ABSTRACT
Sacubitril/valsartan, an angiotensin receptor‐neprilysin inhibitor, has demonstrated a superior blood pressure‐lowering effect compared with renin‐angiotensin system inhibitors in several clinical trials. However, there has been no available evidence on the comparison between sacubitril/valsartan and calcium channel blockers (CCBs), a well‐established class of antihypertensive drugs.
In this open‐label, multicenter study, we aimed to demonstrate the efficacy and safety of sacubitril/valsartan versus amlodipine, one of the most widely used CCBs, after 8 weeks of treatment. A total of 359 Japanese patients with essential hypertension (office systolic blood pressure [SBP] ≥ 150 to < 180 mmHg), aged 18–79, were randomly assigned to receive either once‐daily sacubitril/valsartan 200 mg or once‐daily amlodipine 5 mg in a 1:1 allocation ratio. The primary endpoint was the noninferiority of sacubitril/valsartan compared with amlodipine in mean change in 24‐h SBP from baseline to Week 8, followed by a significance test as a secondary endpoint analysis. The mean change in 24‐h SBP in sacubitril/valsartan was noninferior to that in amlodipine (between‐treatment difference −0.62 mmHg [95% confidential interval: −3.23 to 1.98; p = 0.003 for noninferiority; independent t‐test with noninferiority margin 3.0 mmHg]), with no significant difference observed (p = 0.637). There was no significant difference in the incidence of adverse events (AEs). These results suggested that the blood pressure‐lowering effect of sacubitril/valsartan is comparable to that of amlodipine, with no marked differences in tolerability between the two groups. Sacubitril/valsartan, a potent antihypertensive drug comparable to amlodipine, is expected to improve blood pressure control in clinical practice.
Keywords: ambulatory blood pressure monitoring, angiotensin receptor‐neprilysin inhibitor, calcium channel blocker, essential hypertension, sacubitril/valsartan
1. Introduction
Essential hypertension represents elevated blood pressure (BP), which increases the risk for cardiovascular and renal events. The number of patients with hypertension worldwide was estimated at 1.3 billion in 2019 [1]. In Japan, it is reported that only 12 million patients have adequate BP control out of an estimated 43 million patients with hypertension [2]. These reports highlight a pressing need to improve the control rates of BP.
Current guidelines recommend five major drug classes for the treatment of hypertension: angiotensin‐converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), beta‐blockers, calcium channel blockers (CCBs), and diuretics (thiazides and thiazide‐like diuretics such as chlortalidone and indapamide) [2, 3]. Sacubitril/valsartan is an angiotensin receptor‐neprilysin inhibitor, a new type of drug class for antihypertensive treatment that inhibits angiotensin II signaling through blockade of the angiotensin II receptor‐1 and inhibits neprilysin simultaneously. Sacubitril/valsartan was approved for chronic heart failure with reduced ejection fraction by the U.S. Food and Drug Administration in 2015 and has been approved in more than 120 countries. Among them, the additional indication for hypertension has also been approved in some Asian countries, including Japan. In past clinical trials, sacubitril/valsartan has shown a superior BP‐lowering effect compared with ARBs [4, 5]. However, there is no comparative data available between sacubitril/valsartan and CCBs, another established class of antihypertensive drugs. Among CCBs, amlodipine is commonly used and has proven to be more potent than azilsartan which is more effective at lowering BP than other ARBs [6, 7, 8].
Hence, this study aimed to evaluate the antihypertensive effects and safety of sacubitril/valsartan compared to amlodipine in patients with essential hypertension.
2. Methods
2.1. Study Design and Patients
This was a Prospective, Active‐controlled, RAndomized, comparison of Sacubitril/valsartan with amlodipine, Open‐Label, parallel group (PARASOL) study conducted between February 2023 and October 2023 in Japan.
Japanese patients with essential hypertension, aged 18–79, were recruited. The main inclusion criteria were office systolic blood pressure (SBP) of 150–179 mmHg and not receiving antihypertensive drugs at least 28 days before randomization on Day 0. The main exclusion criteria were office diastolic blood pressure (DBP) of ≥ 110 mmHg, serum potassium of ≥ 5.5 mmol/L, estimated glomerular filtration rate (eGFR) of < 30 mL/min/1.73 m2, and patients with the following self‐reported conditions: those unable to visit study sites alone, women who are breastfeeding, pregnant or possibly pregnant, history of angioedema, moderate/severe liver disease (Child‐Pugh class B or C), known hypersensitivity to dihydropyridine CCBs, secondary hypertension, presence/history of stroke including lacunar infarcts, white coat hypertension, and shift workers.
Patients were randomized using a stratified permuted block design with two factors. One was age stratification (< 65, ≥ 65), and the other was whether or not the patient wears a wrist BP monitor as the allocation factors, which was used in a sub‐study conducted in parallel with this study. Four patient groups, each comprising a combination of two factors, were randomly assigned to each block sequentially. No adjustments were made to ensure equal allocation to each participating center.
This study was conducted under the Clinical Trials Act in Japan and the Declaration of Helsinki. The study protocol was approved by the certified review board at Osaka University (CRB5180007). The study was registered with the Japan Registry of Clinical Trials (jRCTs051220165). All patients provided written informed consent prior to participation.
2.2. Study Treatments
Patients in the sacubitril/valsartan group orally received sacubitril valsartan sodium hydrate 200 mg in the morning once daily, and patients in the amlodipine group received amlodipine besylate 5 mg in the same regimen for 10 weeks. Throughout the study period, patients did not take any antihypertensive medication except sacubitril/valsartan and amlodipine. They were also prohibited from taking any drugs that could potentially affect BP, including phosphodiesterase‐5 inhibitors (e.g., sildenafil and vardenafil).
2.3. Study Measurements
Ambulatory BP (ABP) was monitored every 30 min for 24 h according to the Japanese ABP measurement guideline [9] using TM‐2441 (A&D Company, Limited, Japan) at baseline (Week ‐4 to ‐1) and Week 8, and office BP was measured at baseline, Weeks 4 and 8. The ambulatory BP monitor (ABPM) was certified for accuracy by the British and Irish Hypertension Society (https://inconf.sharepoint.com/:x:/s/External‐Sharing/ETJGwZ4mj‐RKmOFQp5YhN2sBzRJ7q5LTi9fvBy1gKfeBNQ?rtime=IFjsTrzs3Eg) based on the verification study according to ISO protocols [10]. All ABPM devices were newly purchased for this study.
