Abstract
A technique is examined for determining amino groups with 2,4,6-trinitrobenzenesulphonic acid, in which the extinction at 420nm of sulphite complexes of the trinitrophenylated amino groups is measured. The sensitivity of the method is 5–200nmol of amino group. The method is especially suitable for checking the extent of blocking or unblocking of amino groups in proteins and peptides, owing to the short time required for reaction (5min at room temperature). The reaction of the reagent with thiol groups has been studied and was found to proceed 30–50 times faster than with ∈-amino groups of model compounds. The ∈420 of a trinitrophenylated thiol group was found to be 2250m−1·cm−1. The reaction with several amino acids, peptides and proteins is presented. The ∈420 of a typical α-amino group was found to be 22000m−1·cm−1 and that of an ∈-amino group, 19200m−1·cm−1. Difficulties inherent in the analysis of constituent amino group reactions in proteins are discussed.
Full text
PDF
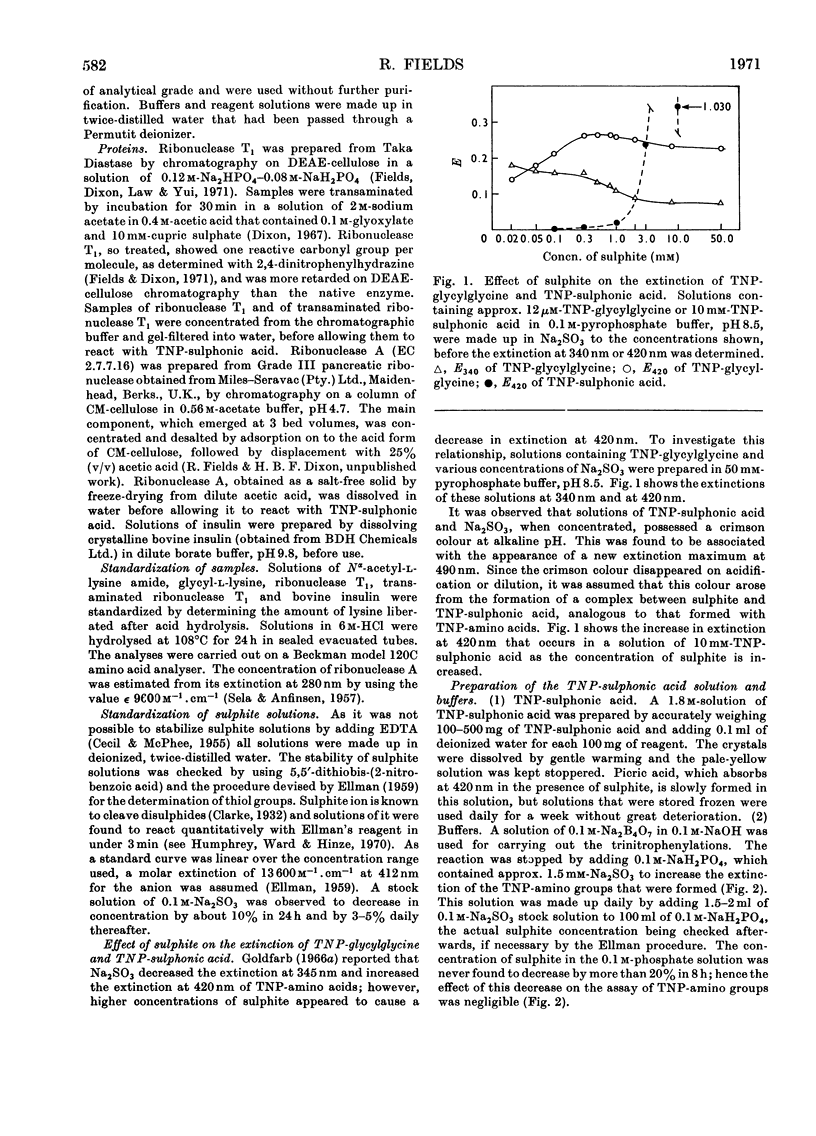
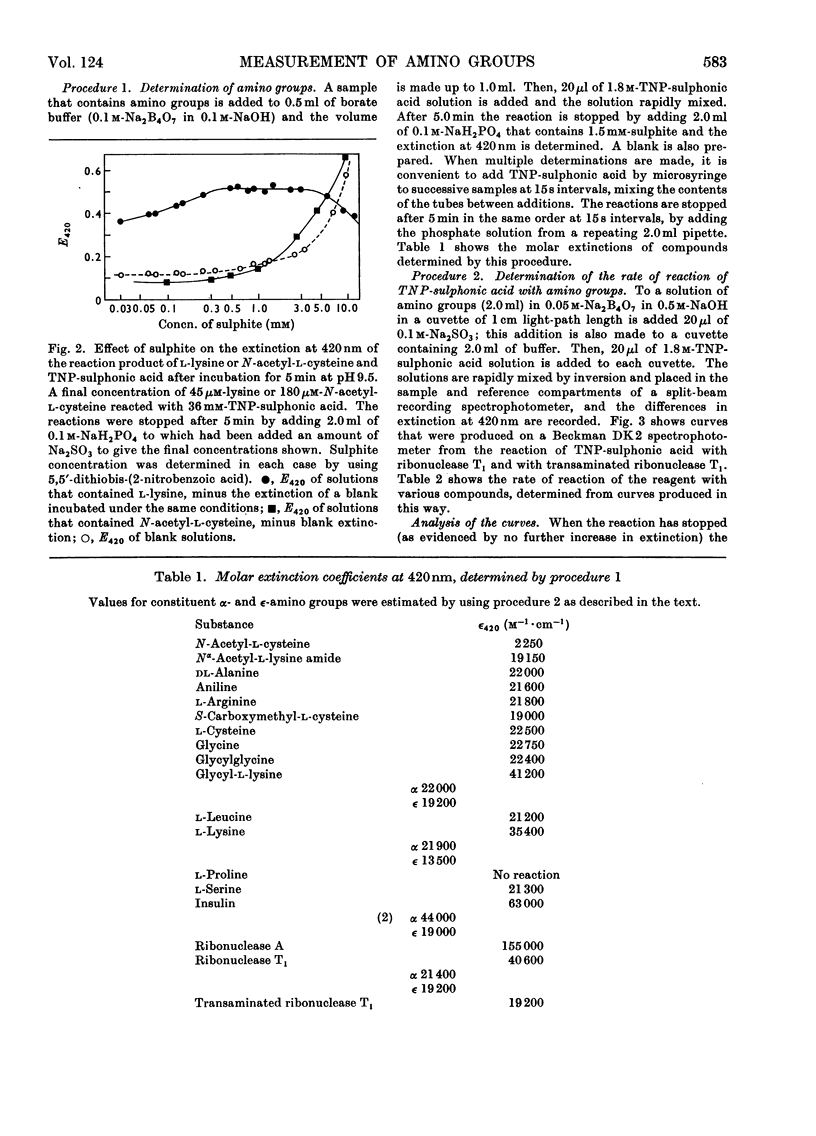
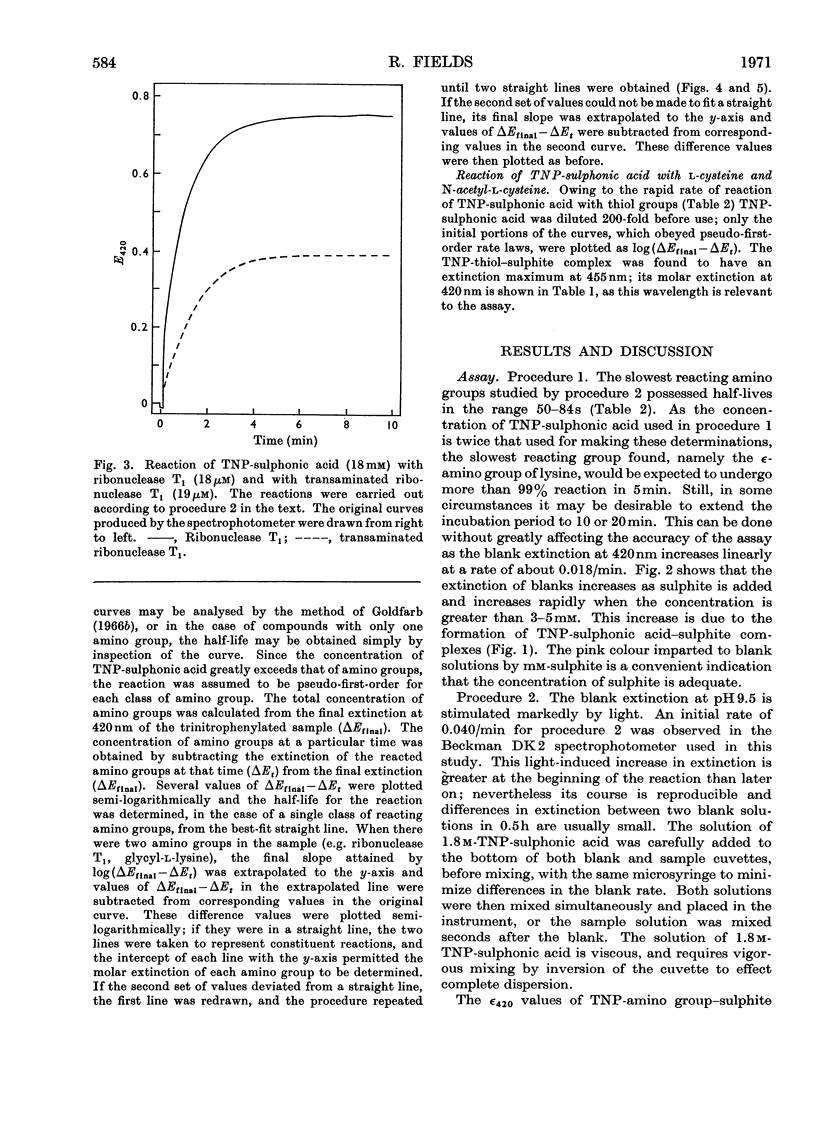
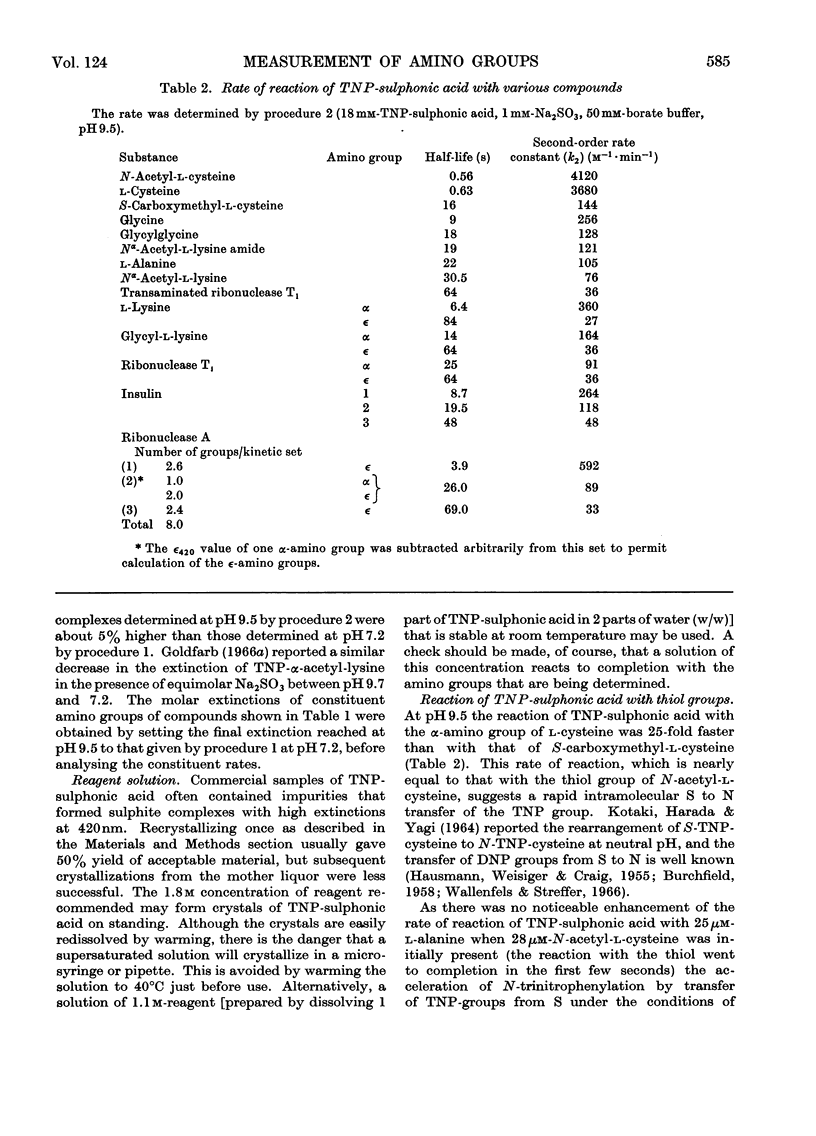


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Perham R. N. The reactivity of thiol groups and the subunit structure of aldolase. Biochem J. 1970 Apr;117(2):291–298. doi: 10.1042/bj1170291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CECIL R., McPHEE J. R. A kinetic study of the reactions on some disulphides with sodium sulphite. Biochem J. 1955 Jul;60(3):496–506. doi: 10.1042/bj0600496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRUZ-COKE E. A colour reaction of vitamin E with vitamin K and cysteine. Nature. 1958 Jan 4;181(4601):49–49. doi: 10.1038/181049a0. [DOI] [PubMed] [Google Scholar]
