Abstract
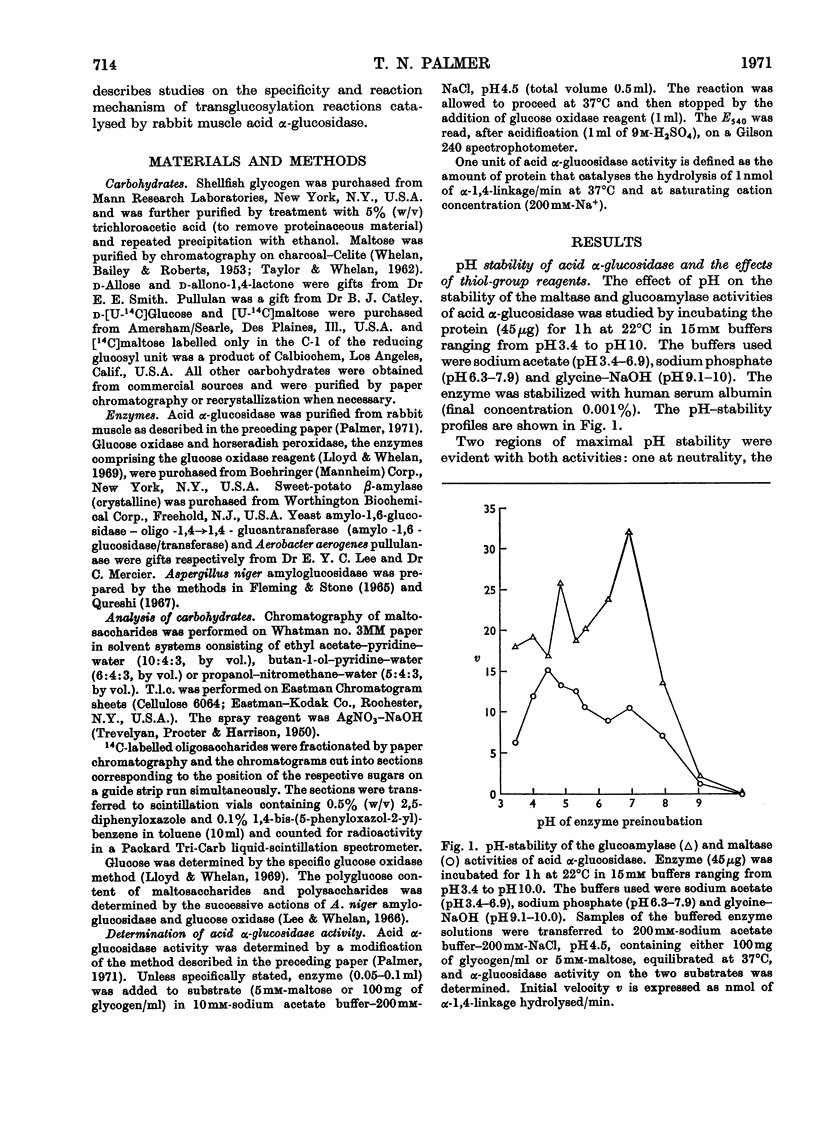
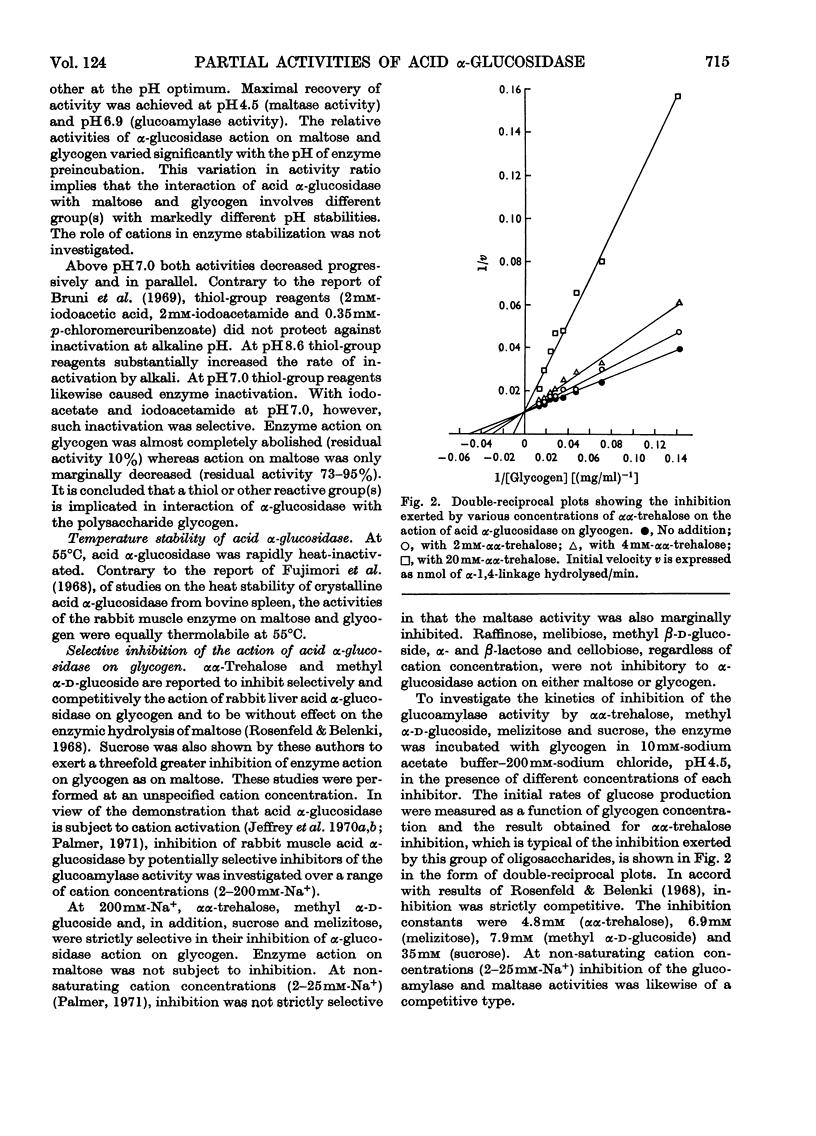
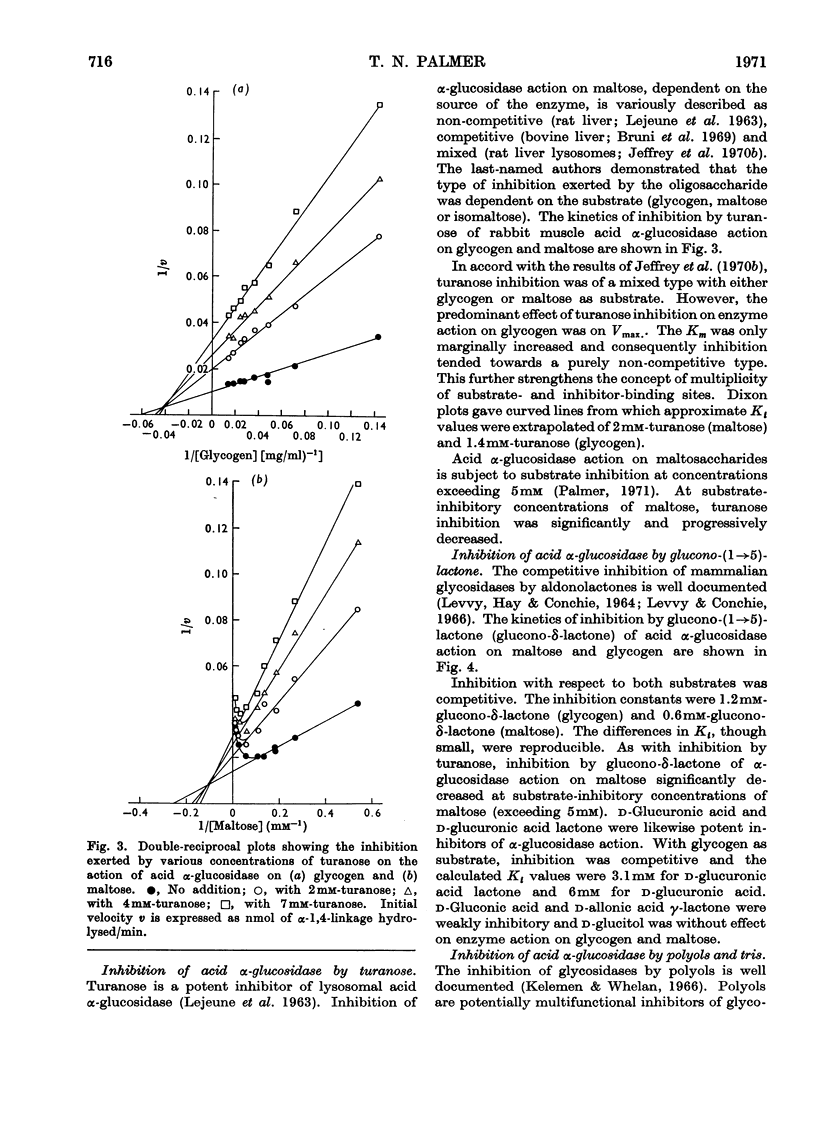
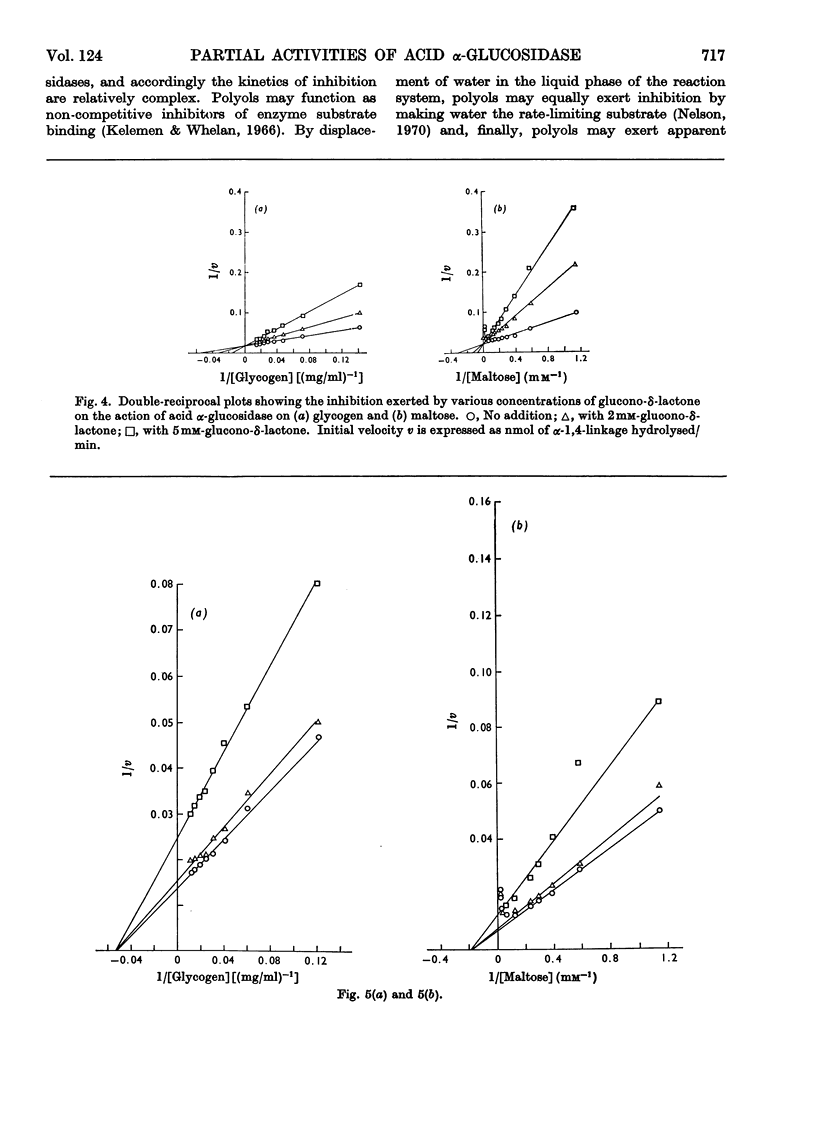
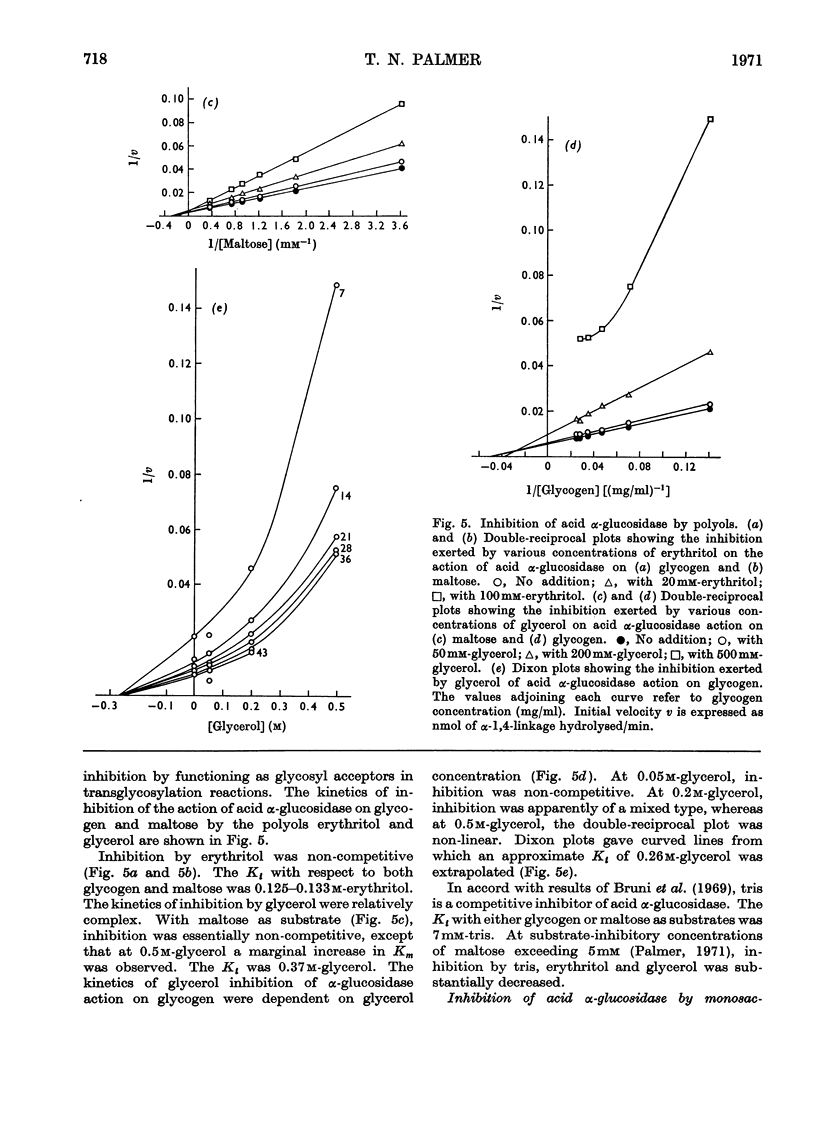
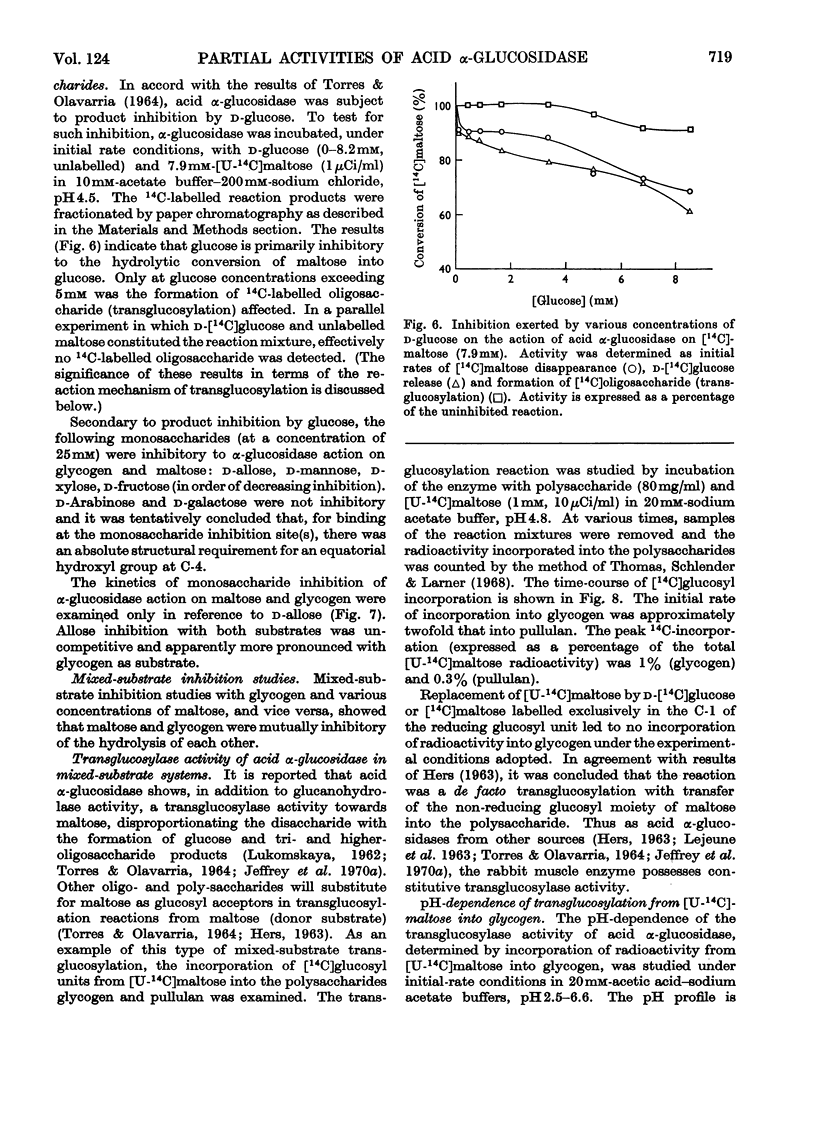
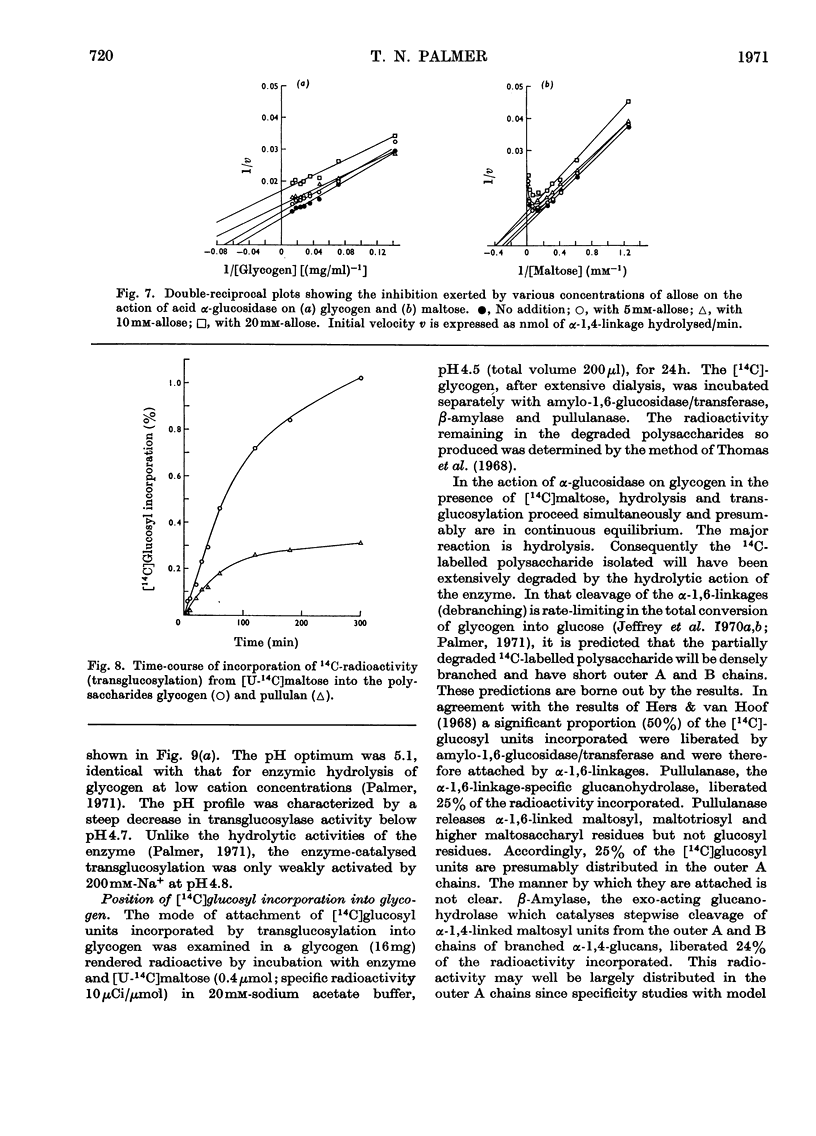
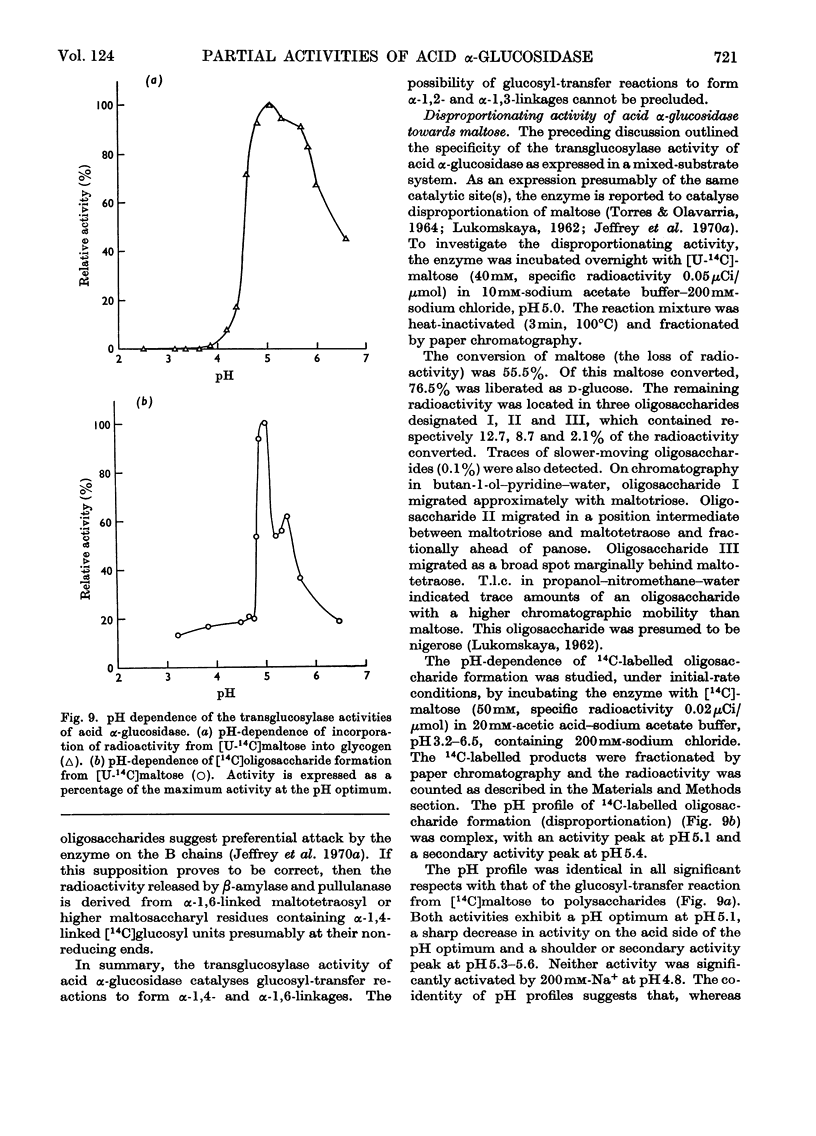
1. The maltase and glucoamylase activities of acid α-glucosidase purified from rabbit muscle exhibited marked differences in certain physicochemical properties. These included pH stability, inactivation by thiol-group reagents, inhibition by αα-trehalose, methyl α-d-glucoside, sucrose, turanose, polyols, glucono-δ-lactone and monosaccharides, pH optimum and the kinetics and pH-dependence of cation activation. 