Abstract
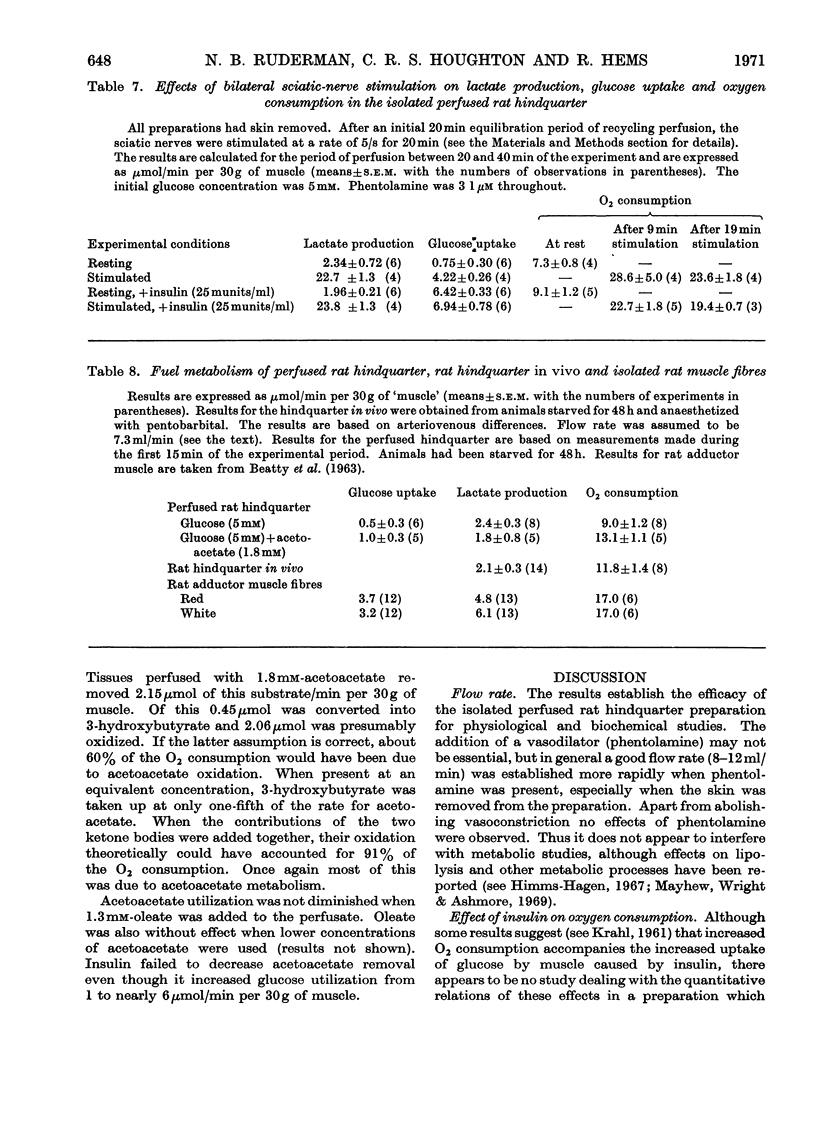
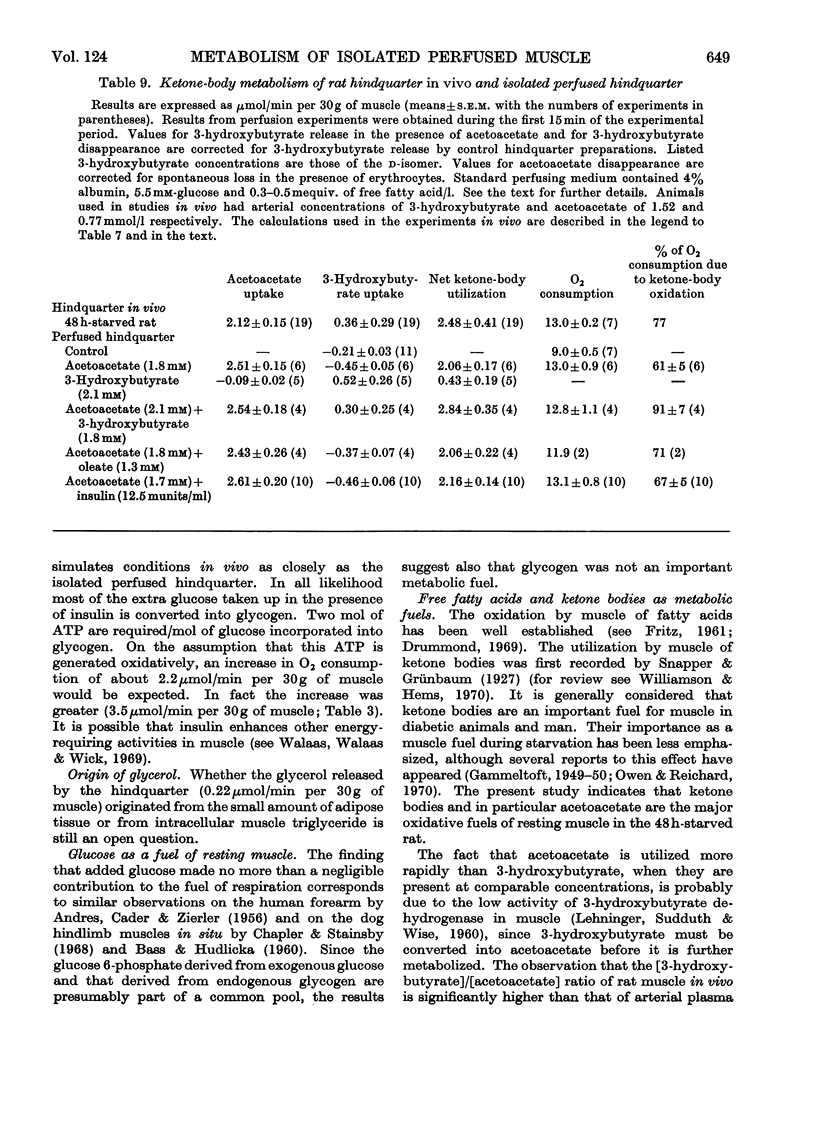
1. The metabolic integrity of a new isolated rat hindquarter preparation was studied. The hindquarter was perfused with a semi-synthetic medium containing aged human erythrocytes. More than 95% of the oxidative metabolism of the preparation was due to muscle, the remainder being due to bone, adipose tissue and, where present, skin. 2. Consumption of O2, glucose utilization, glycerol release and lactate production were similar in the presence and in the absence of the skin, indicating that the latter contributed little to the overall metabolism of the preparation. 3. After 40min of perfusion, tissue concentrations of creatine phosphate, ATP and ADP were similar to those found in muscle taken directly from intact animals. The muscle also appeared normal under the electron microscope. 4. The hindquarter did not lose K+ to the medium during a 30min perfusion. In the presence of insulin it had a net K+ uptake. 5. Insulin caused a sixfold increase in glucose uptake, stimulated O2 consumption by nearly 40% and depressed glycerol release to less than half the control value. 6. Bilateral sciatic-nerve stimulation caused severalfold increases in O2 consumption and lactate production. In the absence of insulin nerve stimulation also enhanced glucose uptake; in the presence of insulin it did not further increase the already high rate of glucose uptake. 7. Rates of lactate production and O2 consumption of the rat hindquarter in vivo and the isolated perfused hindquarter were very similar. 8. Ketone bodies were a major oxidative fuel in vivo of the hindquarter of a rat starved for 2 days. If the acetoacetate and 3-hydroxybutyrate removed by the tissue were completely oxidized, they would have accounted for 77% of the O2 consumption. 9. Acetoacetate accounted for 84% of the ketone bodies removed by the hindquarter in vivo even though its arterial concentration was half that of 3-hydroxybutyrate. 10. Similar rates of acetoacetate and 3-hydroxybutyrate utilization were observed in the perfused hindquarter. 11. Acetoacetate utilization by the perfused hindquarter was not diminished by the addition of either oleate or insulin to the perfusate. 12. Oxidation of glucose to CO2 accounted for less than 4% of the O2 consumed by the perfused hindquarter in both the presence and the absence of insulin. 13. The results indicate that the isolated perfused hindquarter is a useful tool for studying muscle metabolism. They also suggest that ketone bodies, if present in sufficient concentration, are the preferred oxidative fuel of resting muscle.