Morning BP represents the average BP for the first 2 h after wake‐up (if missed, 7 a.m. to 9 a.m.), daytime BP represents the average from wake‐up time to bedtime (if missed, 9 a.m. to 9 p.m.), and nighttime BP represents the average from bedtime to wake‐up time (if missed, 1 a.m. to 6 a.m.). For nocturnal BP dipping status, dipper was defined as a fall of nighttime SBP ≥ 10% compared to daytime SBP (nighttime SBP/daytime SBP ≤ 0.9 at baseline). A nocturnal fall < 10% (nighttime SBP/daytime SBP > 0.9 at baseline) was defined as non‐dipper.
Blood and urine samples were collected for laboratory tests at baseline and Week 8. Serum samples for N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) and plasma samples for atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) were stored at 2°C–8°C and < −10°C, respectively. The urine albumin‐to‐creatinine ratio (UACR) was calculated by dividing the urinary albumin values by the urinary creatinine concentrations. All the above tests were performed at the central laboratory, LSI Medience Corporation, Japan. For cyclic guanosine monophosphate (cGMP) assessment, urine samples were stored at −80°C and measured using Cyclic GMP ELISA Kit (Item No. 581021, Cayman Chemical Company, USA). cGMP level was normalized by urinary creatinine.
2.4. Efficacy Assessment
The primary endpoint was the noninferiority of sacubitril/valsartan compared to amlodipine in the mean change in 24‐h SBP from baseline to Week 8. The secondary endpoints were changes in other BP parameters measured by ABPM and office BP from baseline to Week 8, and BP control and response rate at Week 8. BP control was defined as follows. Ambulatory BP control: 24‐h SBP/24‐h DBP < 130/80 mmHg for patients < 75 years of age, and < 140/90 mmHg for ≥ 75 years of age. Office BP control: SBP/DBP < 130/80 mmHg for patients < 75 years of age, and < 140/90 mmHg for ≥ 75 years of age. SBP response rate was defined as follows. Ambulatory SBP: 24‐h SBP < 130 mmHg at Week 8 for patients < 75 years of age, as 24‐h SBP < 140 mmHg for ≥ 75 years of age, or a reduction ≥ 20 mmHg from baseline. Office SBP: SBP < 130 mmHg at Week 8 for patients < 75 years of age, as < 140 mmHg for ≥ 75 years of age, or a reduction ≥ 20 mmHg from baseline. DBP response rate was defined as follows. Ambulatory DBP: 24‐h DBP < 80 mmHg at Week 8 for patients < 75 years of age, as 24‐h DBP < 90 mmHg for ≥ 75 years of age, or a reduction ≥ 10 mmHg from baseline. Office DBP: SBP/DBP < 80 mmHg at Week 8 for patients < 75 years of age, as < 90 mmHg for ≥ 75 years of age, or a reduction ≥ 10 mmHg from baseline.
The exploratory endpoints were changes in biomarkers including cGMP, ANP, BNP, NT‐proBNP, UACR, and eGFR from baseline to Week 8.
2.5. Safety Assessment
Investigator‐reported adverse events (AEs) were collected during 10 weeks of study treatment and tabulated according to preferred terms and system organ classes of the Medical Dictionary for Regulatory Activities/Japanese Edition (MedDRA/J) version 27.0.
2.6. Statistical Analysis
The full analysis set (FAS) consisted of patients who received at least one dose of test/control drug and had at least one observation/measurement. Per protocol set (PPS) was a subset of FAS excluding patients who deviated from inclusion/exclusion criteria, whose drug compliance by Week 8 was less than 70%, and who got a wrong prescription. The safety analysis set (SAS) consisted of patients who received at least one dose of test/control drug. The efficacy analysis was mainly performed with FAS, and the safety analysis was performed with SAS. Missing values were not complemented.
The primary endpoint of noninferiority in mean change in 24‐h SBP from baseline to Week 8 was assessed using an independent t‐test with a one‐sided significance level of 0.025 with the noninferiority margin of 3.0 mmHg. For the analysis of secondary efficacy and exploratory endpoints, summary statistics were calculated for measured values and changes from baseline to Week 8. An independent t‐test with a two‐sided significance level of 0.05 was used to evaluate changes from baseline to Week 8 between the two groups. Subgroup analyses stratified by baseline characteristics were examined the interactions using a generalized linear model. For safety analysis, the incidences of AEs up to Week 10 were tabulated, and Fisher's exact test was used to compare treatments. All statistical analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, North Carolina, USA).
2.7. Sample Size Calculation
The null hypothesis for the primary endpoint was set as follows: the BP‐lowering effect of sacubitril/valsartan is inferior to that of amlodipine by 3.0 mmHg or greater. In this study, the noninferiority margin was defined by the following two perspectives. First, because a previous study has reported that the difference in changes in 24‐h SBP was 9.0 mmHg between sacubitril/valsartan and placebo [4], we defined noninferiority margin as below 4.5 mmHg, the half value of the effect of sacubitril valsartan. Second, for clinically meaningful differences, the lower limit was set at 4.0 mmHg, referring to previous reports [2, 11]. Based on the above, the noninferiority margin of this study was set at 3.0 mmHg.
Considering the result of a phase III study of sacubitril/valsartan in Japan, the required number of patients for the analysis was determined as 310 (one‐sided significance level of 0.025; ≥ 80% power; standard deviation (SD) = 9.4 mmHg). The SD value was derived from the group difference in change in 24‐h mean SBP when comparing sacubitril/valsartan 200 mg and olmesartan 20 mg in our previous study [12]. Assuming the dropout rate is 12%, the target number of patients was set at 350.
3. Results
3.1. Demographic and Baseline Characteristics
Of the 359 randomized patients, 182 patients received sacubitril/valsartan, and 177 patients received amlodipine. Patient disposition is shown in Figure S1. A total of ten patients (seven patients in the sacubitril/valsartan group and three patients in the amlodipine group) were prematurely discontinued. Among them, one patient in the sacubitril/valsartan group discontinued due to an AE, and the remaining six patients in the sacubitril/valsartan group and three patients in the amlodipine group discontinued due to investigators’ decision for reasons other than AEs or requests from patients. Demographics and baseline characteristics were well‐balanced in the two groups (Table 1). Most patients were male (76% in the sacubitril/valsartan group and 73.9% in the amlodipine group), mean ages were 58.4 and 58.0, and the proportion of patients aged ≥ 75 was 4.5% and 4.0%, respectively. Mean 24‐h SBP/DBP and Office SBP/DBP were 152.2/96.6 and 154.2/97.4, 154.3/95.0, and 154.9/95.2 mmHg. The mean duration of hypertension was 1632.1 days and 1564.6 days, and 73.7% and 70.5% of the patients in each group were on antihypertensive medication.