- Fields R., Dixon H. B., Law G. R., Yui C. Purification of ribonuclease T 1 by diethylaminoethylcellulose chromatography. Biochem J. 1971 Feb;121(4):591–596. doi: 10.1042/bj1210591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields R., Dixon H. B. Micro method for determination of reactive carbonyl groups in proteins and peptides, using 2,4-dinitrophenylhydrazine. Biochem J. 1971 Feb;121(4):587–589. doi: 10.1042/bj1210587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R. B., Radda G. K. The reaction of 2,4,6-trinitrobenzenesulphonic acid with amino acids, Peptides and proteins. Biochem J. 1968 Jul;108(3):383–391. doi: 10.1042/bj1080383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb A. R. A kinetic study of the reactions of amino acids and peptides with trinitrobenzenesulfonic acid. Biochemistry. 1966 Aug;5(8):2570–2574. doi: 10.1021/bi00872a013. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTAKI A., HARADA M., YAGI K. REACTION BETWEEN SULFHYDRYL COMPOUNDS AND 2,4,6-TRINITROBENZENE-1-SULFONIC ACID. J Biochem. 1964 May;55:553–561. [PubMed] [Google Scholar]
- Kartha G., Bello J., Harker D. Tertiary structure of ribonuclease. Nature. 1967 Mar 4;213(5079):862–865. doi: 10.1038/213862a0. [DOI] [PubMed] [Google Scholar]
- SELA M., ANFINSEN C. B. Some spectrophotometric and polarimetric experiments with ribonuclease. Biochim Biophys Acta. 1957 May;24(2):229–235. doi: 10.1016/0006-3002(57)90186-5. [DOI] [PubMed] [Google Scholar]
- Singer S. J. Covalent labeling of active sites. Adv Protein Chem. 1967;22:1–54. doi: 10.1016/s0065-3233(08)60040-6. [DOI] [PubMed] [Google Scholar]


