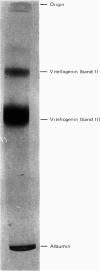
Abstract
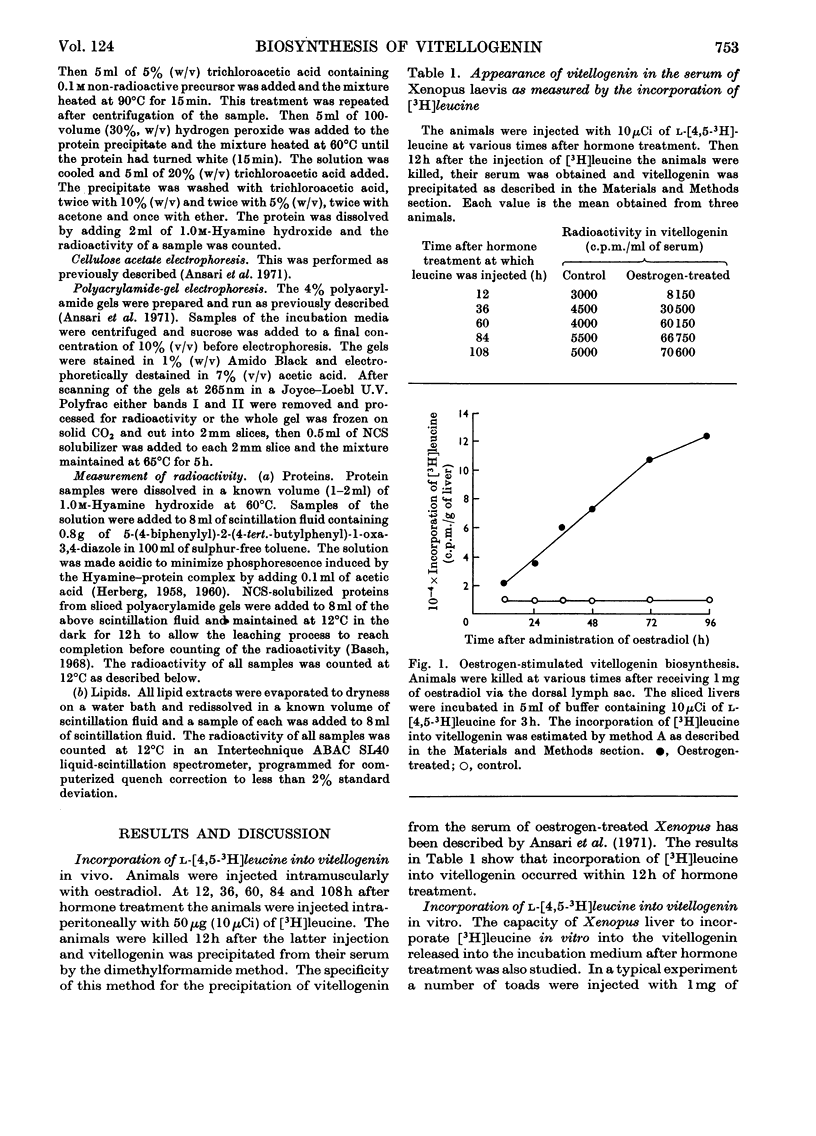
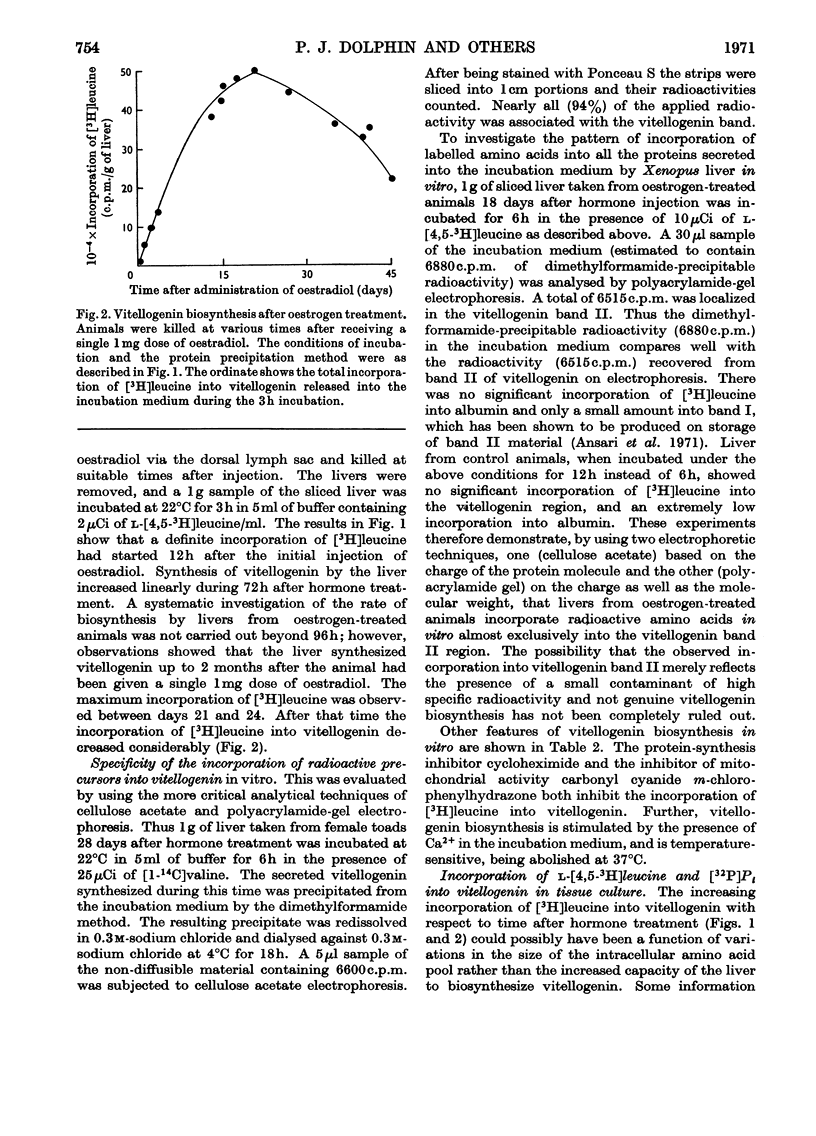
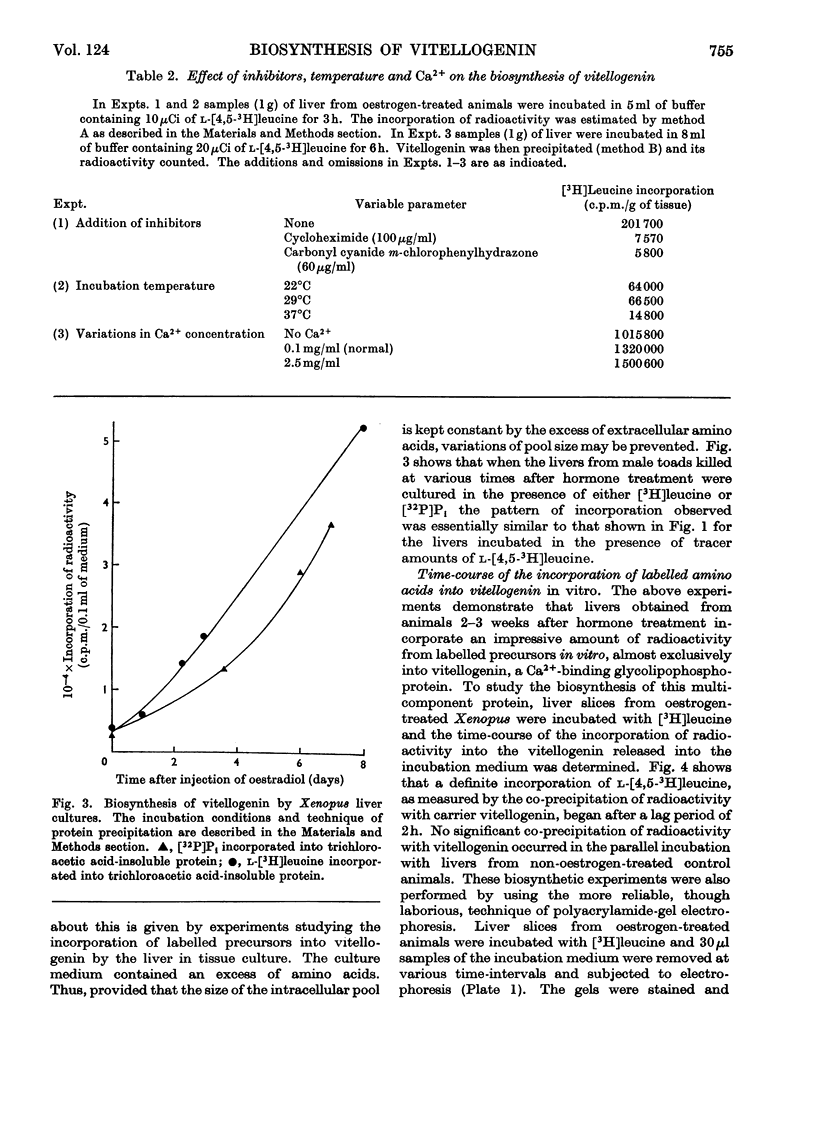
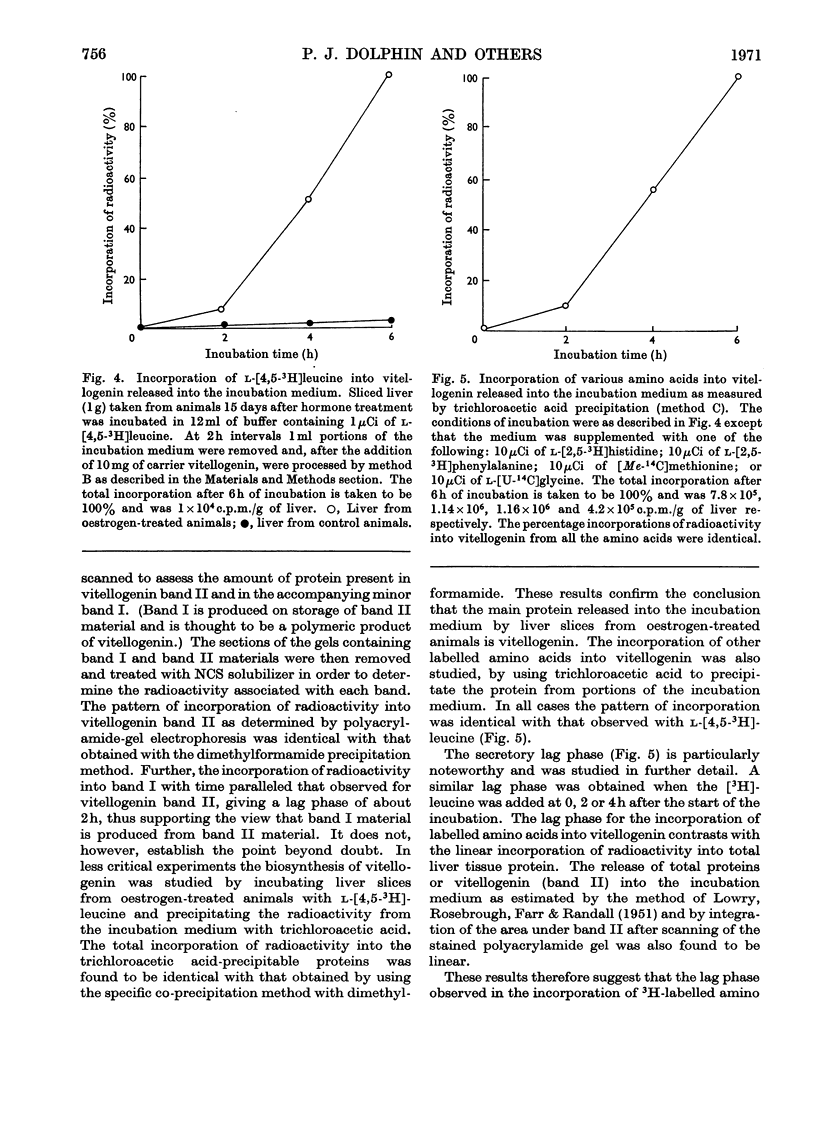
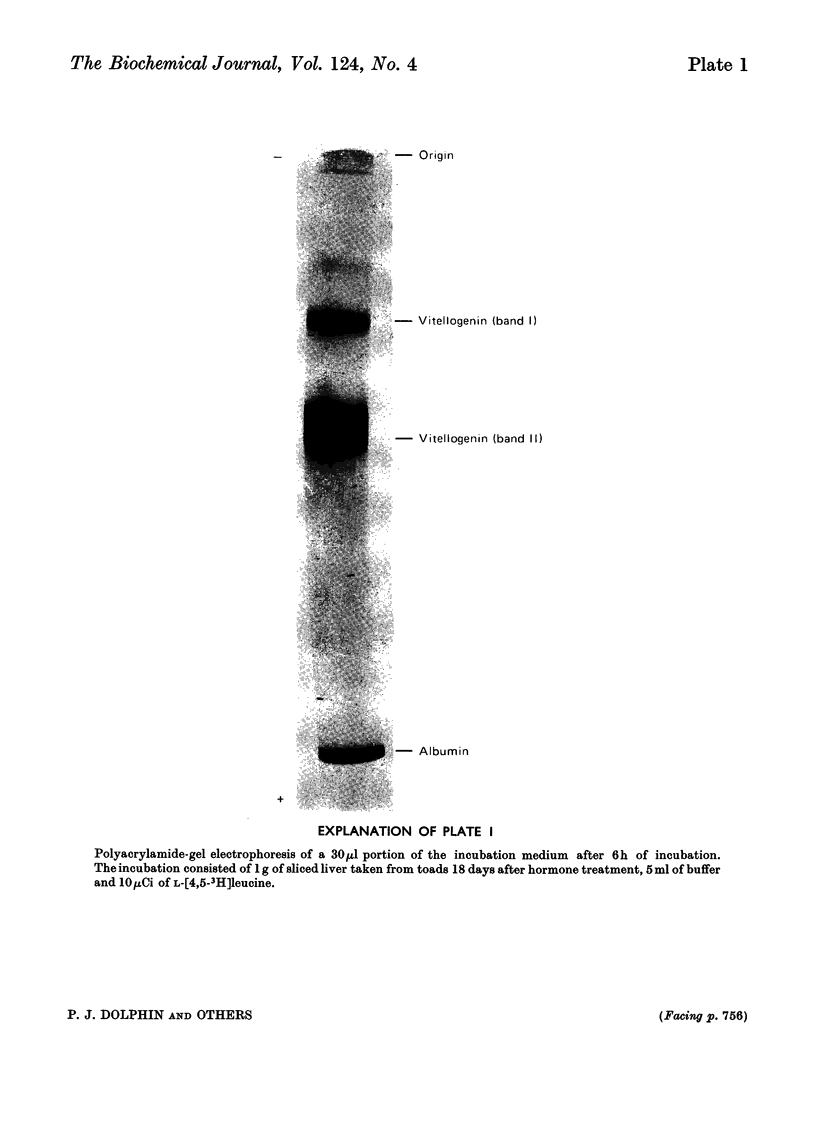
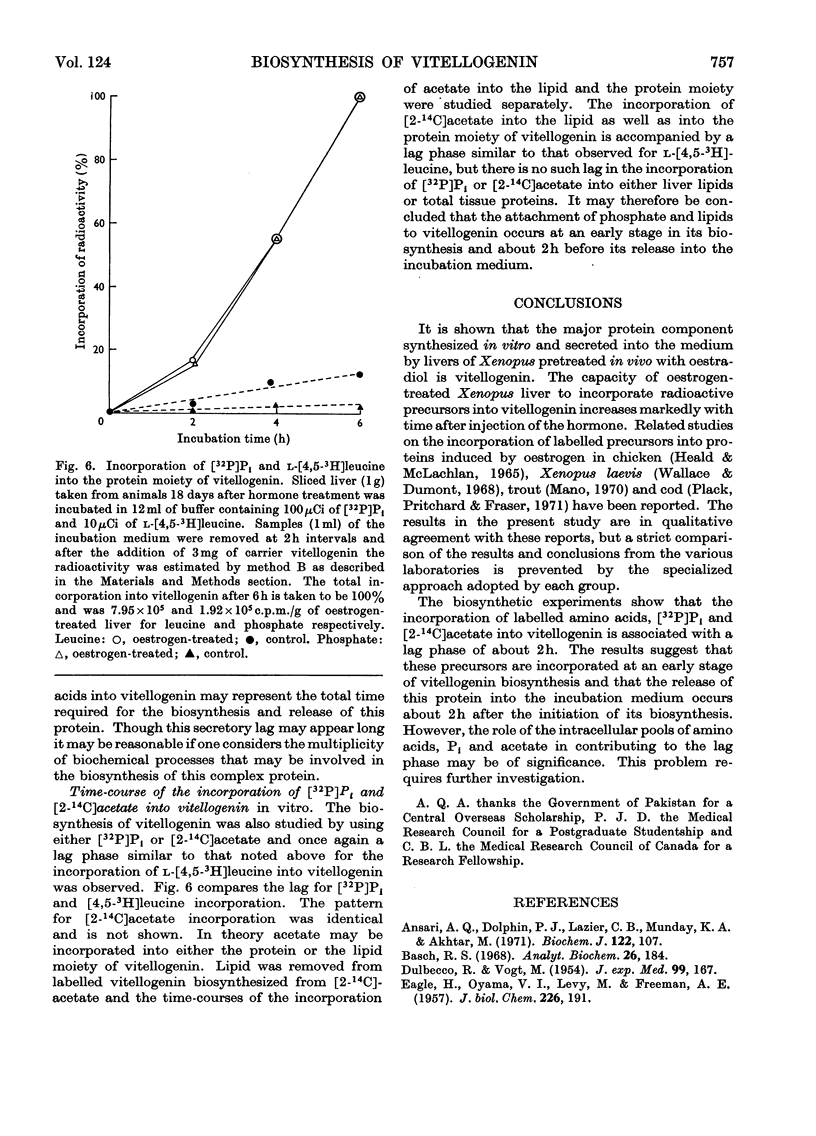
1. Oestrogen treatment induces the formation of a Ca2+-binding glycolipophosphoprotein, vitellogenin, in Xenopus laevis. 2. The incorporation of l-[4,5-3H]-leucine into vitellogenin in vivo and in vitro was observed 12–24h after hormone treatment and increased progressively up to 21 days after treatment. 3. Vitellogenin is shown to be the major protein component biosynthesized and released into the incubation medium in vitro by livers from oestrogen-treated animals. 