Abstract
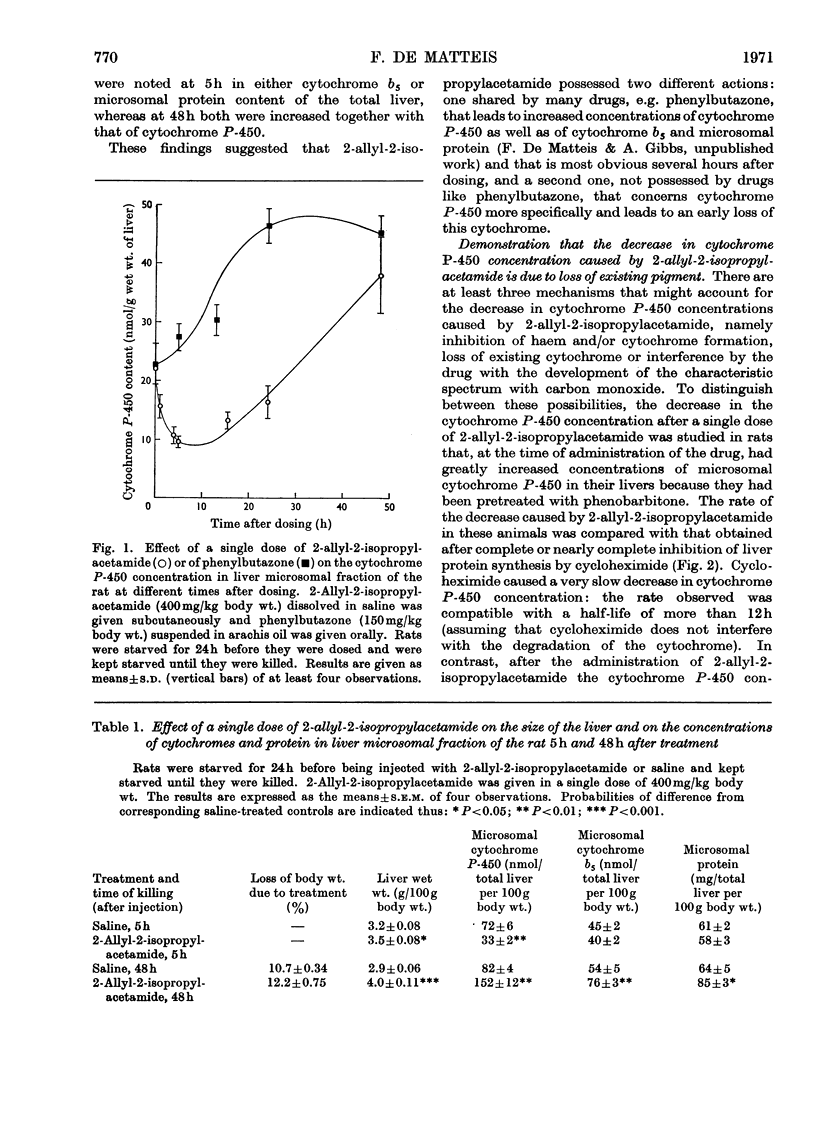
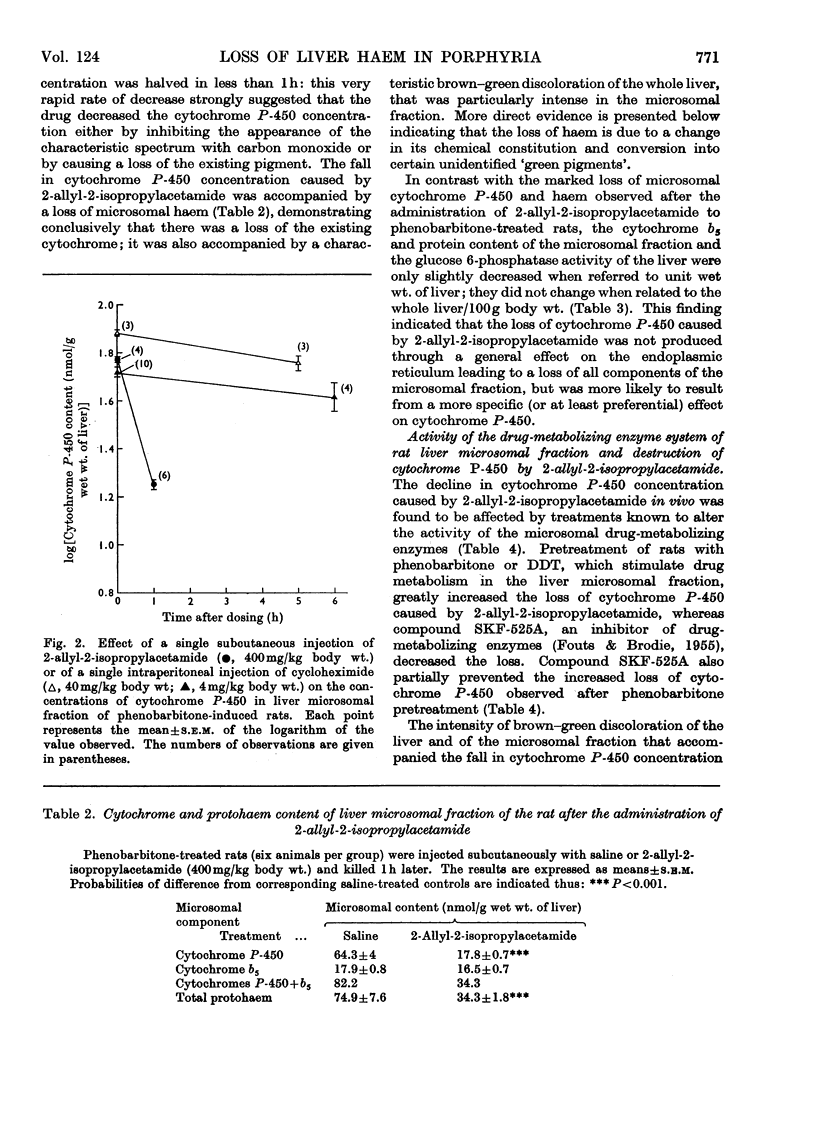
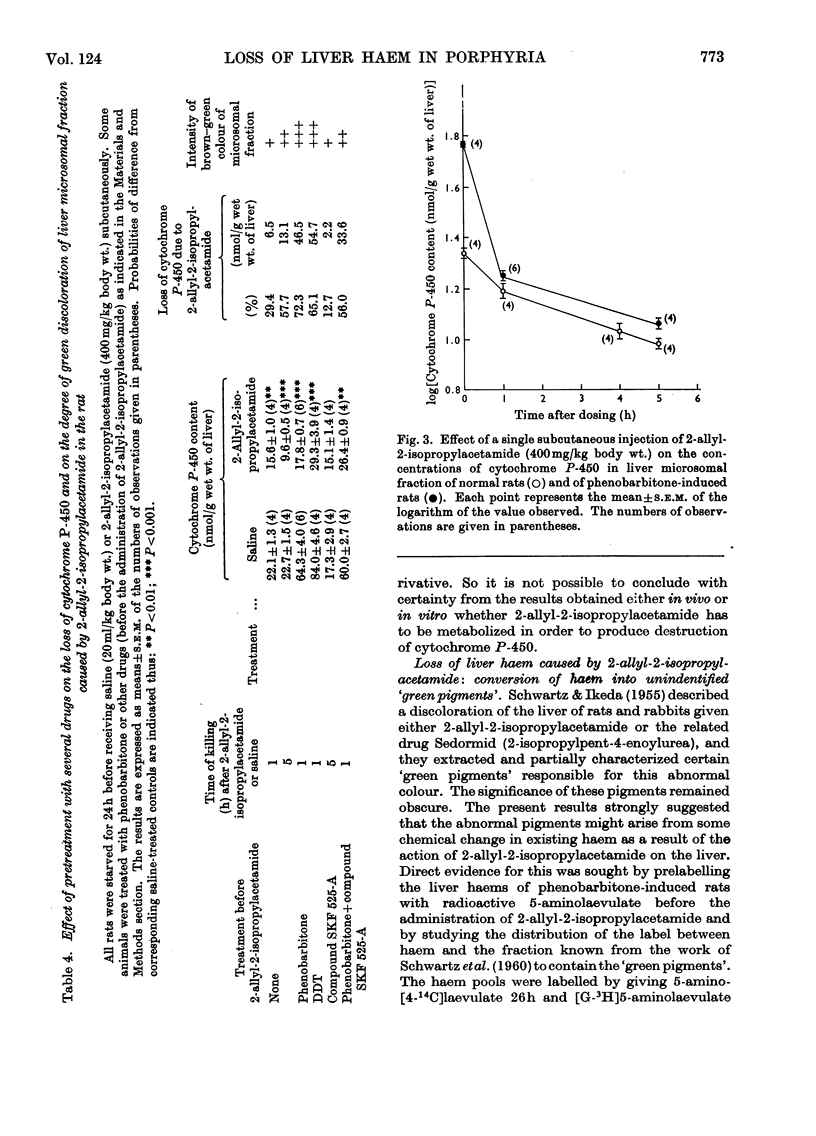
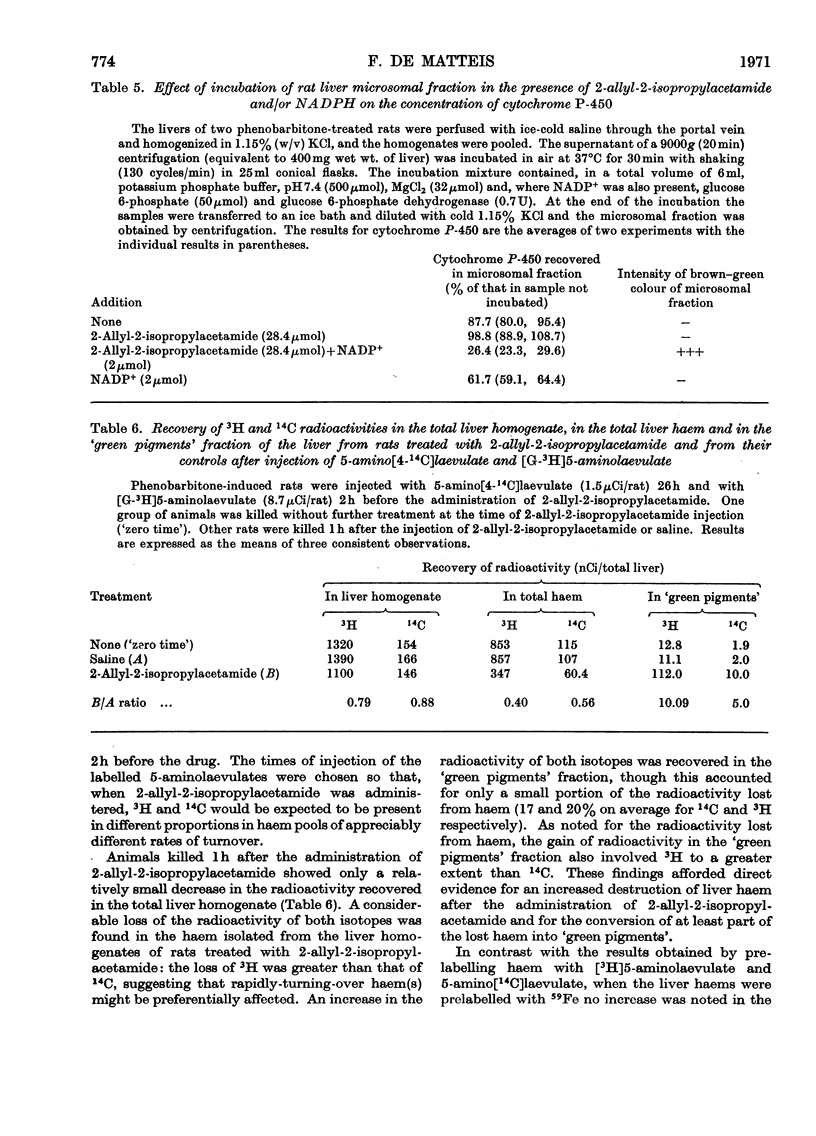
1. The effect of a single dose of 2-allyl-2-isopropylacetamide on the cytochrome P-450 concentration in rat liver microsomal fraction was studied. The drug caused a rapid loss of cytochrome P-450 followed by a gradual increase to above the normal concentration. 2. The loss of cytochrome P-450 was accompanied by a loss of microsomal haem and by a brown–green discoloration of the microsomal fraction suggesting that a change in the chemical constitution of the lost haem had taken place. Direct evidence for this