TABLE 1.
Demographics and baseline characteristics.
| Items | Sacubitril/valsartan | Amlodipine | p value a | ||
|---|---|---|---|---|---|
| (N = 179) | (N = 176) | ||||
| Gender—n (%) | 0.646 | ||||
| Male | 136 | (76.0) | 130 | (73.9) | |
| Female | 43 | (24.0) | 46 | (26.1) | |
| Age—years | 0.693 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 58.4 | (9.1) | 58.0 | (8.7) | |
| Median (Q1, Q3) | 59.0 | (53.0, 64.0) | 58.5 | (51.0, 64.0) | |
| Age—n (%) | 0.968 | ||||
| < 65 years | 138 | (77.1) | 136 | (77.3) | |
| ≥ 65 | 41 | (22.9) | 40 | (22.7) | |
| Age—n (%) | 0.818 | ||||
| < 75 years | 171 | (95.5) | 169 | (96.0) | |
| ≥ 75 | 8 | (4.5) | 7 | (4.0) | |
| BMI (kg/m2) | 0.419 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 25.86 | (4.00) | 25.53 | (3.68) | |
| Median (Q1, Q3) | 25.38 | (23.05, 28.03) | 24.90 | (23.11, 27.59) | |
| BMI (kg/m2)—n (%) | 0.125 | ||||
| < 25 | 79 | (44.1) | 92 | (52.3) | |
| ≥ 25 | 100 | (55.9) | 84 | (47.7) | |
| Dipper/Non‐dipper—n (%) | 0.062 | ||||
| Dipper | 114 | (63.7) | 95 | (54.0) | |
| Non‐dipper | 64 | (35.8) | 80 | (45.5) | |
| Unknown | 1 | (0.6) | 1 | (0.6) | |
| 24‐h SBP (mmHg) | 0.196 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 152.2 | (13.5) | 154.2 | (16.1) | |
| Median (Q1, Q3) | 151.1 | (142.7, 159.2) | 152.1 | (144.0, 163.0) | |
| 24‐h DBP (mmHg) | 0.461 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 96.6 | (9.2) | 97.4 | (10.0) | |
| Median (Q1, Q3) | 96.5 | (89.9, 103.0) | 97.1 | (91.2, 103.9) | |
| Office SBP (mmHg) | 0.676 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 154.3 | (13.6) | 154.9 | (13.0) | |
| Median (Q1, Q3) | 153.0 | (147.0, 163.0) | 154.0 | (147.0, 162.0) | |
| Office DBP (mmHg) | 0.893 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 95.0 | (10.1) | 95.2 | (10.6) | |
| Median (Q1, Q3) | 95.0 | (88.0, 103.0) | 95.5 | (89.0, 102.5) | |
| Classification of blood pressure levels at randomization—n (%) | 0.788 | ||||
| Grade 1 b | 87 | (48.6) | 83 | (47.2) | |
| Grade 2 c | 71 | (39.7) | 72 | (40.9) | |
| Other | 21 | (11.7) | 21 | (11.9) | |
| History of antihypertensive treatment—n (%) | 0.490 | ||||
| No | 47 | (26.3) | 52 | (29.5) | |
| Yes | 132 | (73.7) | 124 | (70.5) | |
| Unknown | 0 | (0.0) | 0 | (0.0) | |
| Duration of hypertension (days) | 0.792 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 1632.1 | (2334.4) | 1564.6 | (2484.6) | |
| Median (Q1, Q3) | 761.0 | (1.0, 1927.0) | 437.5 | (1.0, 1992.0) | |
| eGFR (mL/min/1.73 m2) | 0.223 | ||||
| n | 179 | 176 | |||
| Mean (SD) | 71.362 | (12.145) | 73.191 | (15.844) | |
| Median (Q1, Q3) | 70.650 | (62.440, 78.000) | 70.535 | (63.420, 81.975) | |
| eGFR (mL/min/1.73 m2)—n (%) | 0.844 | ||||
| < 60 | 34 | (19.0) | 32 | (18.2) | |
| ≥ 60 | 145 | (81.0) | 144 | (81.8) | |
| UACR (mg/gCr) | 0.567 | ||||
| n | 178 | 175 | |||
| Mean (SD) | 41.66 | (125.14) | 51.52 | (191.45) | |
| Median (Q1, Q3) | 12.80 | (6.70, 25.60) | 11.70 | (7.20, 24.30) | |
| UACR (mg/gCr)—n (%) | 0.554 | ||||
| < 30 | 141 | (78.8) | 143 | (81.3) | |
| ≥ 30 | 37 | (20.7) | 32 | (18.2) | |
| Unknown | 1 | (0.6) | 1 | (0.6) | |
| History of illness | NA | ||||
| Cerebrovascular accident | 0 | (0.0) | 0 | (0.0) | |
| Cardiac failure | 0 | (0.0) | 0 | (0.0) | |
| Myocardial infarction | 0 | (0.0) | 0 | (0.0) | |
| Atrial fibrillation | 0 | (0.0) | 0 | (0.0) | |
| Diabetes mellitus | 1 | (0.6) | 1 | (0.6) | |
| Dyslipidemia | 18 | (10.1) | 26 | (14.8) | |
| Renal impairment | 1 | (0.6) | 2 | (1.1) | |
| Hyperuricemia | 15 | (8.4) | 25 | (14.2) | |
| Peripheral arterial disease | 0 | (0.0) | 0 | (0.0) | |
| Coronary artery disease other than myocardial infarction | 1 | (0.6) | 0 | (0.0) | |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; NA, not applicable; Q1, first quartile; Q3, third quartile; SBP, systolic blood pressure; SD, standard deviation; UACR, urine albumin‐to‐creatinine ratio.
Chi‐squared test for categorical variables and independent t‐test for continuous variables.
office SBP 140–159 mmHg and/or office DBP 90–99 mmHg.
office SBP 160–179 mmHg and/or office DBP 100–109 mmHg.