Full text
PDF

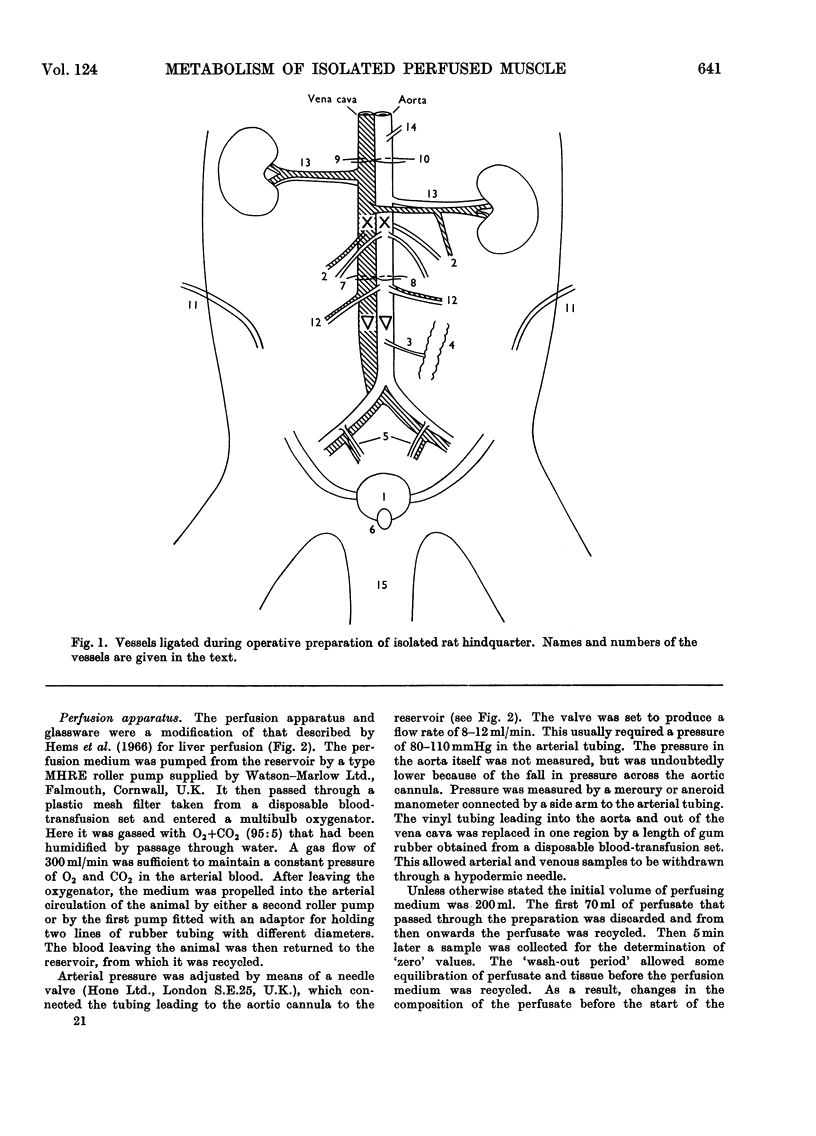
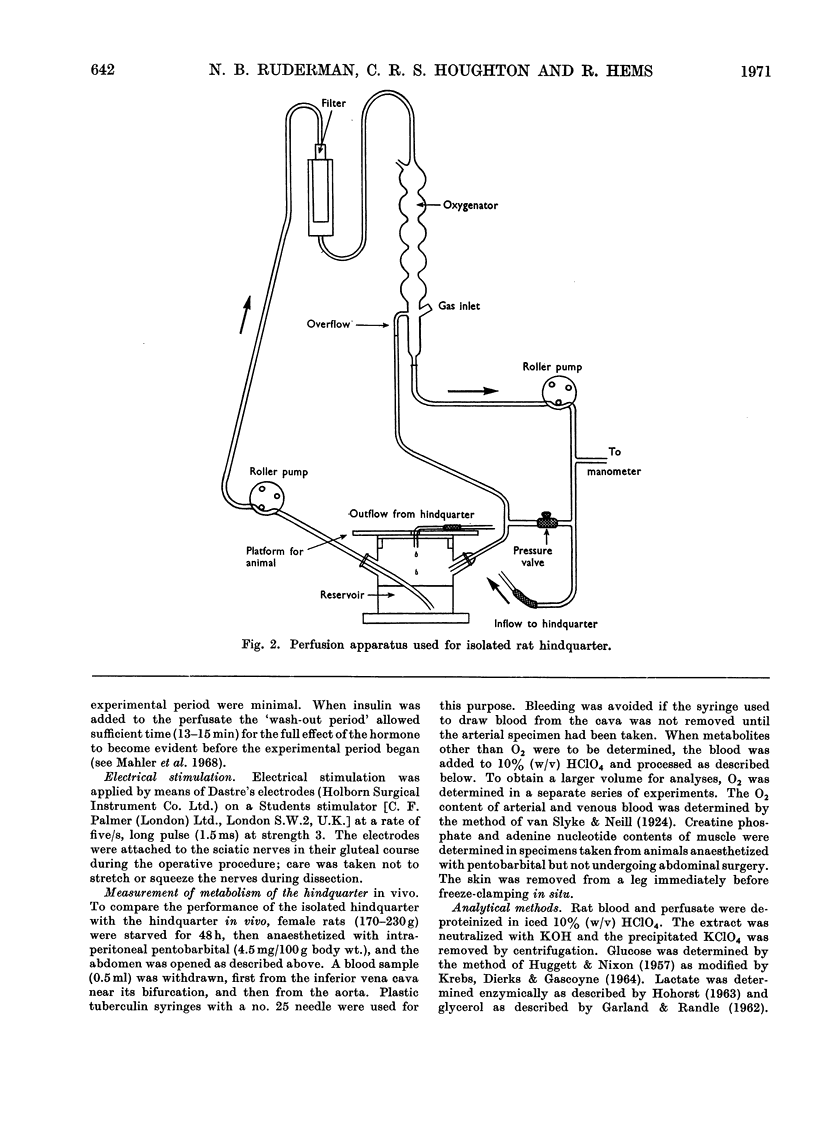



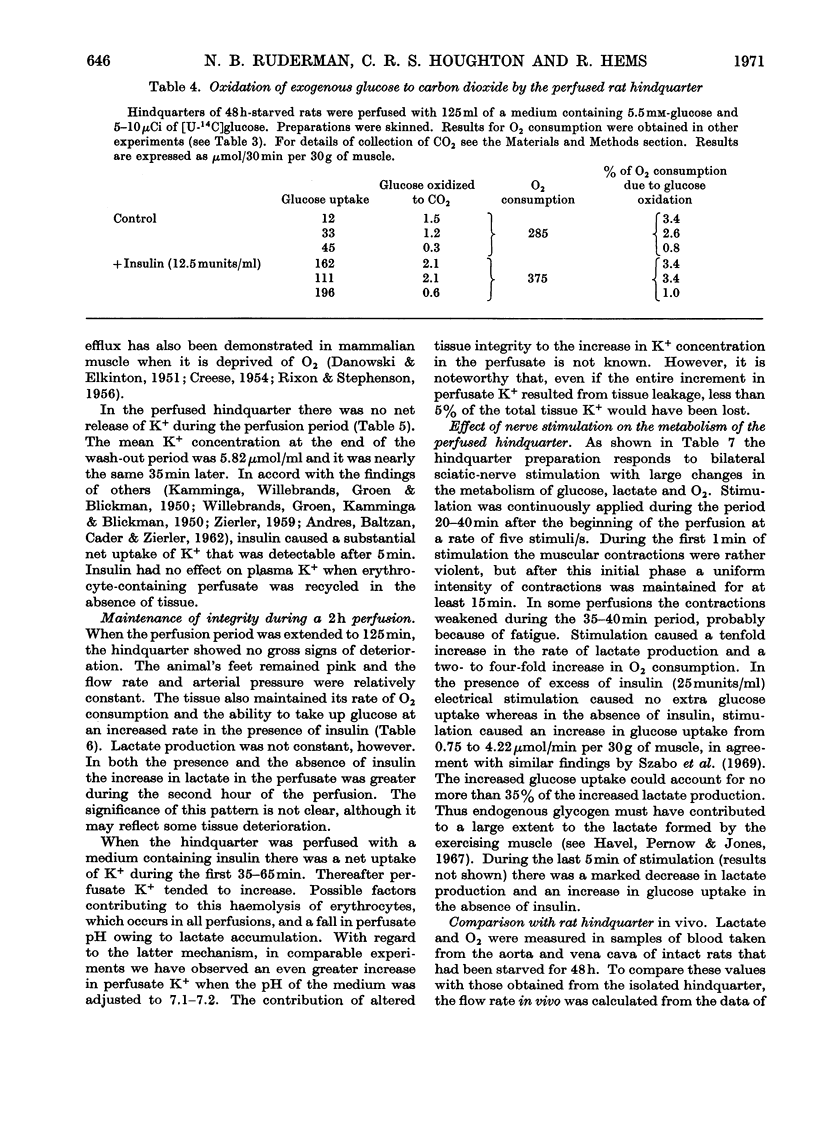
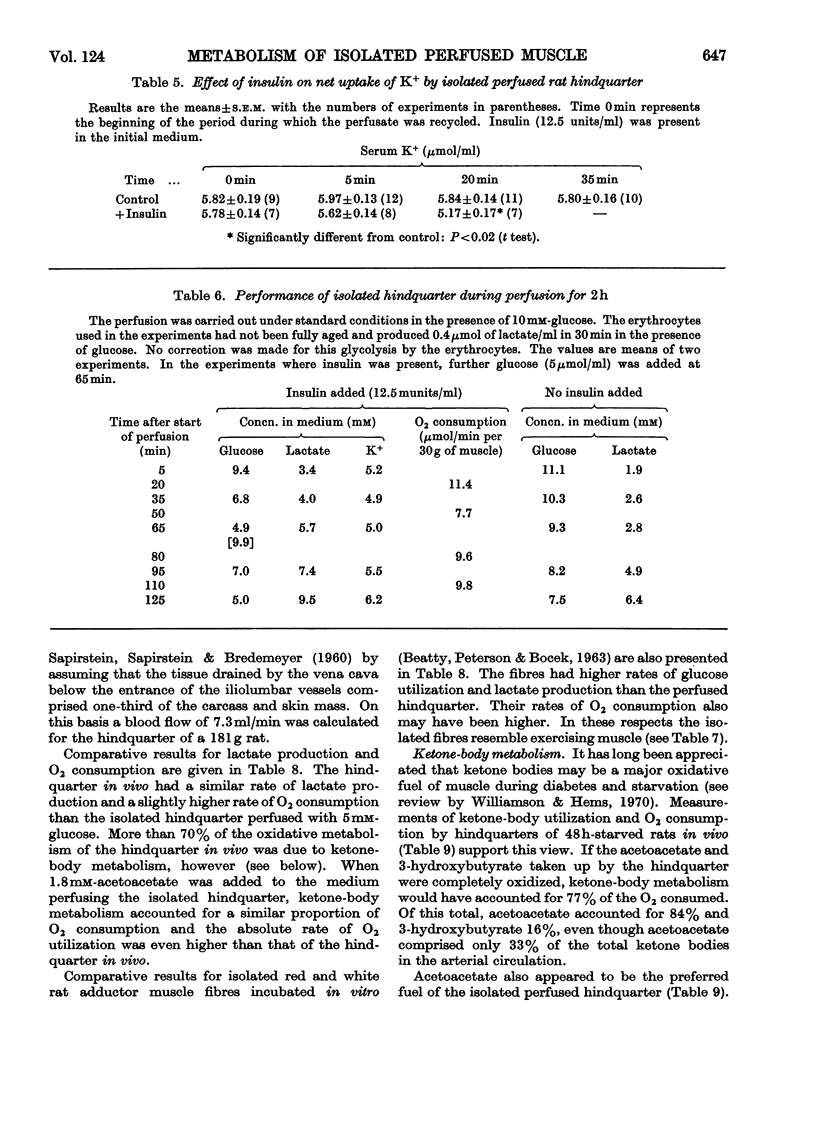


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRES R., BALTZAN M. A., CADER G., ZIERLER K. L. Effect of insulin on carbohydrate metabolism and on potassium in the forearm of man. J Clin Invest. 1962 Jan;41:108–115. doi: 10.1172/JCI104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRES R., CADER G., ZIERLER K. L. The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state; measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest. 1956 Jun;35(6):671–682. doi: 10.1172/JCI103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATTY C. H., PETERSON R. D., BOCEK R. M. Metabolism of red and white muscle fiber groups. Am J Physiol. 1963 May;204:939–942. doi: 10.1152/ajplegacy.1963.204.5.939. [DOI] [PubMed] [Google Scholar]
- Burn J. H., Dale H. H. The vaso-dilator action of histamine, and its physiological significance. J Physiol. 