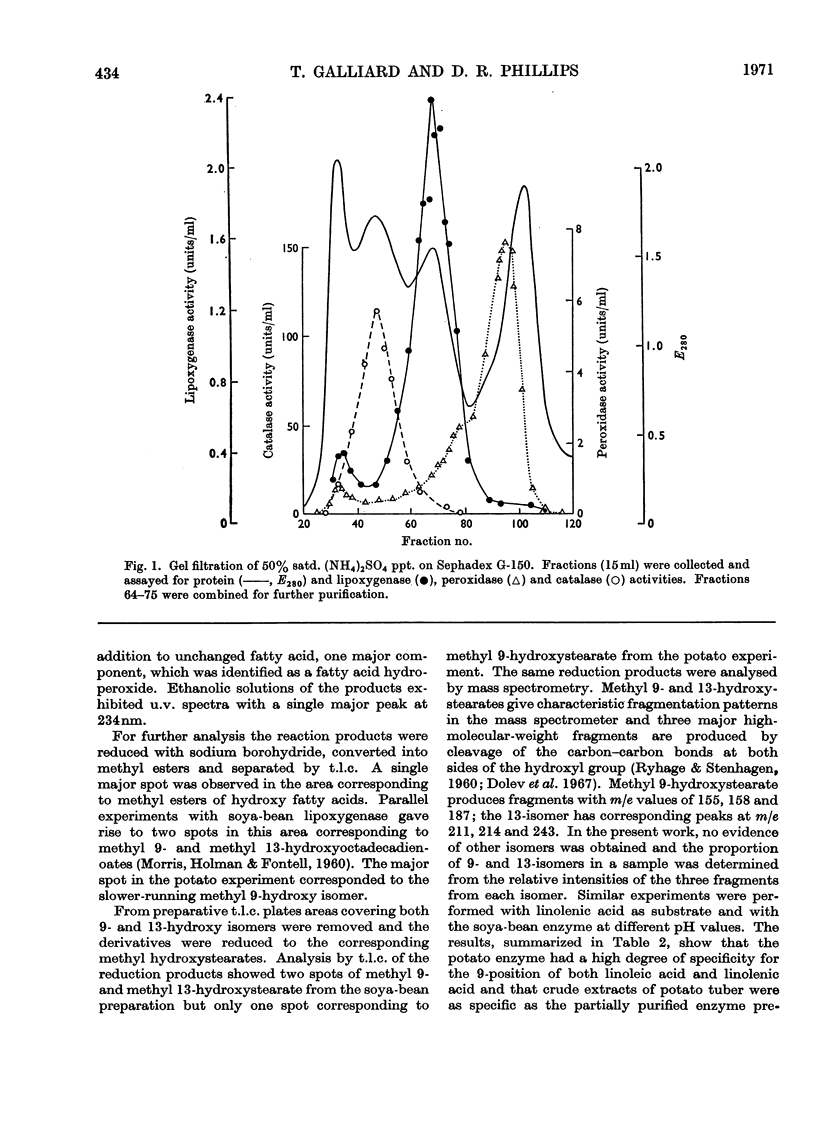
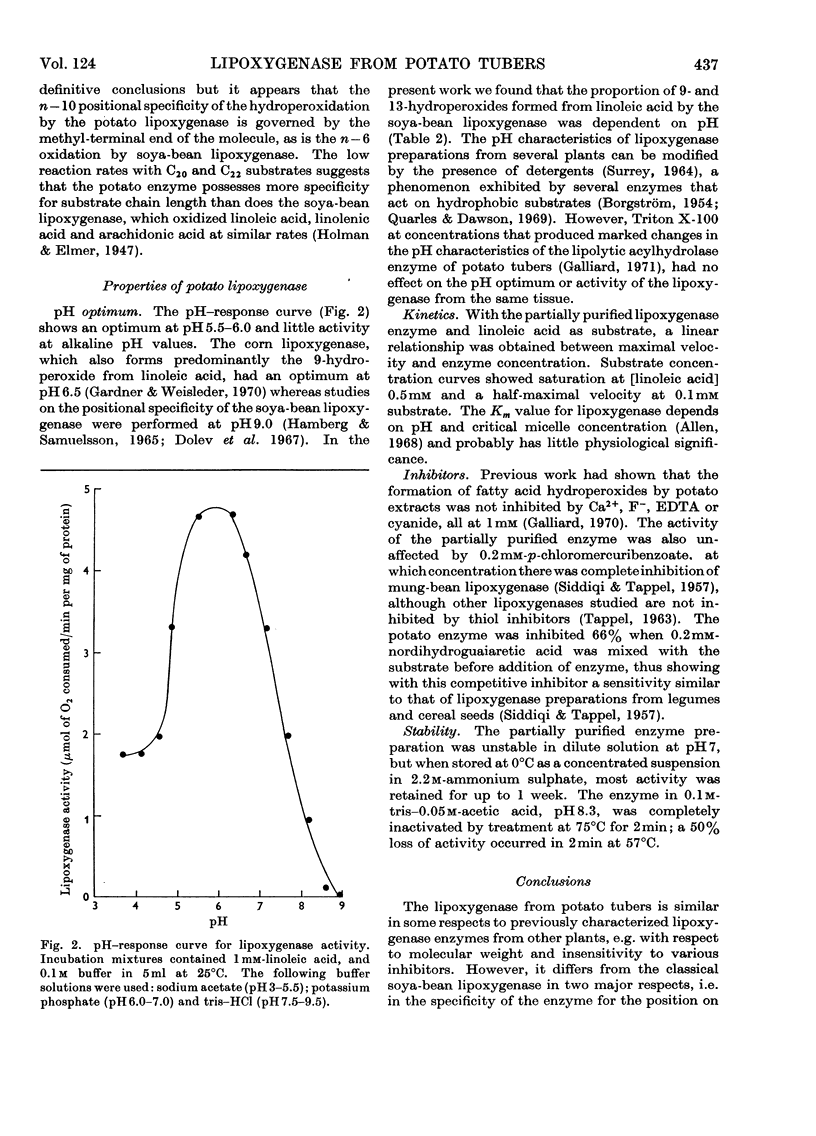
Abstract
A lipoxygenase (EC 1.13.1.13) was partially purified from potato tubers and was shown to differ from previously characterized soya-bean lipoxygenases in the positional specificity and pH characteristics of the oxygenation reaction. The potato enzyme converted linoleic acid almost exclusively (95%) into 9-d-hydroperoxyoctadeca-trans-10,cis-12-dienoic acid. The 13-hydroperoxy isomer was only a minor product (5%). Linolenic acid was an equally effective substrate, which was also oxygenated specifically at the 9-position. The enzyme had a pH optimum at 5.5–6.0 and was inactive at pH9.0. A half-maximal velocity was obtained at a linoleic acid concentration of 0.1mm. No inhibition was observed with EDTA (1mm) and cyanide (1mm) or with p-chloromercuribenzoate (0.2mm). Haemoproteins were not involved in the lipoxygenase activity. The molecular weight of the enzyme was estimated from gel filtration to be approx. 105. Preliminary evidence suggested that the enzyme oxygenated the n–10 position of fatty acids containing a penta(n–3, n–6)diene structure.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. C. Soybean Lipoxygenase. 1. Purification, and the effect of organic solvents upon kinetics of the reaction. Eur J Biochem. 1968 Apr 3;4(2):201–208. doi: 10.1111/j.1432-1033.1968.tb00194.x. [DOI] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- BORGSTROM B. Effect of tauro-cholic acid on the pH/activity curve of rat pancreatic lipase. Biochim Biophys Acta. 1954 Jan;13(1):149–150. doi: 10.1016/0006-3002(54)90290-5. [DOI] [PubMed] [Google Scholar]