3.2. Efficacy
The mean 24‐h SBP reduction in sacubitril/valsartan from baseline to Week 8 was noninferior to that in amlodipine (between‐treatment difference −0.62 mmHg [95% confidential interval (CI): −3.23 to 1.98; p = 0.003 for noninferiority; independent t‐test with noninferiority margin 3.0 mmHg]), and the following significance test showed no differences between treatments (Figure 1 and Table S1). The results obtained in PPS were also consistent with those in FAS (between‐treatment difference −0.50 mmHg [95% CI: −3.11 to 2.11; p = 0.004 for noninferiority]). Hourly average ambulatory BPs are shown in Figure 1, and the morning, daytime, and nighttime BP changes are detailed in Table S1. The missing data for body movement and others was 3.1% daytime and 1.9% nighttime of ABP measurements taken every 30 min. None of the timeframes showed significant differences between mean BP changes in the two groups.
FIGURE 1.

Changes in 24‐h SBP (A) and DBP (B) from baseline to Week 8, and hourly average ambulatory SBP (C) and DBP (D) at baseline and Week 8. Error bars represent standard error. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
In the subgroup analysis classified by nocturnal BP dipping status, no between‐treatment differences were observed in any timeframes, including nighttime BP in non‐dippers (Table S2 and Figure S2).
Another subgroup analysis by patients’ baseline characteristics is also shown in Table S3. These subgroup analyses showed no significant interaction effect for stratified baseline characteristics, except for baseline UACR. In the UACR ≥ 30 mg/gCr subgroup, the reduction in 24‐h SBP was greater in the amlodipine group than in the sacubitril/valsartan group.
BP control rates and response rates for both ABPM and office BP, representing the proportions of patients who met the target BP/pre‐defined response at Week 8, are shown in Figure 2. BP control rate as measured by ABPM tended to be higher in the sacubitril/valsartan group, but the difference was not statistically significant (Figure 2). Waterfall plots highlighted the distribution of individual BP changes, where both drugs also showed similar BP‐lowering trends in both 24‐h SBP and DBP (Figure 3).
FIGURE 2.

Proportion of patients who achieved ambulatory or office BP control and response rate at Week 8. (A) BP control was defined as follows. Ambulatory BP control: 24‐h SBP/24‐h DBP < 130/80 mmHg for patients < 75 years of age, and < 140/90 mmHg for ≥ 75 years of age. Office BP control: SBP/DBP < 130/80 mmHg for patients < 75 years of age, and < 140/90 mmHg for ≥ 75 years of age. (B) SBP response rate was defined as follows. Ambulatory SBP: 24‐h SBP < 130 mmHg at Week 8 for patients < 75 years of age, as 24‐h SBP < 140 mmHg for ≥ 75 years of age, or a reduction ≥ 20 mmHg from baseline. Office SBP: SBP < 130 mmHg at Week 8 for patients < 75 years of age, as < 140 mmHg for ≥ 75 years of age, or a reduction ≥ 20 mmHg from baseline. (C) DBP response rate was defined as follows. Ambulatory DBP: 24‐h DBP < 80 mmHg at Week 8 for patients < 75 years of age, as 24‐h DBP < 90 mmHg for ≥ 75 years of age, or a reduction ≥ 10 mmHg from baseline. Office DBP: SBP/DBP < 80 mmHg at Week 8 for patients < 75 years of age, as < 90 mmHg for ≥ 75 years of age, or a reduction ≥ 10 mmHg from baseline. p values obtained from Fisher's exact test. ABPM indicates ambulatory blood pressure monitoring; BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
FIGURE 3.
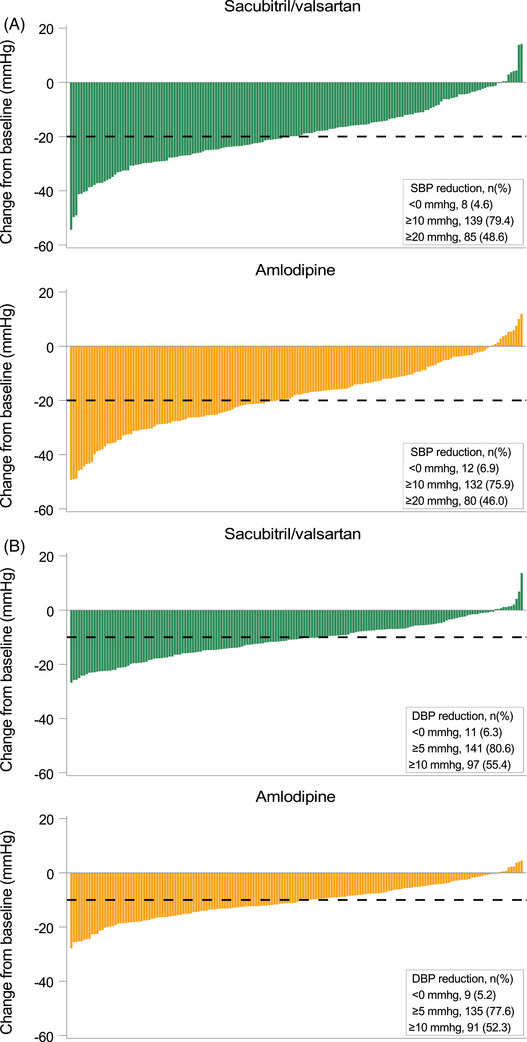
Waterfall plot of individual (A) 24‐h SBP and (B) 24‐h DBP changes from baseline to Week 8. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
In this study, we excluded patients with white coat hypertension based on patient's self‐report. However, three patients’ baseline ABPs met the criteria for white coat hypertension. The results of the analysis after excluding the three patients were the same as the results of the analysis including the three patients (data not shown).
3.3. Safety
Investigator‐reported AEs in this study are summarized in Table 2. Overall, the incidences of AEs and serious adverse events (SAEs) in the sacubitril/valsartan group up to Week 10 were comparable to those in the amlodipine group. A total of two SAEs were reported: clavicle fracture in the sacubitril/valsartan group, which was considered not related to sacubitril/valsartan, and aspartate aminotransferase increased in the amlodipine group, which was considered related to amlodipine. No deaths occurred during the study. Table S4 summarizes adverse drug reactions (ADRs) related to test/control drugs. For hypotension‐related events including dizziness, BP decreased, orthostatic hypotension, and hypotension, seven events (3.8%) in the sacubitril/valsartan group and three events (1.7%) in the amlodipine group were observed. The severities were mild, except for one moderate dizziness leading to discontinuation of study treatment in the sacubitril/valsartan group. As ADRs are summarized based on the investigator's report, hyperkalemia and angioedema, which were not reported, are not included in Table S4. In fact, no patient in either group had a serum potassium level above 5.5 mmol/L in the laboratory test.