1926 Apr 23;61(2):185–214. doi: 10.1113/jphysiol.1926.sp002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COPENHAVER W. M., MOORE D. H., RUSKA H. Electron microscopic and histochemical observations of muscle degeneration after tourniquet. J Biophys Biochem Cytol. 1956 Nov 25;2(6):755–764. doi: 10.1083/jcb.2.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREESE R. Measurement of cation fluxes in rat diaphragm. Proc R Soc Lond B Biol Sci. 1954 Sep 27;142(909):497–513. doi: 10.1098/rspb.1954.0039. [DOI] [PubMed] [Google Scholar]
- Chapler C. K., Stainsby W. N. Carbohydrate metabolism in contracting dog skeletal muscle in situ. Am J Physiol. 1968 Nov;215(5):995–1004. doi: 10.1152/ajplegacy.1968.215.5.995. [DOI] [PubMed] [Google Scholar]
- DANOWSKI T. S., ELKINTON J. R. Exchanges of potassium related to organs and systems. Pharmacol Rev. 1951 Mar;3(1):42–58. [PubMed] [Google Scholar]
- Drummond G. I. Muscle metabolism. Fortschr Zool. 1967;18(3):359–429. [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J. A rapid enzymatic assay for glycerol. Nature. 1962 Dec 8;196:987–988. doi: 10.1038/196987a0. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J. Sympathetic regulation of metabolism. Pharmacol Rev. 1967 Sep;19(3):367–461. [PubMed] [Google Scholar]