4. The biosynthesis in vitro of vitellogenin was inhibited by cycloheximide and carbonyl cyanide m-chlorophenylhydrazone, stimulated by increased Ca2+ concentrations and decreased by raising the incubation temperature from 22 to 37°C. 5. Incorporation of labelled amino acids into vitellogenin began after approx. 2h. No lag phase was noted for the incorporation of labelled amino acids into total tissue proteins. 6. The incorporation of label from [32P]phosphate and [2-14C]acetate into the protein as well as into the lipid moiety of vitellogenin showed a lag phase similar to that noted for the incorporation of amino acids. 7. These results suggest that the release of vitellogenin into the incubation medium occurs about 2h after the initiation of its biosynthesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A. Q., Dolphin P. J., Lazier C. B., Munday K. A., Akhtar M. Chemical composition of an oestrogen-induced calcium-binding glycolipophosphoprotein in Xenopus laevis. Biochem J. 1971 Mar;122(1):107–113. doi: 10.1042/bj1220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch R. S. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968 Oct 10;26(1):184–188. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H., OYAMA V. I., LEVY M., FREEMAN A. E. Myo-Inositol as an essential growth factor for normal and malignant human cells in tissue culture. J Biol Chem. 1957 May;226(1):191–205. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HEALD P. J., MCLACHLAN P. M. THE SYNTHESIS OF PHOSVITIN IN VITRO BY SLICES OF LIVER FROM THE LAYING HEN. Biochem J. 1965 Jan;94:32–39. doi: 10.1042/bj0940032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERG R. J. Phosphorescence in liquid scintillation counting of proteins. Science. 