Abstract
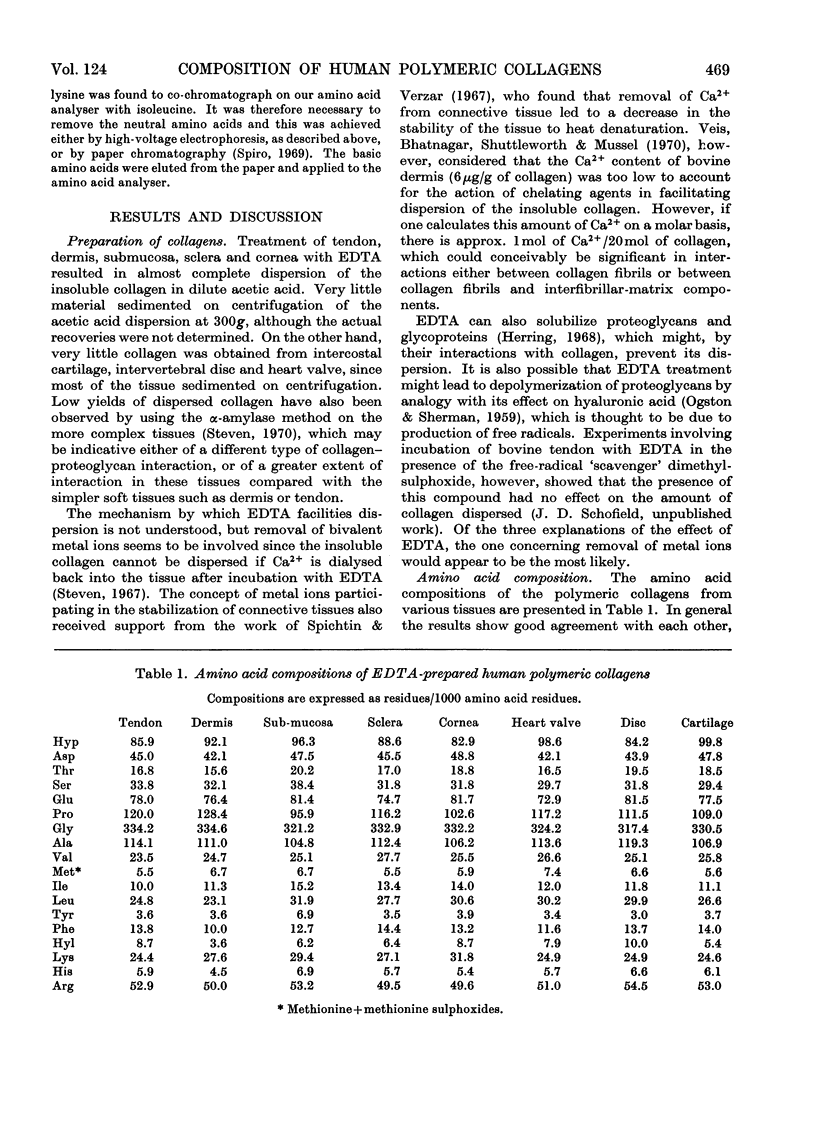
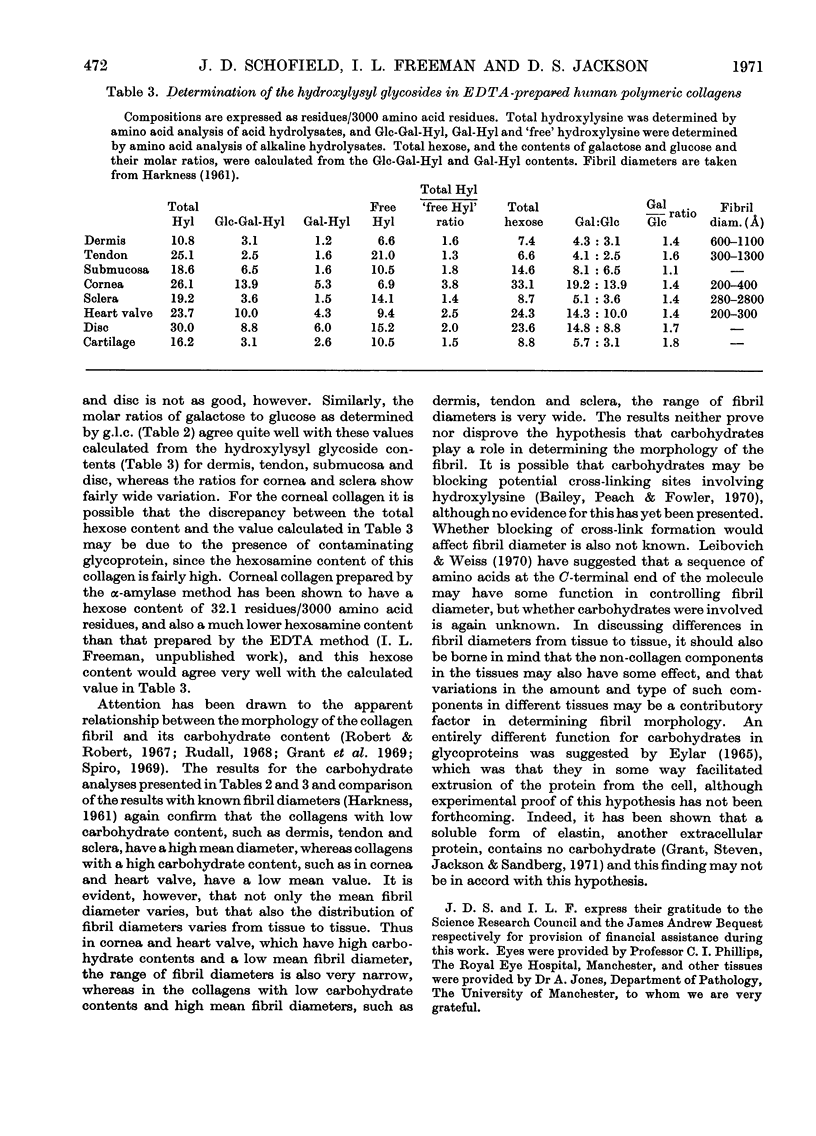
1. Insoluble polymeric collagens from various human tissues were prepared by the EDTA method. Almost all of the collagen from simple soft tissues such as dermis, tendon, submucosa, sclera and cornea could be extracted, whereas the more complex tissues such as intercostal cartilage and intervertebral disc yielded only small amounts of collagen. Amino acid and carbohydrate analysis indicated that most of the preparations were highly purified on the basis of their tyrosine, hexosamine, mannose, xylose and fucose contents. 2. Wide variation in the total hexose content was observed, the lowest being 8.5 residues/3000 amino acid residues for collagen from dermis and the highest being 42.1 residues/3000 in corneal collagen. The molar ratios of sugars also varied, submucosal collagen having a galactose/glucose ratio of 1.0 and corneal collagen having a ratio of 2.3. 3. The presence of glucosylgalactosylhydroxylysine was confirmed in submucosal collagen by compositional and chromatographic analysis of this component after its isolation from alkaline hydrolysates of the collagen. Evidence was also obtained for the presence of galactosylhydroxylysine. 4. Determination of the hydroxylysyl glycosides was carried out and it was observed that the amounts of these components varied widely from tissue to tissue. Corneal collagen contained 19.1 hydroxylysine-linked carbohydrate units/3000 amino acid residues, whereas tendon collagen contained only 4.1 units/3000. Variation in the ratio disaccharide unit/monosaccharide unit was also observed, the ratio being 1.2 in intercostal cartilage collagen and 4.1 in submucosal collagen. The proportion of the total hydroxylysine that was substituted by carbohydrate also varied from tissue to tissue.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Etherington D. J. Action of crude bacterial alpha-amylase on tropocollagen. Biochim Biophys Acta. 1970 Jul 27;214(1):238–241. doi: 10.1016/0005-2795(70)90093-0. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Peach C. M., Fowler L. J. Chemistry of the collagen cross-links. Isolation and characterization of two intermediate intermolecular cross-links in collagen. Biochem J. 1970 May;117(5):819–831. doi: 10.1042/bj1170819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensusan H. B. An investigation of the products of an enzymic hydrolysis of collagens. Biochemistry. 1969 Dec;8(12):4716–4723. doi: 10.1021/bi00840a009. [DOI] [PubMed] [Google Scholar]
- Butler W. T., Cunningham L. W. Evidence for the linkage of a disaccharide to hydroxylysine in tropocollagen. J Biol Chem. 1966 Sep 10;241(17):3882–3888. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D. A comparison of glycopeptides derived from soluble and insoluble collagens. J Biol Chem. 1968 May 10;243(9):2390–2398. [PubMed] [Google Scholar]