2. The results are interpreted in terms of the existence of at least two specific substrate-binding sites or sub-sites. One site is specific for the binding of maltose and probably other oligosaccharides. The second site binds polysaccharides such as glycogen. 3. The sites appear to be in close proximity, since glycogen and maltose are mutually inhibitory substrates and interact directly in transglucosylation reactions. 4. Acid α-glucosidase exhibited intrinsic transglucosylase activity. The enzyme catalysed glucosyl-transfer reactions from [14C]maltose (donor substrate) to polysaccharides (glycogen and pullulan) and to maltose itself (disproportionation). The pH optimum was 5.1, with a shoulder or secondary activity peak at pH5.4. The glucose transferred to glycogen was attached by α-1,4- and α-1,6-linkages. Three major oligosaccharide products of enzyme action on maltose (disproportionation) were detected. 5. The kinetics of enzyme action on [14C]maltose showed that the rate of transglucosylation increased in a sigmoidal fashion as a function of substrate concentration, approximately in parallel with a decrease in the rate of glucose release. 6. The results are interpreted to imply competitive interaction at a specific binding site between maltose and water as glucosyl acceptors. 7. The results are discussed in terms of the possible existence of multiple subgroups of glycogen-storage disease type II.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni C. B., Auricchio F., Covelli I. Acid alpha-D-glucosidase glucohydrolase from cattle liver. J Biol Chem. 1969 Sep 10;244(17):4735–4742. [PubMed] [Google Scholar]
- Chipman D. M., Sharon N. Mechanism of lysozyme action. Science. 1969 Aug 1;165(3892):454–465. doi: 10.1126/science.165.3892.454. [DOI] [PubMed] [Google Scholar]
- Fujimori K., Hizukuri S., Nikuni Z. Studies on acid alpha-1,4-glucosidase from bovine spleen. Biochem Biophys Res Commun. 1968 Sep 6;32(5):811–816. doi: 10.1016/0006-291x(68)90313-6. [DOI] [PubMed] [Google Scholar]
