Abstract
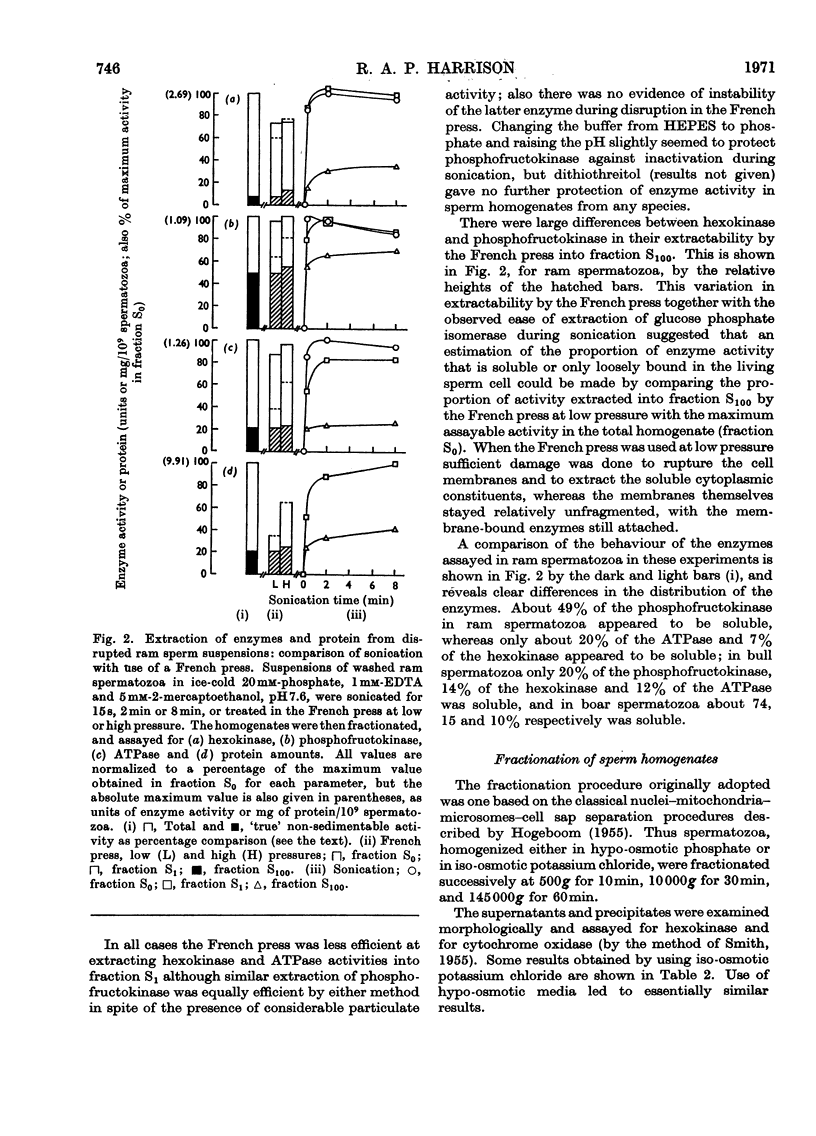
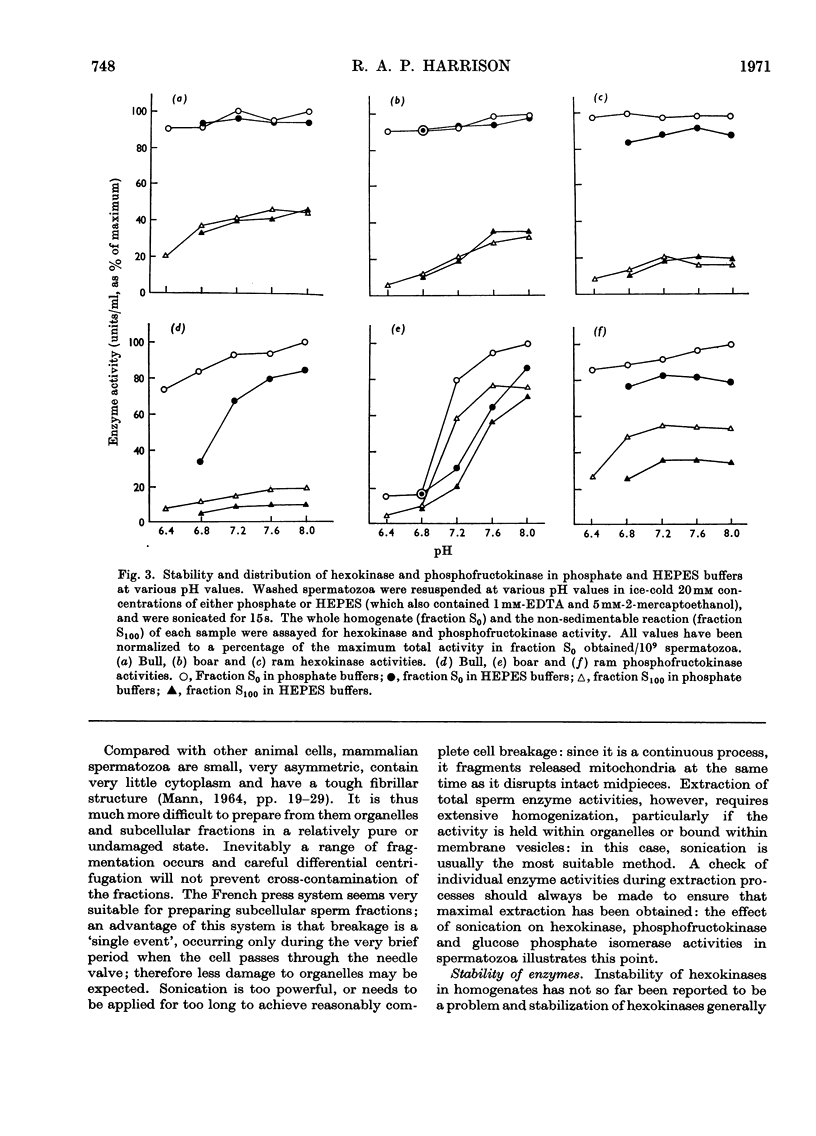
1. Methods of homogenizing suspensions of washed mammalian spermatozoa were studied. The most useful methods were those using sonication and those using a French press. 2. Hexokinase, phosphofructokinase, glucose phosphate isomerase and adenosine triphosphatase activities in ram, bull and boar spermatozoa were investigated by using these two homogenization methods. Glucose phosphate isomerase, representative of soluble cytoplasmic material, was very readily extracted and remained entirely in the supernatant after centrifugation at 145000g for 60min. In contrast, the other three activities were less easily extracted and were sedimented in various proportions under the described conditions of centrifugation. 3. Attempts to obtain subcellular fractions from sperm homogenates by `classical' methods failed, owing apparently to the inhomogeneity of subcellular particles in the homogenates. It is concluded that, after removal of sperm heads, the only meaningful fractionation is a separation of spermatozoal material which sediments at 145000g during 60min from that which does not. 4. The stabilities of hexokinase and phosphofructokinase activities in bull, boar and ram sperm homogenates were investigated. Hexokinases showed very little dependence on the various environments tested, whereas the optimum conditions for phosphofructokinase stability were: a minimum of sonication, the presence of phosphate ions and of a thiol-group protectant, and a pH7.5. Activities of hexokinase, phosphofructokinase and glucose phosphate isomerase per sperm cell were compared with published data on rates of fructolysis by spermatozoa; the potential catalytic activities were shown to be considerably in excess of these rates. However, phosphofructokinase may be the rate-limiting enzyme of glycolysis in vivo in bull and ram spermatozoa.