- Houghton C. R., Ruderman N. B. Acetoacetate as a fuel for perfused rat skeletal muscle. Biochem J. 1971 Jan;121(1):15P–16P. doi: 10.1042/bj1210015pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Dierks C., Gascoyne T. Carbohydrate synthesis from lactate in pigeon-liver homogenate. Biochem J. 1964 Oct;93(1):112–121. doi: 10.1042/bj0930112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- LEHNINGER A. L., SUDDUTH H. C., WISE J. B. D-beta-Hydroxybutyric dehydrogenase of muitochondria. J Biol Chem. 1960 Aug;235:2450–2455. [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Mayhew D. A., Wright P. H., Ashmore J. Regulation of insulin secretion. Pharmacol Rev. 1969 Sep;21(3):183–212. [PubMed] [Google Scholar]
- OLSON R. E. Effect of pyruvate and acetoacetate on the metabolism of fatty acids by the perfused rat heart. Nature. 1962 Aug 11;195:597–599. doi: 10.1038/195597b0. [DOI] [PubMed] [Google Scholar]
- RIXON R. H., STEVENSON J. A. The water and electrolyte metabolism of rat diaphragm in vitro. Can J Biochem Physiol. 1956 Sep;34(5):1069–1083. [PubMed] [Google Scholar]