1958 Jul 25;128(3317):199–200. doi: 10.1126/science.128.3317.199. [DOI] [PubMed] [Google Scholar]
- Hechter O., Yoshinaga K., Halkerston I. D., Birchall K. Estrogen-like anabolic effects of cyclic 3',5'-adenosine monophosphate and other nucleotides in isolated rat uterus. Arch Biochem Biophys. 1967 Nov;122(2):449–465. doi: 10.1016/0003-9861(67)90219-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mano Y. Mechanism of phosphorylation of phosphoprotein during oogenesis in trout in vivo. Biochim Biophys Acta. 1970 Feb 24;201(2):284–294. doi: 10.1016/0304-4165(70)90302-8. [DOI] [PubMed] [Google Scholar]
- Munday K. A., Ansari A. Q., Oldroyd D., Akhtar M. Oestrogen-induced calcium-binding protein in Xenopus laevis. Biochim Biophys Acta. 1968 Oct 29;166(3):748–751. doi: 10.1016/0005-2787(68)90393-6. [DOI] [PubMed] [Google Scholar]
- Plack P. A., Pritchard D. J., Fraser N. W. Egg proteins in cod serum. Natural occurrence and induction by injections of oestradiol 3-benzoate. Biochem J. 1971 Mar;121(5):847–856. doi: 10.1042/bj1210847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudack D., Wallace R. A. On the site of phosvitin synthesis in Xenopus laevis. Biochim Biophys Acta. 1968 Jan 29;155(1):299–301. doi: 10.1016/0005-2787(68)90361-4. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. 8. The estrogen-induced hepatic synthesis of a serum lipophosphoprotein and its selective uptake by the ovary and trasformation into yolk platelet proteins in Xenopus laevis. Dev Biol. 1969 May;19(5):498–526. doi: 10.1016/0012-1606(69)90085-2. [DOI] [PubMed] [Google Scholar]