Abstract
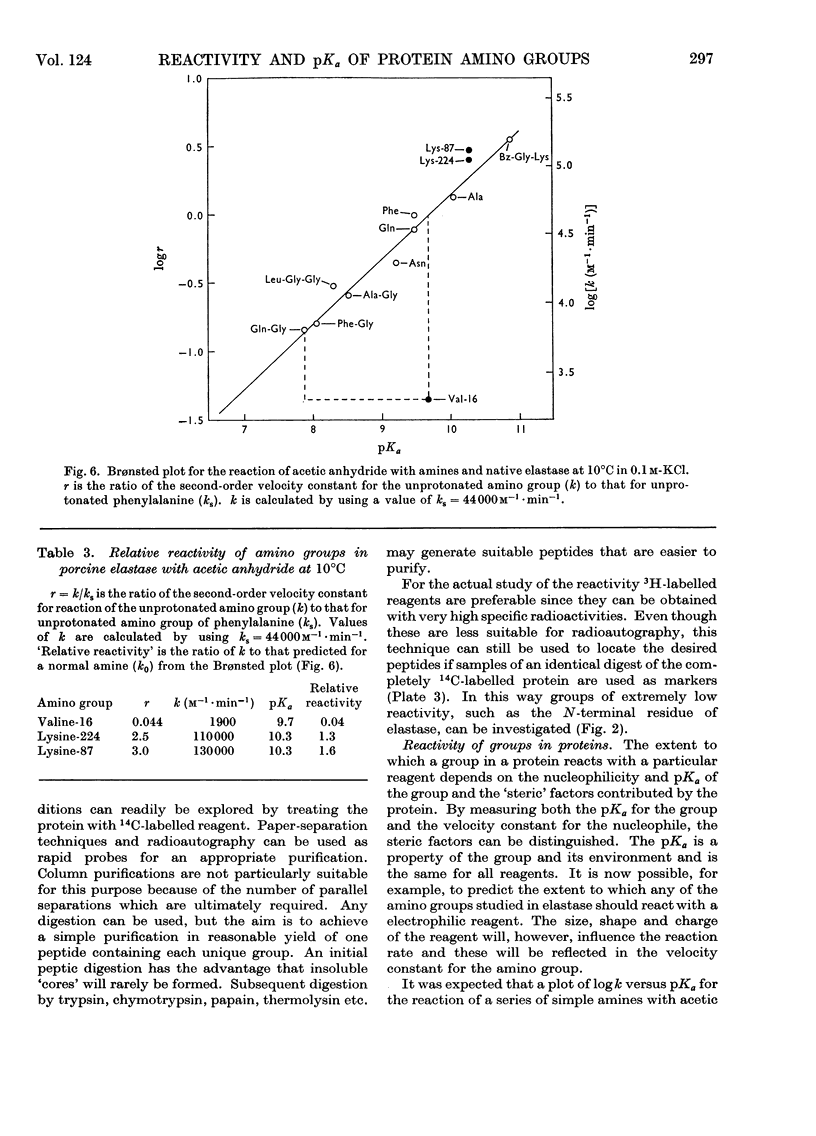
1. A method is described for determining the ionization constants and reactivities of individual amino groups in proteins. The principle is that in the presence of a trace amount of radioactive label, the various reactive groups in a protein molecule will compete for the label and the amount incorporated into any one group will be determined by its nucleophilicity, pK and micro-environment. The relative amounts of label incorporated into various groups will be proportional to their second-order rate constants and by comparing these rate constants with those expected on the basis of a linear free-energy relationship obtained with a series of standard compounds, the micro-environment can be defined for a particular amino group. 2. The method consists of treating a protein and an internal standard with a limiting amount of radioactive reagent and then with an excess of unlabelled reagent to yield a chemically homogeneous but heterogeneously labelled compound. After appropriate enzymic digestion peptides containing each labelled group are isolated and their rates of reaction, relative to the internal standard, are determined from their specific radioactivities. The entire procedure is repeated at several pH values. 3. When the method was applied to the amino groups of porcine elastase by using tritiated acetic anhydride as the labelling reagent, the N-terminus was found to have pKa 9.7 and a much lower than normal reactivity. Lysine-87 and lysine-224 were found to have pKa 10.3 and normal reactivities. At pH values greater than 10.5 there are discontinuities in all the titration curves, indicating that the entire molecule is undergoing a structural reorganization.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRINK N. G., LEWIS U. J., WILLIAMS D. E. Pancreatic elastase: purification, properties, and function. J Biol Chem. 1956 Oct;222(2):705–720. [PubMed] [Google Scholar]
- Brown J. R., Hartley B. S. Location of disulphide bridges by diagonal paper electrophoresis. The disulphide bridges of bovine chymotrypsinogen A. Biochem J. 1966 Oct;101(1):214–228. doi: 10.1042/bj1010214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer S. T., Kraut J., Robertus J. D., Wright H. T., Xuong N. H. Chymotrypsinogen: 2.5-angstrom crystal structure, comparison with alpha-chymotrypsin, and implications for zymogen activation. Biochemistry. 1970 Apr 28;9(9):1997–2009. doi: 10.1021/bi00811a022. [DOI] [PubMed] [Google Scholar]
- Gertler A., Hofmann T. Acetyl-L-alanyl-L-alanyl-L-alanine methyl ester: a new highly specific elastase substrate. Can J Biochem. 1970 Mar;48(3):384–386. doi: 10.1139/o70-061. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. J., Davis R. W. The pK of specific groups of proteins. I. The alpha-amino group of the alpha chain of human CO-hemoglobin. J Biol Chem. 1967 May 10;242(9):2005–2012. [PubMed] [Google Scholar]
- Kaplan H., Dugas H. Evidence that the activity of elastase is not dependent on the ionization of its N-terminal amino group. Biochem Biophys Res Commun. 1969 Mar 10;34(5):681–685. doi: 10.1016/0006-291x(69)90792-x. [DOI] [PubMed] [Google Scholar]
- Kaplan H. Properties of the isoleucyl amino-terminus of alpha-chymotrypsin. Biochem Biophys Res Commun. 1971 Mar 19;42(6):1042–1049. doi: 10.1016/0006-291x(71)90009-x. [DOI] [PubMed] [Google Scholar]
- Kaplan H., Symonds V. B., Dugas H., Whitaker D. R. A comparison of properties of the alpha-lytic protease of Sorangium sp. and porcine elastase. Can J Biochem. 1970 Jun;48(6):649–658. doi: 10.1139/o70-104. [DOI] [PubMed] [Google Scholar]
- Oppenheimer H. L., Labouesse B., Hess G. P. Implication of an ionizing group in the control of conformation and activity of chymotrypsin. J Biol Chem. 1966 Jun 10;241(11):2720–2730. [PubMed] [Google Scholar]
- Shotton D. M., Hartley B. S. Amino-acid sequence of porcine pancreatic elastase and its homologies with other serine proteinases. Nature. 1970 Feb 28;225(5235):802–806. doi: 10.1038/225802a0. [DOI] [PubMed] [Google Scholar]
- Smillie L. B., Hartley B. S. Histidine sequences in the active centres of some 'serine' proteinases. Biochem J. 1966 Oct;101(1):232–241. doi: 10.1042/bj1010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. C., Shotton D. M., Cox J. M., Muirhead H. Three-dimensional Fourier synthesis of tosyl-elastase at 3.5 å resolution. Nature. 1970 Feb 28;225(5235):806–811. doi: 10.1038/225806a0. [DOI] [PubMed] [Google Scholar]