Full text
PDF
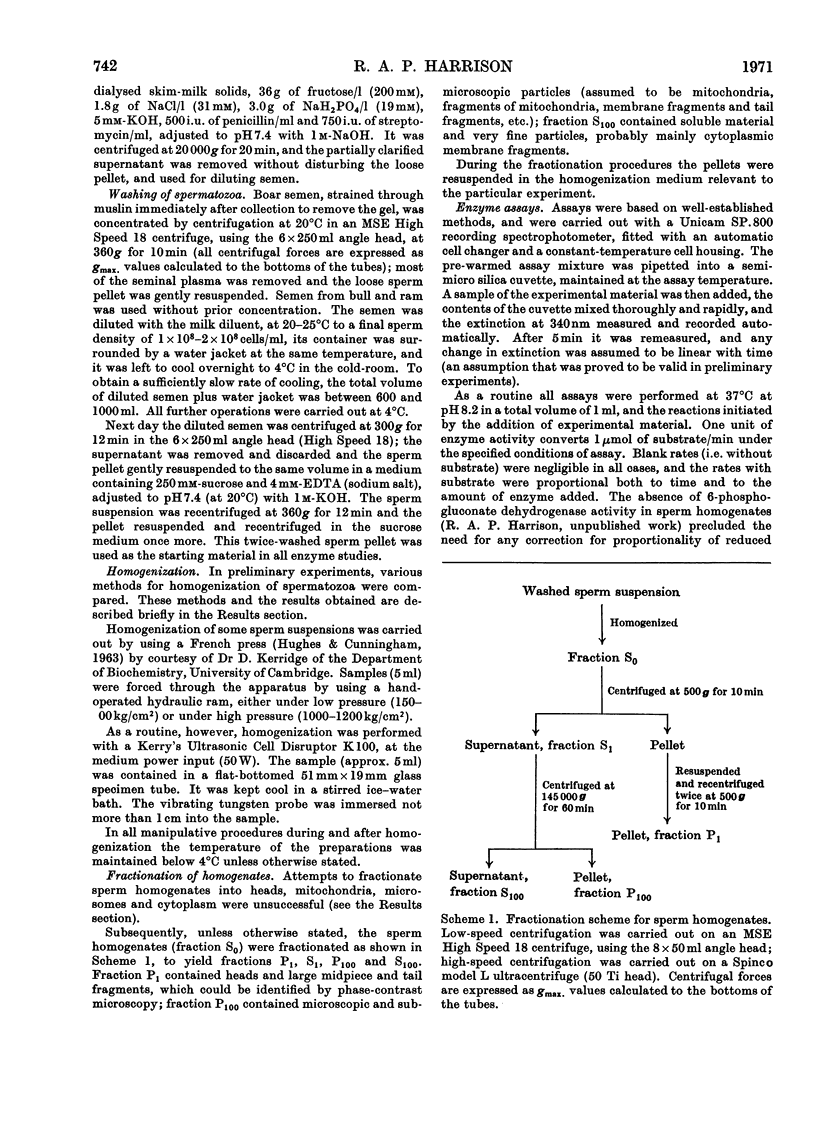

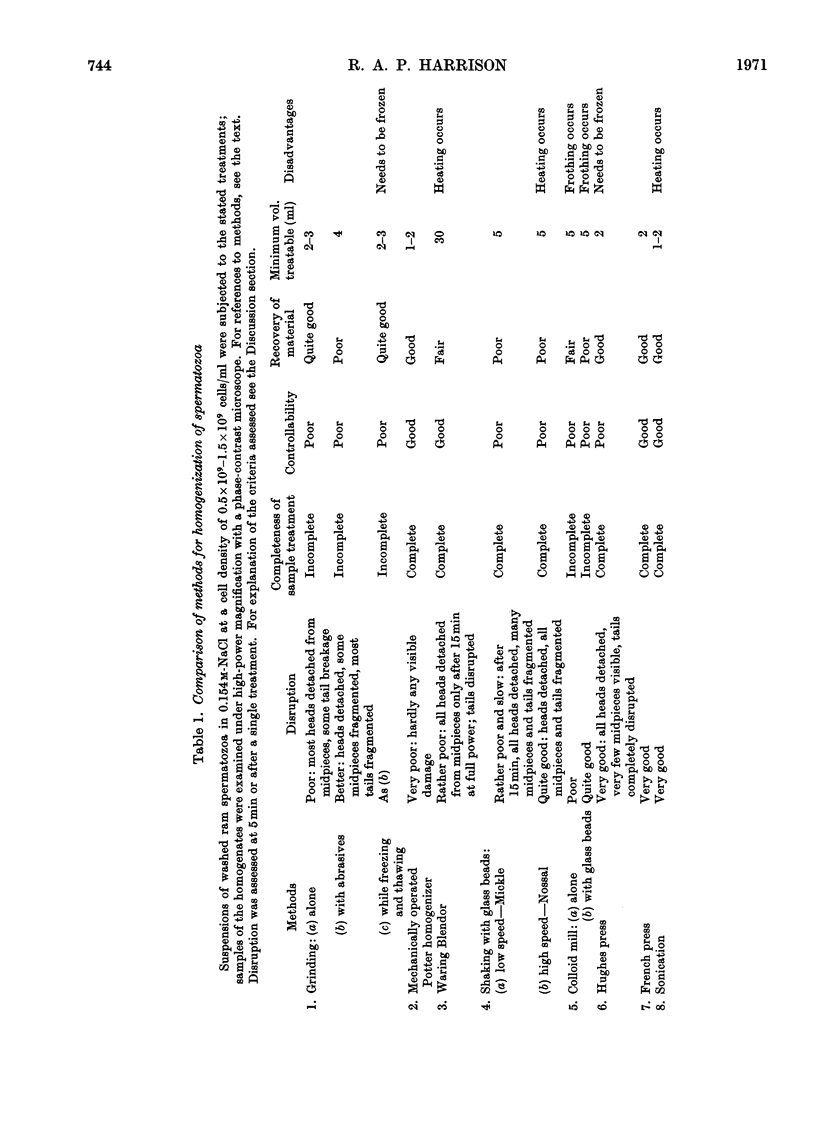
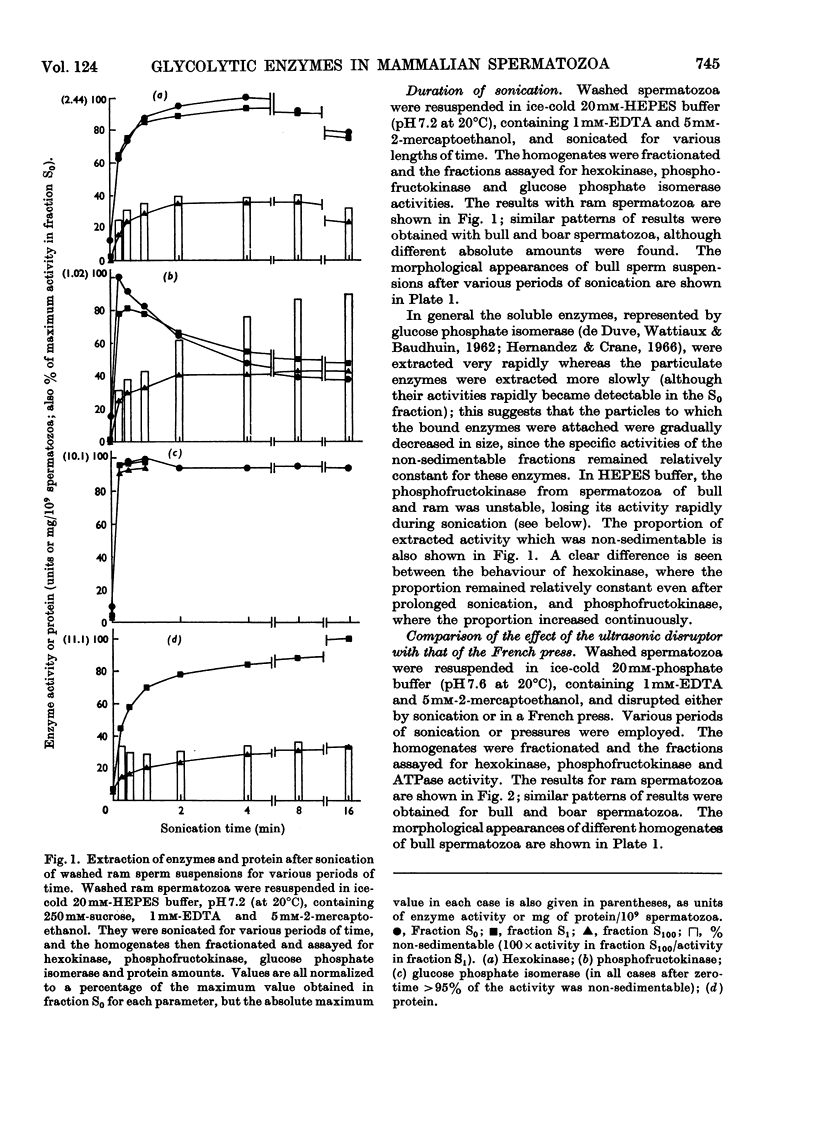



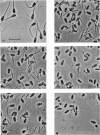
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlfors C. E., Mansour T. E. Studies on heart phosphofructokinase. Desensitization of the enzyme to adenosine triphosphate inhibition. J Biol Chem. 1969 Mar 10;244(5):1247–1251. [PubMed] [Google Scholar]
- Avigad G. Inhibition of glucose 6-phosphate dehydrogenase by adenosine 5'-triphosphate. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1543–1547. doi: 10.1073/pnas.56.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell E. B. Iso-antibody formation against rabbit spermatozoa and its effect on fertility. J Reprod Fertil. 1969 Dec;20(3):519–522. doi: 10.1530/jrf.0.0200519. [DOI] [PubMed] [Google Scholar]
- Brock D. J. Purification and properties of sheep liver phosphofructokinase. Biochem J. 1969 Jun;113(2):235–242. doi: 10.1042/bj1130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen J., Ovlisen B. Lactate dehydrogenase isoenzymes of human semen. Biochem J. 1965 Nov;97(2):513–517. doi: 10.1042/bj0970513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Hernandez A., Crane R. K. Association of heart hexokinase with subcellular structure. Arch Biochem Biophys. 1966 Jan;113(1):223–229. doi: 10.1016/0003-9861(66)90176-7. [DOI] [PubMed] [Google Scholar]
- Hoskins D. D., Stephens D. T. Regulatory properties of primate sperm phosphofructokinase. Biochim Biophys Acta. 1969 Nov 4;191(2):292–302. doi: 10.1016/0005-2744(69)90248-4. [DOI] [PubMed] [Google Scholar]