- Badami R. C., Morris L. J. The oxygenated fatty acid of Calendula seed oil. J Am Oil Chem Soc. 1965 Dec;42(12):1119–1121. doi: 10.1007/BF02636925. [DOI] [PubMed] [Google Scholar]
- Dolev A., Rohwedder W. K., Dutton H. J. Quantitative separation of methyl 9-hydroxystearate from methyl 13-hydroxystearate by column chromatography on Silica gel. Lipids. 1966 May;1(3):231–233. doi: 10.1007/BF02531879. [DOI] [PubMed] [Google Scholar]
- Eriksson C. E., Svensson S. G. Lipoxygenase from peas, purification and properties of the enzyme. Biochim Biophys Acta. 1970 Mar 18;198(3):449–459. doi: 10.1016/0005-2744(70)90123-3. [DOI] [PubMed] [Google Scholar]
- Eriksson C. J., Olsson P. A., Svensson S. G. Oxidation of fatty acids by heat treated hemoproteins. Lipids. 1970 Mar;5(3):365–366. doi: 10.1007/BF02531471. [DOI] [PubMed] [Google Scholar]
- Galliard T. The enzymic deacylation of phospholipids and galactolipids in plants. Purification and properties of a lipolytic acyl-hydrolase from potato tubers. Biochem J. 1971 Feb;121(3):379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the lipoxidase catalyzed oxygenation of unsaturated fatty acids. Biochem Biophys Res Commun. 1965 Dec 21;21(6):531–536. doi: 10.1016/0006-291x(65)90517-6. [DOI] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the specificity of the oxygenation of unsaturated fatty acids catalyzed by soybean lipoxidase. J Biol Chem. 1967 Nov 25;242(22):5329–5335. [PubMed] [Google Scholar]
- Holman R. T., Egwim P. O., Christie W. W. Substrate specificity of soybean lipoxidase. J Biol Chem. 1969 Mar 10;244(5):1149–1151. [PubMed] [Google Scholar]
