Abstract
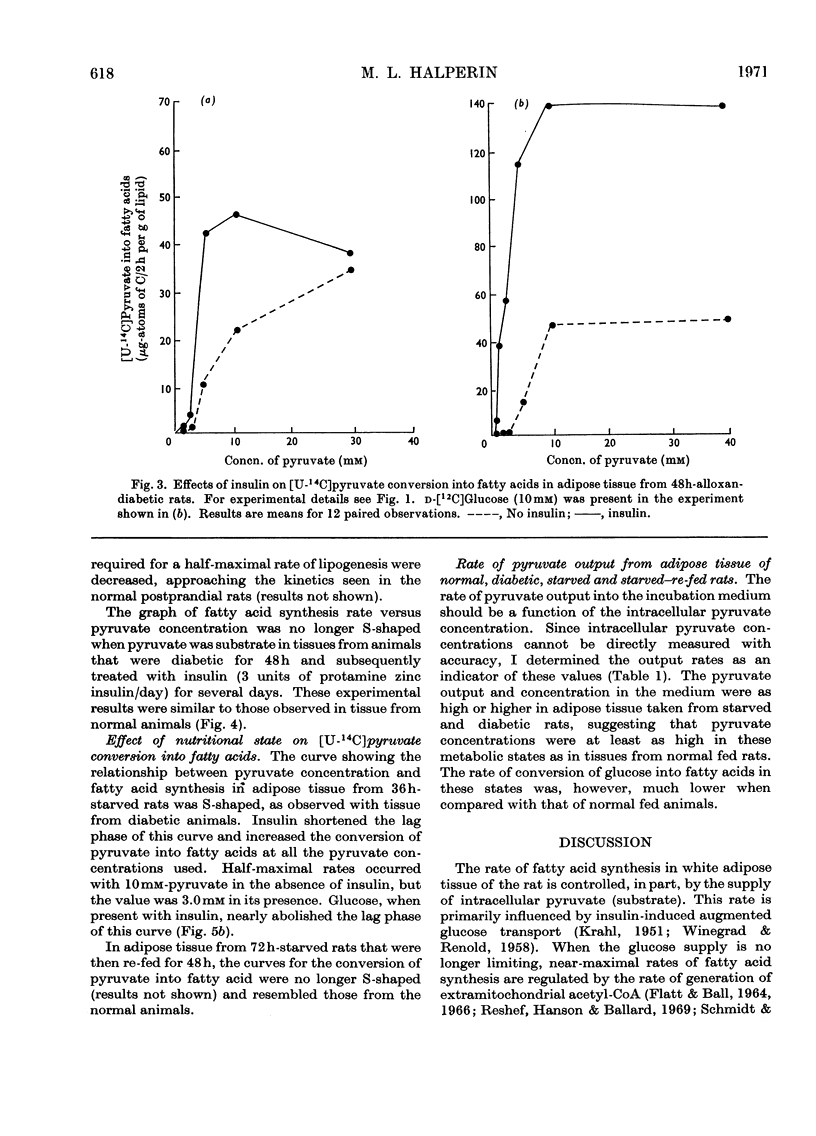
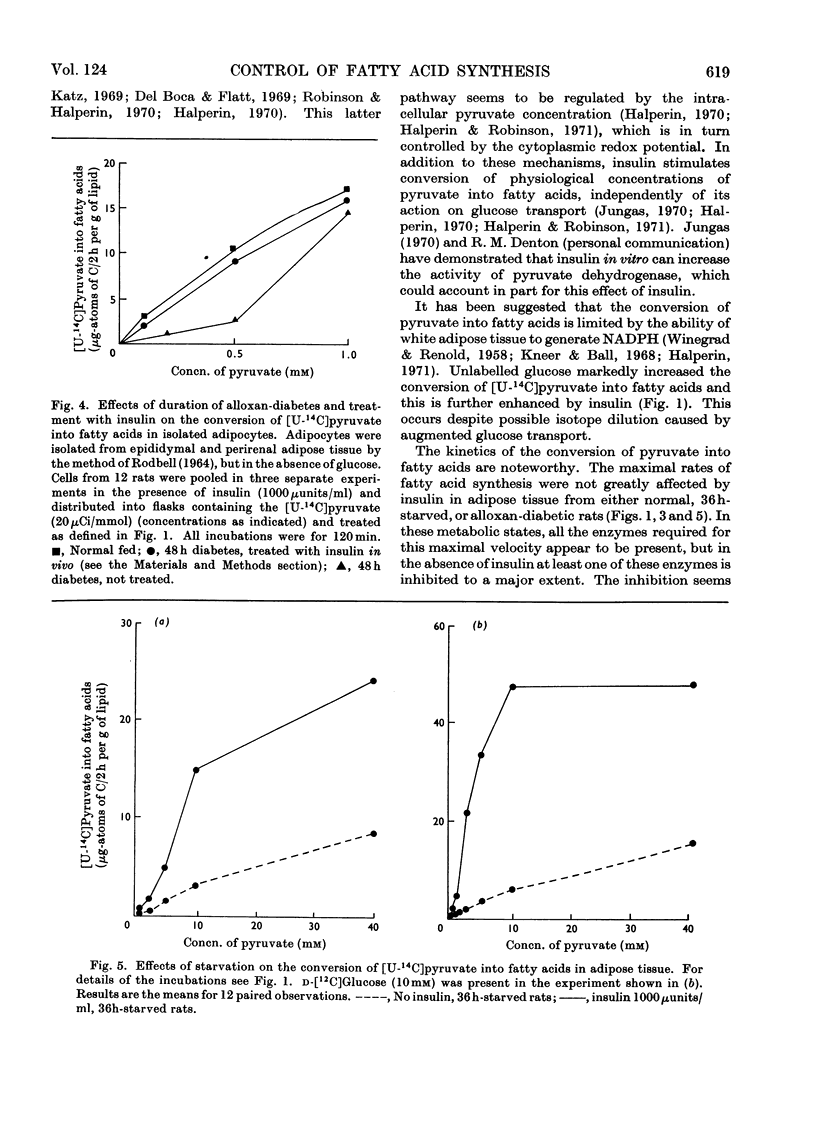
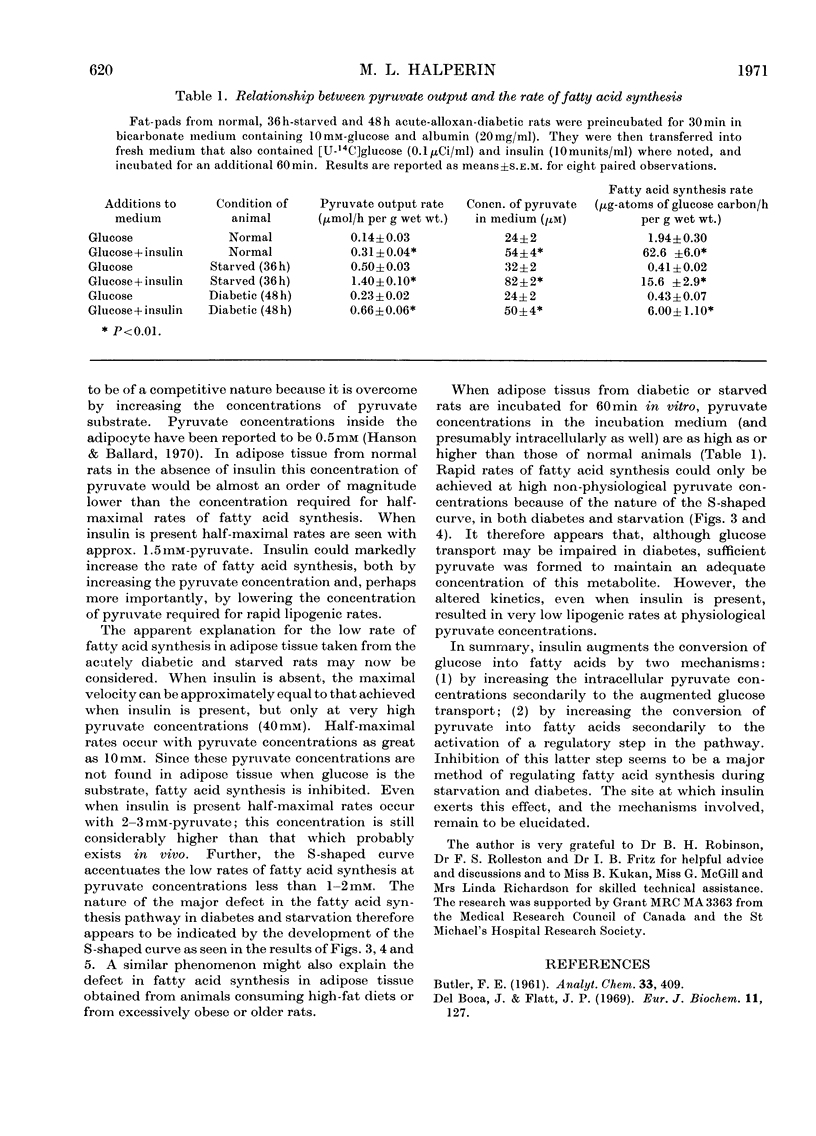
The effect of insulin on the conversion of pyruvate into fatty acids in the presence and in the absence of glucose was studied in epididymal adipose tissue of the rat. 1. In adipose tissue from the normal rat, conversion of pyruvate into fatty acids is directly related to its concentration, the maximal rates occurring with 40mm- and the half-maximal rates with approx. 4mm-pyruvate. Insulin treatment did not greatly influence the maximal rates, but the half-maximal rates were at much lower pyruvate concentrations. This effect of insulin could be seen with physiological concentrations of this hormone (50–100μunits/ml). 2. In adipose tissue from acute-alloxan-diabetic and 36h-starved rats the conversion of pyruvate into fatty acids was almost zero until its concentration exceeded 3mm and then increased markedly as the concentration of pyruvate was increased. The lag phase of this S-shaped curve was decreased but not eliminated when insulin was present. This could account for the very low rates of glucose conversion into fatty acids in these metabolic states. Maximum rates of fatty acid synthesis were similar in the presence and in the absence of insulin, but only when 30–40mm-pyruvate was employed. Re-feeding of the starved rats or insulin treatment of the diabetic rats in vivo for several days restored these patterns to normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Del Boca J., Flatt J. P. Fatty acid synthesis from glucose and acetate and the control of lipogenesis in adipose tissue. Eur J Biochem. 