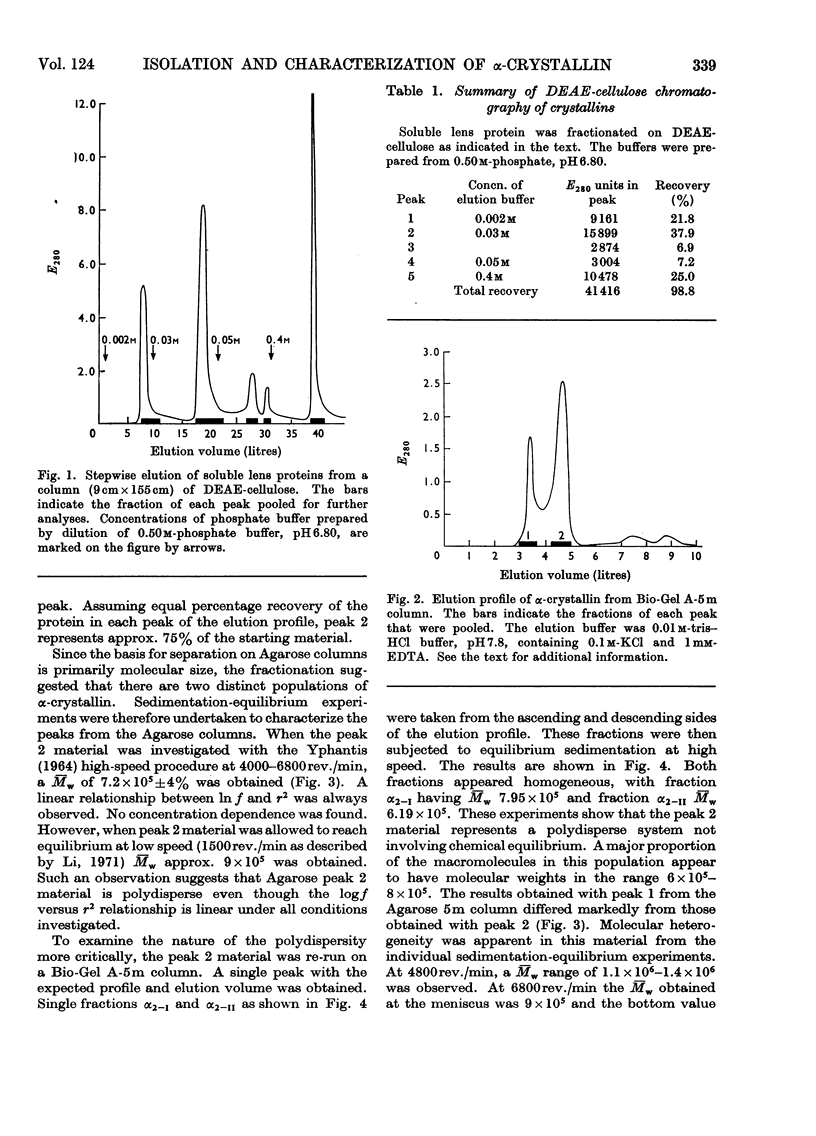
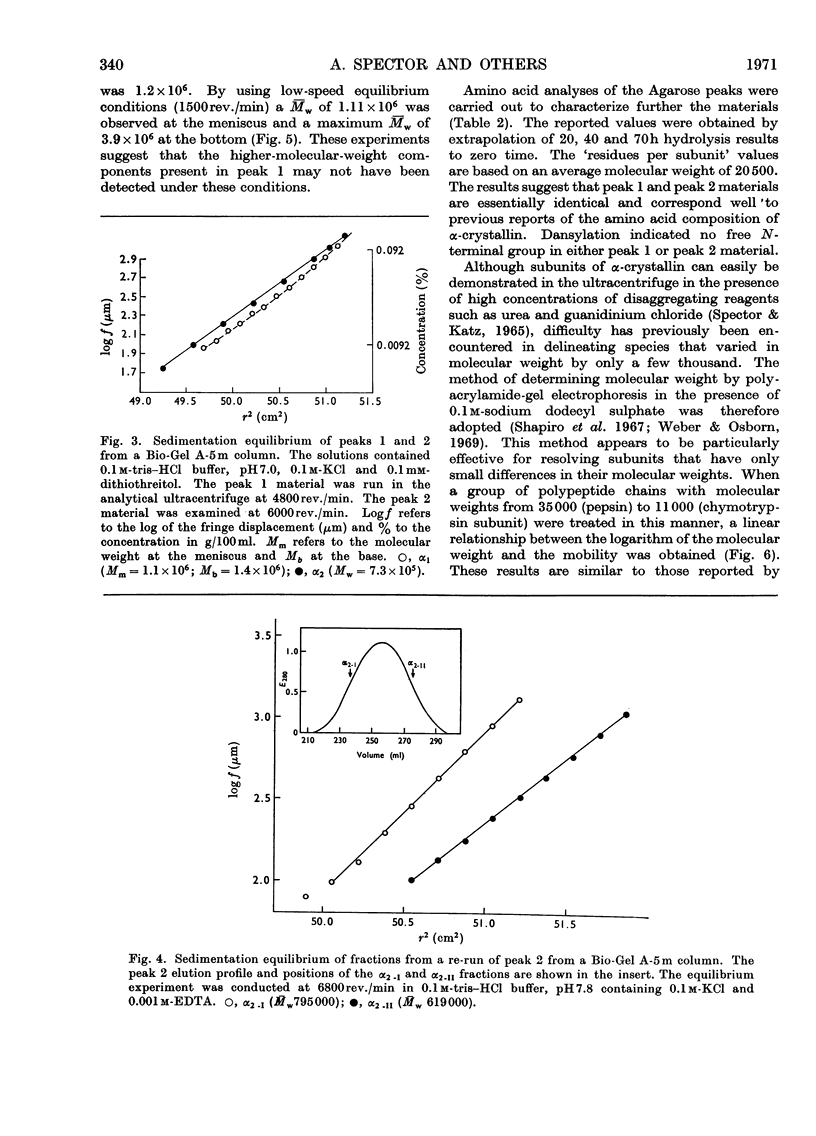
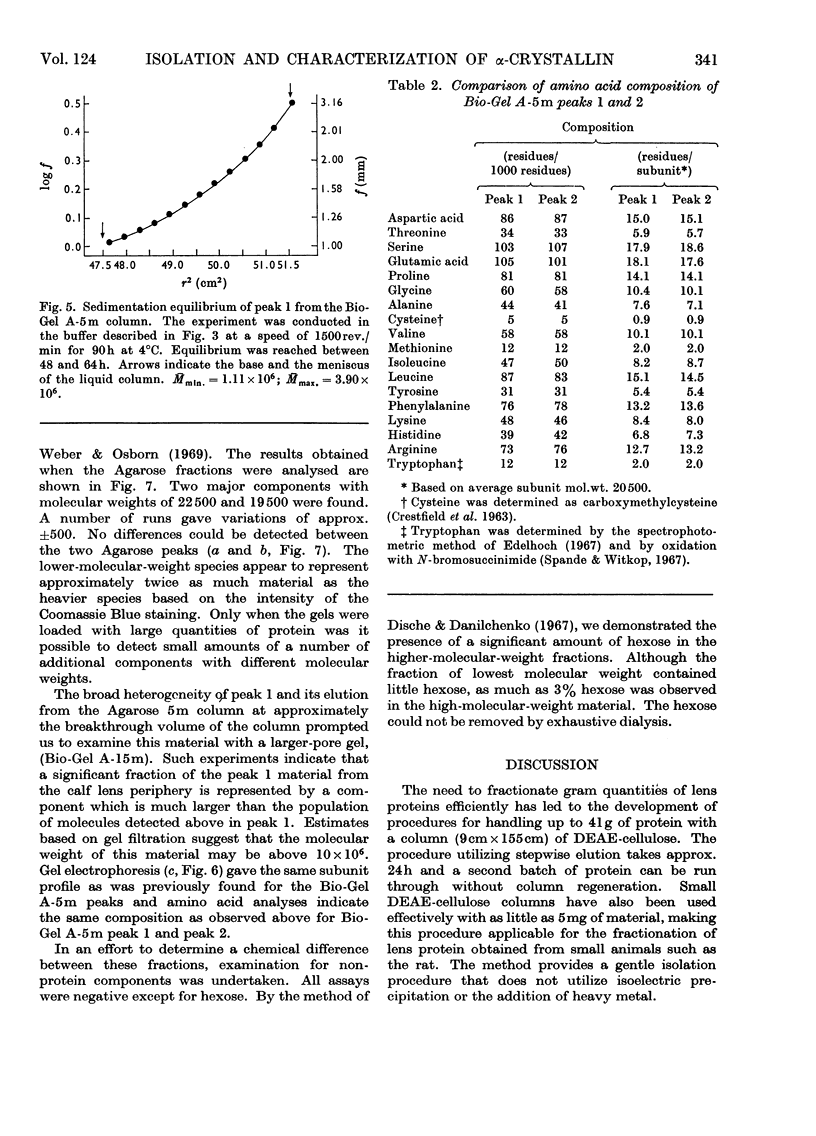
Abstract
α-Crystallin was isolated from calf lens periphery by chromatography on DEAE-cellulose and gel filtration. Three distinct populations of macromolecules have been isolated with molecular weights in the ranges approx. 6×105−9×105, 0.9×106−4×106 and greater than 10×106. The concentration of macromolecules at the molecular-weight limits of a population are very low. The members of the different populations do not appear to be in equilibrium with each other. Further, in those molecular-weight fractions investigated, no equilibrium between members of the same population was observed. The population of lowest molecular weight comprises 65–75% of the total material. The amino acid and subunit composition of the different-sized fractions appear very similar, if not identical. The only chemical difference observed between the fractions is the presence of significant amounts of sugar in the higher-molecular-weight fractions. Subunit molecular weights of approx. 19.5×103 and 22.5×103 were observed for all α-crystallin fractions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augusteyn R. C., Spector A. -Crystallin. Fractionation of subunits and sequence studies on an isolated polypeptide. Biochem J. 1971 Sep;124(2):345–355. doi: 