- Cunningham L. W., Ford J. D., Segrest J. P. The isolation of identical hydroxylysyl glycosides from hydrolysates of soluble collagen and from human urine. J Biol Chem. 1967 May 25;242(10):2570–2571. [PubMed] [Google Scholar]
- Drake M. P., Davison P. F., Bump S., Schmitt F. O. Action of proteolytic enzymes on tropocollagen and insoluble collagen. Biochemistry. 1966 Jan;5(1):301–312. doi: 10.1021/bi00865a039. [DOI] [PubMed] [Google Scholar]
- EASTOE J. E. The amino acid composition of mammalian collagen and gelatin. Biochem J. 1955 Dec;61(4):589–600. doi: 10.1042/bj0610589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- FLEISCHMAJER R., FISHMAN L. AMINO-ACID COMPOSITION OF HUMAN DERMAL COLLAGEN. Nature. 1965 Jan 16;205:264–266. doi: 10.1038/205264a0. [DOI] [PubMed] [Google Scholar]
- FULLER K. W., NORTHCOTE D. H. A micro method for the separation and determination of polysaccharides by zone electrophoresis. Biochem J. 1956 Dec;64(4):657–663. doi: 10.1042/bj0640657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman I. L., Steven F. S., Jackson D. S. Isolation and amino acid composition of bovine corneal polymeric collagens. Biochim Biophys Acta. 1968 Jan 22;154(1):252–254. doi: 10.1016/0005-2795(68)90286-9. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Freeman I. L., Schofield J. D., Jackson D. S. Variations in the carbohydrate content of human and bovine polymeric collagens from various tissues. Biochim Biophys Acta. 1969 May 6;177(3):682–685. doi: 10.1016/0304-4165(69)90345-6. [DOI] [PubMed] [Google Scholar]
- Grant M. E., Jackson D. S. Carbohydrate content of bovine collagen preparations. Biochem J. 1968 Jul;108(4):587–591. doi: 10.1042/bj1080587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M. E., Steven F. S., Jackson D. S., Sandberg L. B. Carbohydrate content of insoluble elastins prepared from adult bovine and calf ligamentum nuchae and tropoelastin isolated from copper-deficient porcine aorta. Biochem J. 1971 Jan;121(2):197–202. doi: 10.1042/bj1210197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS R. D. Biological functions of collagen. Biol Rev Camb Philos Soc. 1961 Nov;36:399–463. doi: 10.1111/j.1469-185x.1961.tb01596.x. [DOI] [PubMed] [Google Scholar]
- Herring G. M. The chemical structure of tendon, cartilage, dentin and bone matrix. Clin Orthop Relat Res. 1968 Sep-Oct;60:261–299. [PubMed] [Google Scholar]
- JACKSON D. S., LEACH A. A., JACOBS S. The amino acid composition of the collagen fractions of rabbit skin. Biochim Biophys Acta. 1958 Feb;27(2):418–420. doi: 10.1016/0006-3002(58)90356-1. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Electron microscope studies of the effects of endo- and exopeptidase digestion on tropocollagen. A novel concept of the role of terminal regions in fibrillogenesis. Biochim Biophys Acta. 1970 Sep 29;214(3):445–454. doi: 10.1016/0005-2795(70)90303-x. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A., LIKINS R. C. The conversion of lysine to hydroxylysine and its relation to the biosynthesis of collagen in several tissues of the rat. J Biol Chem. 1957 Nov;229(1):101–109. [PubMed] [Google Scholar]