TABLE 2.
Summary of adverse events.
| Items | Sacubitril/valsartan | Amlodipine | p value b | ||||
|---|---|---|---|---|---|---|---|
| (N = 182) | (N = 177) | ||||||
| n (%) | 95% CI a | n (%) | 95% CI a | ||||
| Adverse events | 34 | (18.7) | (13.3, 25.1) | 31 | (17.5) | (12.2, 23.9) | 0.786 |
| Serious adverse events | 1 | (0.5) | (0.0, 3.0) | 1 | (0.6) | (0.0, 3.1) | 1.000 |
| Adverse drug reactions | 14 | (7.7) | (4.3, 12.6) | 9 | (5.1) | (2.4, 9.4) | 0.390 |
Abbreviations: CI, confidence interval; n, the number of patients.
Clopper–Pearson confidential interval.
p values obtained from Fisher's exact test.
3.4. Laboratory Tests
Laboratory test results are shown in Table 3. Significant increases in cGMP and ANP from baseline to Week 8 were observed in the sacubitril/valsartan group, while eGFR was significantly elevated in amlodipine. Decreases in NT‐proBNP and UACR were shown in both groups, but there were no differences between treatments in the changes. Serum potassium, creatinine, and uric acid levels slightly decreased in both groups at Week 8, with a significantly greater decrease in the amlodipine group.
TABLE 3.
Laboratory tests.
| Analysis set: FAS | Sacubitril/valsartan | Amlodipine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 179) | (N = 176) | Between‐treatment difference | |||||||
| Biomarker | n | Geometric mean | Ratio of geometric mean and 95% CI b | n | Geometric mean | Ratio of geometric mean a and 95% CI b | Ratio of geometric mean c | 95% CI b | p value d |
| cGMP (nmol/mg·Cre) | |||||||||
| Baseline | 179 | 0.665 | — | 176 | 0.646 | — | — | — | — |
| Week 8 | 175 | 0.931 | 1.393 (1.317, 1.473) | 173 | 0.601 | 0.926 (0.875, 0.980) | 1.504 | (1.390, 1.627) | < 0.001 |
| ANP (pg/mL) | |||||||||
| Baseline | 179 | 18.19 | — | 176 | 16.80 | — | — | — | — |
| Week 8 | 175 | 39.06 | 2.14 (2.00, 2.30) | 173 | 14.65 | 0.87 (0.81, 0.93) | 2.46 | (2.24, 2.71) | < 0.001 |
| BNP (pg/mL) | |||||||||
| Baseline | 179 | 10.67 | — | 176 | 10.16 | — | — | — | — |
| Week 8 | 174 | 10.81 | 1.01 (0.94, 1.08) | 172 | 8.94 | 0.88 (0.82, 0.94) | 1.15 | (1.05, 1.27) | 0.003 |
| NT‐proBNP (pg/mL) | |||||||||
| Baseline | 179 | 51.12 | — | 176 | 54.22 | — | — | — | — |
| Week 8 | 174 | 34.36 | 0.67 (0.62, 0.73) | 172 | 34.76 | 0.64 (0.59, 0.70) | 1.05 | (0.93, 1.18) | 0.413 |
| UACR (mg/gCr) | |||||||||
| Baseline | 178 | 15.10 | — | 175 | 14.72 | — | — | — | — |
| Week 8 | 174 | 12.53 | 0.82 (0.73, 0.91) | 169 | 11.91 | 0.80 (0.73, 0.88) | 1.02 | (0.88, 1.18) | 0.781 |
| eGFR (mL/min/1.73 m2) | |||||||||
| Baseline | 179 | 70.348 | — | 176 | 71.667 | — | — | — | — |
| Week 8 | 175 | 70.618 | 1.003 (0.987, 1.019) | 173 | 74.193 | 1.033 (1.016, 1.050) | 0.971 | (0.949, 0.993) | 0.010 |
| Analysis set: SAS | Sacubitril/valsartan | Amlodipine | |||||||
|---|---|---|---|---|---|---|---|---|---|
| (N = 182) | (N = 177) | ||||||||
| Biochemistry | n | Arithmetic mean | Mean change and 95% CI | n | Arithmetic mean | Mean change and 95% CI | Mean difference | 95% CI | p value e |
| Sodium (mmol/L) | |||||||||
| Baseline | 182 | 141.9 | — | 177 | 141.7 | — | — | — | — |
| Week 8 | 175 | 141.8 | −0.1 (−0.4, 0.1) | 173 | 141.3 | −0.5 (−0.8, −0.2) | 0.4 | (0.0, 0.7) | 0.061 |
| Potassium (mmol/L) | |||||||||
| Baseline | 182 | 4.06 | — | 177 | 3.98 | — | — | — | — |
| Week 8 | 175 | 3.98 | −0.07 (−0.13, −002) | 173 | 3.84 | −0.15 (−0.20, −0.09) | 0.07 | (0.00, 0.15) | 0.049 |
| Creatinine (mg/dL) | |||||||||
| Baseline | 182 | 0.831 | — | 177 | 0.818 | — | — | — | — |
| Week 8 | 175 | 0.830 | −0.001 (−0.013, 0.011) | 173 | 0.791 | −0.024 (−0.036, −0.012) | 0.023 | (0.006, 0.040) | 0.007 |
| Uric acid (mg/dL) | |||||||||
| Baseline | 182 | 5.91 | — | 177 | 6.14 | — | — | — | — |
| Week 8 | 175 | 5.75 | −0.14 (−0.28, 0.00) | 173 | 5.75 | −0.38 (−0.50, −0.27) | 0.24 | (0.06, 0.43) | 0.009 |
Abbreviations: ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; cGMP, cyclic guanosine monophosphate; CI, confidence interval; eGFR, estimated glomerular filtration rate; FAS, full analysis set; NA, not applicable; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; SAS, safety analysis set; UACR, urine albumin‐to‐creatinine ratio.
The ratio of the geometric mean was calculated by dividing the geometric mean at Week 8 by that at baseline.
95% CI for the geometric mean.
The ratio of the geometric mean was calculated by dividing the geometric mean in sacubitril/valsartan by that in amlodipine.
p values were calculated by an independent t‐test with a two‐sided significance level of 0.05 for logarithmic values.
p values were calculated by an independent t‐test with a two‐sided significance level of 0.05.
