5137–5144. [PubMed] [Google Scholar]