- KOCH R. B., STERN B., FERRARI C. G. Linoleic acid and trilinolein as substrates for soybean lipoxidase (s). Arch Biochem Biophys. 1958 Nov;78(1):165–179. doi: 10.1016/0003-9861(58)90325-4. [DOI] [PubMed] [Google Scholar]
- Koch R. B. Calcium ion activation of lipoxidase. Arch Biochem Biophys. 1968 Apr;125(1):303–307. doi: 10.1016/0003-9861(68)90665-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Purdy R. H., Axelrod L. R. The formation of 5 alpha- and 5 beta-androstane-3,17-dione from testosterone by human placental microsomes. Biochim Biophys Acta. 1970 Jun 9;210(1):196–198. doi: 10.1016/0005-2760(70)90080-9. [DOI] [PubMed] [Google Scholar]
- Quarles R. H., Dawson R. M. A shift in the optimum pH of pospholipase D produced by activating long-chain anions. Biochem J. 1969 May;112(5):795–799. doi: 10.1042/bj1120795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIOQUE E., HOLMAN R. T. Characterization of the ketodienes formed in the oxidation of linoleate by lipoxidase. Arch Biochem Biophys. 1962 Dec;99:522–528. doi: 10.1016/0003-9861(62)90301-6. [DOI] [PubMed] [Google Scholar]
- Veldink G. A., Vliegenthart J. F., Boldingh J. Proof of the enzymatic formation of 9-hydroperoxy-10-trans, 12-cis-octadecadienoic acid from linoleic acid by soya lipoxygenase. Biochim Biophys Acta. 1970 Feb 10;202(1):198–199. doi: 10.1016/0005-2760(70)90235-3. [DOI] [PubMed] [Google Scholar]
- Veldink G. A., Vliegenthart J. F., Boldingh J. The enzymic conversion of linoleic acid hydroperoxide by flax-seed hydroperoxide isomerase. Biochem J. 1970 Nov;120(1):55–60. doi: 10.1042/bj1200055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman D. C., Vick B. A. Specificity of flaxseed lipoxidase. Lipids. 1970 Apr;5(4):392–397. doi: 10.1007/BF02532104. [DOI] [PubMed] [Google Scholar]