- Kemp R. G., Forest P. B. Reactivity of the sulfhydryl groups of muscle phosphofructokinase. Biochemistry. 1968 Jul;7(7):2596–2602. doi: 10.1021/bi00847a022. [DOI] [PubMed] [Google Scholar]
- LING K. H., MARCUS F., LARDY H. A. PURIFICATION AND SOME PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHOFRUCTOKINASE. J Biol Chem. 1965 May;240:1893–1899. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Layzer R. B., Rowland L. P., Bank W. J. Physical and kinetic properties of human phosphofructokinase from skeletal muscle and erythrocytes. J Biol Chem. 1969 Jul 25;244(14):3823–3831. [PubMed] [Google Scholar]
- Linford E. The hexokinase of boar spermatozoa. Biochem J. 1968 Dec;110(4):45P–46P. doi: 10.1042/bj1100045pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSOUR T. E. STUDIES ON HEART PHOSPHOFRUCTOKINASE. ACTIVE AND INACTIVE FORMS OF THE ENZYME. J Biol Chem. 1965 May;240:2165–2172. [PubMed] [Google Scholar]
- MOHRI H., MOHRI T., ERNSTER L. ISOLATI N AND ENZYMIC PROPERTIES OF THE MIDPIECE OF BULL SPERMATOZOA. Exp Cell Res. 1965 May;38:217–246. doi: 10.1016/0014-4827(65)90400-3. [DOI] [PubMed] [Google Scholar]
- Mansour T. E., Ahlfors C. E. Studies on heart phosphofructokinase. Some kinetic and physical properties of the crystalline enzyme. J Biol Chem. 1968 May 25;243(10):2523–2533. [PubMed] [Google Scholar]