10.1042/bj1240345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., JONGKIND J. F., WISSE J. H. Splitting and recombination of alpha-crystallin. Exp Eye Res. 1962 Jun;1:300–305. doi: 10.1016/s0014-4835(62)80015-3. [DOI] [PubMed] [Google Scholar]
- BONT W. S., JONGKIND J. F., WISSE J. H., BLOEMENDAL H. The effect of urea on lens proteins. Biochim Biophys Acta. 1962 May 21;59:512–514. doi: 10.1016/0006-3002(62)90216-0. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- De Groot K., Hoenders H. J., Gerding J. J., Bloemendal H. The molecular weight of the polypeptide chains of alpha-crystallin. Biochim Biophys Acta. 1970 Apr 28;207(1):202–205. doi: 10.1016/0005-2795(70)90152-2. [DOI] [PubMed] [Google Scholar]
- Dische Z., Danilchenko A. Modifications of two color reactions of hexoses with cysteine and sulfuric acid. Anal Biochem. 1967 Oct;21(1):119–124. doi: 10.1016/0003-2697(67)90090-5. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Hoenders H. J., de Groot K., Gerding J. J., Bloemendal H. The effect of denaturing agents on the molecular weight of bovine alpha-crystallin. Biochim Biophys Acta. 1969 Aug 12;188(1):162–163. doi: 10.1016/0005-2795(69)90058-0. [DOI] [PubMed] [Google Scholar]
- McMEEKIN T. L., MARSHALL K. Specific volumes of proteins and the relationship to their amino acid contents. Science. 1952 Aug 8;116(3006):142–143. doi: 10.1126/science.116.3006.142. [DOI] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]