1969 Nov;11(1):127–134. doi: 10.1111/j.1432-1033.1969.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLATT J. P., BALL E. G. STUDIES ON THE METABOLISM OF ADIPOSE TISSUE. XV. AN EVALUATION OF THE MAJOR PATHWAYS OF GLUCOSE CATABOLISM AS INFLUENCED BY INSULIN AND EPINEPHRINE. J Biol Chem. 1964 Mar;239:675–685. [PubMed] [Google Scholar]
- Flatt J. P., Ball E. G. Studies on the metabolism of adipose tissue. XIX. An evaluation of the major pathways of glucose catabolism as influenced by acetate in the presence of insulin. J Biol Chem. 1966 Jun 25;241(12):2862–2869. [PubMed] [Google Scholar]
- Halperin M. L. An additional role for insulin in the control of fatty acid synthesis independent of glucose transport. Can J Biochem. 1970 Nov;48(11):1228–1233. doi: 10.1139/o70-191. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Denton R. M. Regulation of glycolysis and L-glycerol 3-phosphate concentration in rat epididymal adipose tissue in vitro. Role of phosphofructokinase. Biochem J. 1969 Jun;113(1):207–214. doi: 10.1042/bj1130207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin M. L. Fatty acid synthesis from pyruvate in white adipose tissue: the effect of norepinephrine. Can J Biochem. 1971 Jun;49(6):736–741. doi: 10.1139/o71-104. [DOI] [PubMed] [Google Scholar]
- Halperin M. L., Robinson B. H. Mechanism of insulin action on control of fatty acid synthesis independent of glucose transport. Metabolism. 1971 Jan;20(1):78–86. doi: 10.1016/0026-0495(71)90061-8. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Ballard F. J. The concentrations of metabolites of glucose in rat adipose tissue cells in vitro. Biochim Biophys Acta. 1970 Feb 24;201(2):196–204. doi: 10.1016/0304-4165(70)90293-x. [DOI] [PubMed] [Google Scholar]