- STEVEN F. S. THE NISHIHARA TECHNIQUE FOR THE SOLUBILIZATION OF COLLAGEN. APPLICATION TO THE PREPARATION OF SOLUBLE COLLAGENS FROM NORMAL AND RHEUMATOID CONNECTIVE TISSUE. Ann Rheum Dis. 1964 Jul;23:300–301. doi: 10.1136/ard.23.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., Cunningham L. W. Variations in human urinary O-hydroxylysyl glycoside levels and their relationship to collagen metabolism. J Clin Invest. 1970 Aug;49(8):1497–1509. doi: 10.1172/JCI106367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spichtin H., Verzár F. Calcium as stabilizing factor of the collagen macromolecule. Experientia. 1969 Jan 15;25(1):9–11. doi: 10.1007/BF01903859. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Characterization and quantitative determination of the hydroxylysine-linked carbohydrate units of several collagens. J Biol Chem. 1969 Feb 25;244(4):602–612. [PubMed] [Google Scholar]
- Spiro R. G. The structure of the disaccharide unit of the renal glomerular basement membrane. J Biol Chem. 1967 Oct 25;242(20):4813–4823. [PubMed] [Google Scholar]
- Steven F. S., Grant M. E., Ayad S., Weiss J. B., Leibovich S. J. The action of crude bacterial alpha-amylase on tropocollagen and on polymeric collagen. Biochim Biophys Acta. 1970 Sep 29;214(3):564–565. doi: 10.1016/0005-2795(70)90321-1. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S. Purification and amino acid composition of monomeric and polymeric collagens. Biochem J. 1967 Aug;104(2):534–536. doi: 10.1042/bj1040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S., Schofield J. D., Bard J. B. Polymeric collagen isolated from the human intestinal submucosa. Gut. 1969 Jun;10(6):484–487. doi: 10.1136/gut.10.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967 Aug 15;140(3):522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]
- Veis A., Bhatnagar R. S., Shuttleworth C. A., Mussell S. The solubilization of mature, polymeric collagen fibrils by lyotropic relaxation. Biochim Biophys Acta. 1970 Jan 20;200(1):97–112. doi: 10.1016/0005-2795(70)90048-6. [DOI] [PubMed] [Google Scholar]
- WALSER M., BODENLOS L. J. Composition of skin as compared with muscle. Am J Physiol. 1954 Jul;178(1):91–96. doi: 10.1152/ajplegacy.1954.178.1.91. [DOI] [PubMed] [Google Scholar]
- Weiss J. B., Smith I. Sensitive location reagent for the simultaneous detection of sugars, amino sugars and sialic acids. Nature. 1967 Aug 5;215(5101):638–638. doi: 10.1038/215638a0. [DOI] [PubMed] [Google Scholar]