- Philipson B. Distribution of protein within the normal rat lens. Invest Ophthalmol. 1969 Jun;8(3):258–270. [PubMed] [Google Scholar]
- RESNIK R. A. Lens proteins. I. Alpha crystallin of calf lens. Am J Ophthalmol. 1957 Nov;44(5 Pt 2):357–362. [PubMed] [Google Scholar]
- SPECTOR A., KATZ E. THE DEAGGREGATION OF BOVINE LENS ALPHA-CRYSTALLIN. J Biol Chem. 1965 May;240:1979–1985. [PubMed] [Google Scholar]
- SPECTOR A. METHODS OF ISOLATION OF ALPHA, BETA, AND GAMMA CRYSTALLINS AND THEIR SUBGROUPS. Invest Ophthalmol. 1964 Apr;3:182–193. [PubMed] [Google Scholar]
- SPECTOR A. THE SOLUBLE PROTEINS OF THE LENS. Invest Ophthalmol. 1965 Aug;4:579–591. [PubMed] [Google Scholar]
- Schoenmakers J. G., Gerding J. J., Bloemendal H. The subunit structure of alpha-crystallin. Isolation and characterization of the S-carboxymethylated acidic subunits from adult and embryonic origin. Eur J Biochem. 1969 Dec;11(3):472–481. doi: 10.1111/j.1432-1033.1969.tb00797.x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Spector A., Wandel T., Li L. K. The purification and characterization of the highly labeled protein fraction from calf lens. Invest Ophthalmol. 1968 Apr;7(2):179–190. [PubMed] [Google Scholar]
- Wannemacher C. F., Spector A. Protein synthesis in the core of calf lens. Exp Eye Res. 1968 Oct;7(4):623–625. doi: 10.1016/s0014-4835(68)80018-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]