- Jungas R. L. Effect of insulin on fatty acid ynthesis from pyruvate, lactage, or endogenous sources in adipose tissue: evidence for the hormonal regulation of pyruvate dehydrogenase. Endocrinology. 1970 Jun;86(6):1368–1375. doi: 10.1210/endo-86-6-1368. [DOI] [PubMed] [Google Scholar]
- KRAHL M. E. The effect of insulin and pituitary hormones on glucose uptake in muscle. Ann N Y Acad Sci. 1951 Dec;54(4):649–670. doi: 10.1111/j.1749-6632.1951.tb46620.x. [DOI] [PubMed] [Google Scholar]
- Kneer P., Ball E. G. Studies on the metabolism of adipose tissue. XXI. An evaluation of the major pathways of pyruvate metabolism. J Biol Chem. 1968 Jun 10;243(11):2863–2870. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Reshef L., Hanson R. W., Ballard F. J. Glyceride-glycerol synthesis from pyruvate. Adaptive changes in phosphoenolpyruvate carboxykinase and pyruvate carboxylase in adipose tissue and liver. J Biol Chem. 1969 Apr 25;244(8):1994–2001. [PubMed] [Google Scholar]
- Robinson B. H., Halperin M. L. Transport of reduced nicotinamide-adenine dinucleotide into mitochondria of rat white adipose tissue. Biochem J. 1970 Jan;116(2):229–233. doi: 10.1042/bj1160229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K., Katz J. Metabolism of pyruvate and L-lactate by rat adipose tissue. J Biol Chem. 1969 Apr 25;244(8):2125–2131. [PubMed] [Google Scholar]
- WINEGRAD A. I., RENOLD A. E. Studies on rat adipose tissue in vitro. I. Effects of insulin on the metabolism of glucose, pyruvate, and acetate. J Biol Chem. 1958 Aug;233(2):267–272. [PubMed] [Google Scholar]