4. Discussion
This is the first prospective randomized trial to compare the BP lowering effect of sacubitril/valsartan with CCB in patients with hypertension. It was demonstrated that treatment with sacubitril/valsartan resulted in a noninferior reduction in 24‐h SBP at Week 8 compared to treatment with amlodipine regardless of patients’ backgrounds. In addition, once‐daily sacubitril/valsartan treatment reduced BP at any timepoints throughout 24‐h, which was comparable to the amlodipine profile. BP control and response rates of sacubitril/valsartan were also noninferior to amlodipine. Amlodipine is known as one of the most potent antihypertensive drugs and is commonly prescribed for hypertensive patients in Japan, which is expected to have reliable efficacy in reducing BP and good safety profiles in patients with various backgrounds [6, 13]. Our results suggested that sacubitril/valsartan, a different drug class than CCB, is a potent antihypertensive drug comparable to amlodipine.
As for patient safety, drug‐related “hypotension‐related events” were reported in both groups, but no marked difference was observed. None of the patients discontinued the study treatment because of an unintended BP decrease, while one patient in the sacubitril/valsartan group discontinued due to moderate dizziness. For the treatment of chronic heart failure, hypotension was more frequent in sacubitril/valsartan than in renin‐angiotensin system inhibitors, so the risk of excessive BP lowering was also indicated for the treatment of hypertension [14, 15]. However, our findings suggested no specific concerns about excessive BP lowering and that sacubitril/valsartan is well‐tolerated in the population like the hypertensive patients enrolled in this study.
Since CCBs including amlodipine have a preventive effect on cerebral cardiovascular (CV) events in addition to their potent BP‐lowering effect, it is an established major drug class in guidelines [2, 16]. NT‐proBNP, a universal surrogate marker predictive of the development of CV events, especially heart failure [17, 18], decreased at Week 8 in both groups in this study. ANP is an endogenous peptide with a cardioprotective effect, and cGMP is a classic intracellular second messenger that works in response to ANP [19, 20]. In this study, these biomarkers elevated only in the sacubitril/valsartan group in response to its neprilysin inhibition, indicating enhanced cardiac protection. Besides, cardiac hypertrophy is also a high‐risk factor for future CV events. A previous study reported that sacubitril/valsartan induced the regression on cardiac hypertrophy greater than ARBs [21], which were reported to have a more potent preventive effect of cardiac hypertrophy than CCBs [22]. Further investigations are warranted to evaluate the long‐term cardiac effects of sacubitril/valsartan in patients with hypertension, however, based on the findings in previous studies and this study, sacubitril/valsartan is expected as an antihypertensive drug which might have more beneficial effect on cardiac hypertrophy than CCBs. The changes from baseline eGFR levels were significantly different between the two groups, resulting from elevated eGFR levels in the amlodipine group while no obvious change was observed in the sacubitril/valsartan group. The increased eGFR by CCB has been reported in a previous study that showed an acute increase in glomerular filtration rate caused by afferent arteriolar vasodilation with amlodipine in participants with baseline urinary protein to creatinine ratio ≤ 0.22 [23]. Therefore, further studies are necessary to clarify their long‐term effects on renal function.
In actual clinical practice, antihypertensive drugs are selected on the grade of hypertension and individual patient background (e.g., lifestyles such as excessive salt intake and the presence of complications of CV disease or renal disease). In Japan, ARB is particularly recommended for hypertensive patients with CKD presenting proteinuria or microalbuminuria, cardiac hypertrophy, and heart failure with reduced ejection fraction [2]. However, ARB's BP‐lowering effect and response rate are not expected to be as strong as CCB's, which might lead to less frequent use of ARB [13]. Many hypertensive cases in Japan are likely to result from excessive salt intake. Indeed, it has been reported that the amount of sodium intake was higher in Asian countries than in other regions [24], and salt sensitivity is high in Asians because of salt‐sensitive gene polymorphism of the renin‐angiotensin system [25, 26, 27]. Diuretics are another major antihypertensive drug with potent sodium excretion and diuretic effect, but the frequency of use is low in Japan, approximately 10% due to concerns about side effects such as hyperuricemia and electrolyte defects [28, 29]. Sacubitril/valsartan promotes sodium excretion via the enhancement of the effect of natriuretic peptides and was reported to increase natriuresis and diuresis [30]. Since no significant events related to electrolyte abnormalities were reported in this study, concerns about electrolyte defects in sacubitril/valsartan were not indicated.
Current guidelines recommend combination therapy with a different class of antihypertensive drugs if the antihypertensive effect is still insufficient, which shows more marked antihypertensive effects compared with doubling the dose of the antihypertensive drug [2]. Furthermore, in order to maintain good adherence to antihypertensive treatment and continue to prevent cardiovascular events for a long time, the key factor is to decrease the number of pills as much as possible along with BP control [31, 32, 33]. In actual clinical practice, the BP control rate in Japan is very low compared with other countries [34], even with two or more drug combination therapies [29, 35]. The findings of this study suggested the possibility that sacubitril/valsartan treatment could contribute to the improvement of BP control. In addition, considering the previous report of the beneficial effect of sacubitril/valsartan add‐on therapy to amlodipine [36], combination of sacubitril/valsartan and amlodipine could contribute to good BP control with a small number of pills.
The study has several limitations, including open‐label treatment. First, the BP assessment was carried out at only three timepoints: baseline, middle, and the end of the treatment period. Since patients at risk for being initiated at 200 mg of sacubitril/valsartan were excluded from this study (e.g., patients > 80 years of age, office SBP < 150 mmHg, or eGFR < 30 mL/min/1.73 m2), hypotension or other safety risks in these patients could not be assessed. In clinical practice, more frequent BP monitoring, including home BP measurement, is recommended after initiation of sacubitril/valsartan to minimize the risk of AEs, especially in these patients. Second, our study was conducted between early spring and autumn. Therefore, no data was available for the treatment in winter, a season with a higher risk of CV events.
5. Conclusions
Sacubitril/valsartan has a beneficial effect of lowering BP, which seems equivalent to amlodipine, one of the most potent antihypertensive drugs. The use of sacubitril/valsartan in hypertensive patients is expected to improve inadequate BP control in actual clinical practice.
Author Contributions
Koichi Yamamoto, Daisuke Yarimizu, and Hiromi Rakugi contributed to the study design, planning of data analysis, data interpretation, and drafting the manuscript. Ayano Shimanishi, Shunsuke Eguchi, Kazuma Iekushi, and Kazuomi Kario contributed to the study design and data interpretation. Yoichi Takami and Yoichi Nozato contributed to data acquisition. All authors reviewed and gave the final approval of the manuscript for submission.