- HERS H. G. alpha-Glucosidase deficiency in generalized glycogenstorage disease (Pompe's disease). Biochem J. 1963 Jan;86:11–16. doi: 10.1042/bj0860011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. I. Purification and properties of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1403–1415. doi: 10.1021/bi00808a015. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. II. Kinetics of action of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1416–1422. doi: 10.1021/bi00808a016. [DOI] [PubMed] [Google Scholar]
- Kelemen M. V., Whelan W. J. Inhibition of glucosidases and galactosidases by polyols. Arch Biochem Biophys. 1966 Nov;117(2):423–428. doi: 10.1016/0003-9861(66)90431-0. [DOI] [PubMed] [Google Scholar]
- LUKOMSKAYA I. S. The synthesis of oligosaccharides with different types of linkage in animal tissues. Enzymologia. 1962 Aug 15;24:327–337. [PubMed] [Google Scholar]
- Lee E. Y., Whelan W. J. Enzymic methods for the microdetermination of glycogen and amylopectin, and their unit-chain lengths. Arch Biochem Biophys. 1966 Sep 26;116(1):162–167. doi: 10.1016/0003-9861(66)90024-5. [DOI] [PubMed] [Google Scholar]
- Levvy G. A., Hay A. J., Conchie J. Inhibition of glycosidases by aldonolactones of corresponding configuration. 4. Inhibitors of mannosidase and glucosidase. Biochem J. 1964 May;91(2):378–384. doi: 10.1042/bj0910378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Nelson T. E. The hydrolytic mechanism of an exo-beta-(1--3)-D-glucanase. J Biol Chem. 1970 Feb 25;245(4):869–872. [PubMed] [Google Scholar]
- Palmer T. N. The substrate specificity of acid -glucosidase from rabbit muscle. Biochem J. 1971 Oct;124(4):701–711. doi: 10.1042/bj1240701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld E. L., Belenki D. M. La dégradation du glycogène et du maltose a l'aide de la gamma-amylase de foie de lapin en présence de sucres divers et de leurs dérivés. Bull Soc Chim Biol (Paris) 1968 Nov 5;50(7):1305–1312. [PubMed] [Google Scholar]
- TORRES H. N., OLAVARRIA J. M. LIVER ALPHA-GLUCOSIDASES. J Biol Chem. 1964 Aug;239:2427–2434. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]