- NELSON L. Enzyme distribution in fragmented bull spermatozoa. II. Succinic dehydrogenase and cytochrome oxidase. Biochim Biophys Acta. 1955 Apr;16(4):494–501. doi: 10.1016/0006-3002(55)90269-9. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- Rikmenspoel R., Caputo R. The Michaelis-Menten constant for fructose and for glucose of hexokinase in bull spermatozoa. J Reprod Fertil. 1966 Dec;12(3):437–444. doi: 10.1530/jrf.0.0120437. [DOI] [PubMed] [Google Scholar]
- Rose I. A., Warms J. V. Mitochondrial hexokinase. Release, rebinding, and location. J Biol Chem. 1967 Apr 10;242(7):1635–1645. [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. The inhibition of brain hexokinase by adenosinediphosphate and sulfhydryl reagents. J Biol Chem. 1954 Feb;206(2):925–936. [PubMed] [Google Scholar]
- Spydevold O., Borrebaek B. Association of epididymal adipose tissue hexokinase with subcellular structure. Biochim Biophys Acta. 1968 Oct 8;167(2):291–301. doi: 10.1016/0005-2744(68)90208-8. [DOI] [PubMed] [Google Scholar]
- Thompson M. F., Bachelard H. S. Cerebral-cortex hexokinase. Comparison of properties of solubilized mitochondrial and cytoplasmic activities. Biochem J. 1970 Jun;118(1):25–34. doi: 10.1042/bj1180025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda K., Racker E. Regulatory mechanisms in carbohydrate metabolism. VII. Hexokinase and phosphofructokinase. J Biol Chem. 1965 Dec;240(12):4682–4688. [PubMed] [Google Scholar]
- Wakid N., Mansour T. E. Factors influencing the stability of heart phosphofructokinase. Mol Pharmacol. 1965 Jul;1(1):53–59. [PubMed] [Google Scholar]