Conflicts of Interest
H.R. has received honoraria from Novartis Pharma K.K., Otsuka Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., and Takeda Pharmaceutical Co. Ltd.; received research funding from Novartis Pharma K.K.; and has received a scholarship or donation from Daiichi Sankyo Co. Ltd., Kyowa Kirin Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., Sumitomo Pharma Co. Ltd., and Takeda Pharmaceutical Co. Ltd. K.K. has received honoraria from Omron Healthcare Co. Ltd., CureApp, Inc., Daiichi Sankyo Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Terumo Corporation, Bayer, Kyowa Kirin Co. Ltd., Viatris Pharmaceuticals Japan Inc., and Novartis Pharma K.K.; received research funding from Omron Healthcare Co. Ltd., Fukuda Denshi Co. Ltd., and Edwards Lifesciences Corporation; and has received a scholarship or donation from Otsuka Pharmaceutical Co. Ltd., Daiichi Sankyo Co. Ltd., Nippon Boehringer Ingelheim Co. Ltd., and Sumitomo Pharma Co. Ltd. K.Y. has received honoraria from Daiichi Sankyo Co. Ltd. and Otsuka Pharmaceutical Co. Ltd.; received research funding from Novartis Pharma K.K. and has received a scholarship or donation from Nippon Boehringer Ingelheim Co. Ltd. and Sumitomo Pharma Co. Ltd. D.Y., A.S., S.E., and K.I. are employees of Novartis Pharma K.K. The remaining authors have nothing to disclose.
Supporting information
Supporting information
Acknowledgments
The authors acknowledge all investigators at the participating centers and all patients for their commitment to the study. The authors would also like to thank I'cros Co., Ltd. for their statistical support, medical writing, and editorial support. This study was funded by Novartis Pharma K.K., which was involved in the study design, planning of the data analysis, interpretation of results, and development of the manuscript, but not involved in the data management and statistical analysis. Data management and statistical analysis were conducted by I'cros Co., Ltd funded by Novartis Pharma K.K.
Funding: This study was funded by Novartis Pharma K.K.
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in this study. The data will be shared upon reasonable request to the corresponding authors.
References
- 1. World Health Organization . Global Report on Hypertension: The Race Against a Silent Killer (Geneva, Switzerland, 2023): 10, https://www.who.int/publications/i/item/9789240081062. [Google Scholar]
- 2. Umemura S., Arima H., Arima S., et al., “The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019),” Hypertension Research 42 (2019): 1235–1481. [DOI] [PubMed] [Google Scholar]
- 3. Mancia G., Kreutz R., Brunström M., et al., “2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA),” Journal of Hypertension 41 (2023): 1874–2071. [DOI] [PubMed] [Google Scholar]
- 4. Ruilope L. M., Dukat A., Böhm M., et al., “Blood‐Pressure Reduction With LCZ696, a Novel Dual‐Acting Inhibitor of the Angiotensin II Receptor and Neprilysin: A Randomised, Double‐Blind, Placebo‐Controlled, Active Comparator Study,” Lancet 375 (2010): 1255–1266. [DOI] [PubMed] [Google Scholar]
- 5. Jhund P. S., Claggett B., Packer M., et al., “Independence of the Blood Pressure Lowering Effect and Efficacy of the Angiotensin Receptor Neprilysin Inhibitor, LCZ696, in Patients With Heart Failure With Preserved Ejection Fraction: An Analysis of the PARAMOUNT Trial,” European Journal of Heart Failure 16 (2014): 671–677. [DOI] [PubMed] [Google Scholar]
- 6. Kario K. and Hoshide S., “Age‐Related Difference in the Sleep Pressure‐Lowering Effect Between an Angiotensin II Receptor Blocker and a Calcium Channel Blocker in Asian Hypertensives: The ACS1 Study,” Hypertension 65 (2015): 729–735. [DOI] [PubMed] [Google Scholar]
- 7. Pradhan A., Tiwari A., Sethi R., et al., “Current Evidence and Perspectives in Management of Hypertension,” International Journal of Hypertension 2019 (2019): 1824621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White W. B., Weber M. A., Sica D., et al., “Effects of the Angiotensin Receptor Blocker Azilsartan Medoxomil Versus Olmesartan and Valsartan on Ambulatory and Clinic Blood Pressure in Patients With Stages 1 and 2 Hypertension,” Hypertension 57 (2011): 413–420. [DOI] [PubMed] [Google Scholar]
- 9. JCS Joint Working Group , “Guidelines for the Clinical Use of 24 Hour Ambulatory Blood Pressure Monitoring (ABPM) (JCS 2010)—Digest Version ‐.” Circulation Journal 76 (2012): 508–519. [DOI] [PubMed] [Google Scholar]
- 10. Kario K., Hoshide S., Saito K., et al., “Validation of the TM‐2441 Ambulatory Blood Pressure Measurement Device According to the ISO 81060‐2,” Blood Pressure Monitoring 24 (2019): 38–41. [DOI] [PubMed] [Google Scholar]
- 11. Blood Pressure Lowering Treatment Trialists' Collaboration , “Pharmacological Blood Pressure Lowering for Primary and Secondary Prevention of Cardiovascular Disease Across Different Levels of Blood Pressure: An Individual Participant‐Level Data Meta‐Analysis.” Lancet 397 (2021): 1625–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kario K., Rakugi H., Yarimizu D., et al., “Twenty‐Four‐Hour Blood Pressure‐Lowering Efficacy of Sacubitril/Valsartan Versus Olmesartan in Japanese Patients With Essential Hypertension Based on Nocturnal Blood Pressure Dipping Status: A Post Hoc Analysis of Data From a Randomized, Double‐Blind Multicenter Study,” Journal of the American Heart Association 12, no. 8 (2023): e027612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ohishi M., Yoshida T., Oh A., et al., “Analysis of Antihypertensive Treatment Using Real‐World Japanese Data – The Retrospective Study of Antihypertensives for Lowering Blood Pressure (REAL) Study,” Hypertension Research 42 (2019): 1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsutsui H., Momomura S.‐I., Saito Y., et al., PARALLEL‐HF Investigators , “Efficacy and Safety of Sacubitril/Valsartan in Japanese Patients With Chronic Heart Failure and Reduced Ejection Fraction – Results From the PARALLEL‐HF Study,” Circulation Journal 85 (2021): 584–594. [DOI] [PubMed] [Google Scholar]