- Ruderman N. B., Herrera M. G. Glucose regulation of hepatic gluconeogenesis. Am J Physiol. 1968 Jun;214(6):1346–1351. doi: 10.1152/ajplegacy.1968.214.6.1346. [DOI] [PubMed] [Google Scholar]
- Ruderman N. B., Toews C. J., Lowy C., Vreeland I., Shafrir E. Inhibition of hepatic gluconeogenesis and fatty acid oxidation by pent-4-enoic acid. Am J Physiol. 1970 Jul;219(1):51–57. doi: 10.1152/ajplegacy.1970.219.1.51. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., MILLER F., BARRNETT R. J. ALDEHYDE FIXATION FOR MORPHOLOGICAL AND ENZYME HISTOCHEMICAL STUDIES WITH THE ELECTRON MICROSCOPE. J Histochem Cytochem. 1964 Feb;12:57–71. doi: 10.1177/12.2.57. [DOI] [PubMed] [Google Scholar]
- SAPIRSTEIN L. A., SAPIRSTEIN E. H., BREDEMEYER A. Effect of hemorrhage on the cardiac output and its distribution in the rat. Circ Res. 1960 Jan;8:135–148. doi: 10.1161/01.res.8.1.135. [DOI] [PubMed] [Google Scholar]
- STENGER R. J., SPIRO D., SCULLY R. E., SHANNON J. M. Ultrastructural and physiologic alterations in ischemic skeletal muscle. Am J Pathol. 1962 Jan;40:1–20. [PMC free article] [PubMed] [Google Scholar]
- Szabo A. J., Mahler R. J., Szabo O. Glucose uptake by the isolated perfused rat hind limb during rest and exercise. Horm Metab Res. 1969 Jul;1(4):156–161. doi: 10.1055/s-0028-1095147. [DOI] [PubMed] [Google Scholar]
- WILLEBRANDS A. F., GROEN J., KAMMINGA C. E., BLICKMAN J. R. Quantitative aspects of the action of insulin on the glucose and potassium metabolism of the isolated rat diaphragm. Science. 1950 Sep 8;112(2906):277–278. doi: 10.1126/science.112.2906.277. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Walaas O., Walaas E., Wick A. N. The stimulatory effect by insulin on the incorporation of 32P radioactive inorganic phosphate into intracellular inorganic phosphate, adenine nucleotides and guanine nucleotides of the intact isolated rat diaphragm. Diabetologia. 1969 Apr;5(2):79–87. doi: 10.1007/BF01212001. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Hyperpolarization of muscle by insulin in a glucose-free environment. Am J Physiol. 1959 Sep;197:524–526. doi: 10.1152/ajplegacy.1959.197.3.524. [DOI] [PubMed] [Google Scholar]