- 15. Solomon S. D., McMurray J. J. V., Anand I. S., et al., “PARAGON‐HF Investigators and Committees. Angiotensin‐Neprilysin Inhibition in Heart Failure With Preserved Ejection Fraction,” New England Journal of Medicine 381 (2019): 1609–1620. [DOI] [PubMed] [Google Scholar]
- 16. Law M. R., Morris J. K., and Wald N. J., “Use of Blood Pressure Lowering Drugs in the Prevention of Cardiovascular Disease: Meta‐Analysis of 147 Randomised Trials in the Context of Expectations From Prospective Epidemiological Studies,” British Medical Journal 338 (2009): b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jia X., Al Rifai M., Ndumele C. E., et al., “Reclassification of Pre‐Heart Failure Stages Using Cardiac Biomarkers: The ARIC Study,” JACC: Heart Failure 11 (2023): 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Doi Y., Ninomiya T., Hata J., et al., “N‐terminal Pro‐Brain Natriuretic Peptide and Risk of Cardiovascular Events in a Japanese Community: The Hisayama Study,” Arteriosclerosis, Thrombosis, and Vascular Biology 31 (2011): 2997–3003. [DOI] [PubMed] [Google Scholar]
- 19. Volpe M., Carnovali M., and Mastromarino V., “The Natriuretic Peptides System in the Pathophysiology of Heart Failure: From Molecular Basis to Treatment,” Clinical Science (London, England: 1979) 130 (2016): 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishikimi T., Maeda N., and Matsuoka H., “The Role of Natriuretic Peptides in Cardioprotection,” Cardiovascular Research 69 (2006): 318–328. [DOI] [PubMed] [Google Scholar]
- 21. Schmieder R. E., Wagner F., Mayr M., et al., “The Effect of Sacubitril/Valsartan Compared to Olmesartan on Cardiovascular Remodelling in Subjects With Essential Hypertension: The Results of a Randomized, Double‐Blind, Active‐Controlled Study,” European Heart Journal 38 (2017): 3308–3317. [DOI] [PubMed] [Google Scholar]
- 22. Ogihara T., Fujimoto A., Nakao K., et al., CASE‐J Trial Group , “ARB Candesartan and CCB Amlodipine in Hypertensive Patients: The CASE‐J Trial,” Expert Review of Cardiovascular Therapy 6 (2008): 1195–1201. [DOI] [PubMed] [Google Scholar]
- 23. Agodoa L. Y., Appel L., Bakris G. L., et al., “Effect of Ramipril vs Amlodipine on Renal Outcomes in Hypertensive Nephrosclerosis: A Randomized Controlled Trial,” Journal of the American Medical Association 285 (2001): 2719–2728. [DOI] [PubMed] [Google Scholar]
- 24. Powles J., Fahimi S., Micha R., et al., “Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE). Global, Regional and National Sodium Intakes in 1990 and 2010: A Systematic Analysis of 24 h Urinary Sodium Excretion and Dietary Surveys Worldwide,” BMJ Open 3 (2013): e003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohishi M., “Sacubitril/Valsartan – A New Weapon for Fighting the Hypertension Paradox,” Hypertension Research 45 (2022): 915–916. [DOI] [PubMed] [Google Scholar]
- 26. Kario K., Chen C.‐H., Park S., et al., “Consensus Document on Improving Hypertension Management in Asian Patients, Taking Into Account Asian Characteristics,” Hypertension 71 (2018): 375–382. [DOI] [PubMed] [Google Scholar]
- 27. Katsuya T., Ishikawa K., Sugimoto K., et al., “Salt Sensitivity of Japanese From the Viewpoint of Gene Polymorphism,” Hypertension Research 26 (2003): 521–525. [DOI] [PubMed] [Google Scholar]
- 28. Ishida T., Oh A., Hiroi S., et al., “Current Prescription Status of Antihypertensive Drugs in Japanese Patients With Hypertension: Analysis by Type of Comorbidities,” Clinical and Experimental Hypertension 41 (2019): 203–210. [DOI] [PubMed] [Google Scholar]
- 29. Kobayashi K., Chin K., Hatori N., et al., “Cross‐Sectional Survey of Hypertension Management in Clinical Practice in Japan: The Kanagawa Hypertension Study 2021 Conducted in Collaboration With Japan Medical Association Database of Clinical Medicine,” Hypertension Research 46 (2023): 2447–2459. [DOI] [PubMed] [Google Scholar]
- 30. Wang T.‐D., Tan R.‐S., Lee H.‐Y., et al., “Effects of Sacubitril/Valsartan (LCZ696) on Natriuresis, Diuresis, Blood Pressures, and NT‐proBNP in Salt‐Sensitive Hypertension,” Hypertension 69 (2017): 32–41. [DOI] [PubMed] [Google Scholar]
- 31. Gradman A. H., Parisé H., Lefebvre P., et al., “Initial Combination Therapy Reduces the Risk of Cardiovascular Events in Hypertensive Patients: A Matched Cohort Study,” Hypertension 61 (2013): 309–318. [DOI] [PubMed] [Google Scholar]
- 32. Rea F., Savaré L., Franchi M., et al., “Adherence to Treatment by Initial Antihypertensive Mono and Combination Therapies,” American Journal of Hypertension 34 (2021): 1083–1091. [DOI] [PubMed] [Google Scholar]
- 33. Weber M. A., Julius S., Kjeldsen S. E., et al., “Blood Pressure Dependent and Independent Effects of Antihypertensive Treatment on Clinical Events in the VALUE Trial,” Lancet 363 (2004): 2049–2451. [DOI] [PubMed] [Google Scholar]
- 34. NCD Risk Factor Collaboration (NCD‐RisC) , “Long‐Term and Recent Trends in Hypertension Awareness, Treatment, and Control in 12 High‐Income Countries: An Analysis of 123 Nationally Representative Surveys,” Lancet 394 (2019): 639–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satoh M., Muroya T., Murakami T., et al., “The Impact of Clinical Inertia on Uncontrolled Blood Pressure in Treated Hypertension: Real‐World, Longitudinal Data From Japan,” Hypertension Research 47 (2024): 598–607. [DOI] [PubMed] [Google Scholar]
- 36. Wang J.‐G., Yukisada K., Sibulo A., et al., “Efficacy and Safety of Sacubitril/Valsartan (LCZ696) Add‐On to Amlodipine in Asian Patients With Systolic Hypertension Uncontrolled With Amlodipine Monotherapy,” Journal of Hypertension 35 (2017): 877–885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals who participated in this study. The data will be shared upon reasonable request to the corresponding authors.