was obtained by prelabelling the liver haems with radioactive 5-aminolaevulate: the drug caused a loss of radioactivity from the haem with an increase of radioactivity in a fraction containing certain un-identified green pigments. 3. Evidence was obtained by a dual-isotopic procedure that rapidly turning-over haem(s) may be preferentially affected. 4. The loss of cytochrome P-450 as well as the loss of microsomal haem and the discoloration of the microsomal fraction were more intense in animals pretreated with phenobarbitone and were much less evident when compound SKF 525-A (2-diethylaminoethyl 3,3-diphenylpropylacetate) was given before 2-allyl-2-isopropylacetamide, suggesting that the activity of the drug-metabolizing enzymes may be involved in these effects. 5. The relevance of the destruction of liver haem to the increased activity of 5-aminolaevulate synthetase caused by 2-allyl-2-isopropylacetamide is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- 'Carra P. O., Colleran E. HAEM catabolism and coupled oxidation of haemproteins. FEBS Lett. 1969 Nov 29;5(4):295–298. doi: 10.1016/0014-5793(69)80372-8. [DOI] [PubMed] [Google Scholar]
- ALDRIDGE W. N. Adenosine triphosphatase in the microsomal fraction from rat brain. Biochem J. 1962 Jun;83:527–533. doi: 10.1042/bj0830527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNHAM B. F., PIERCE W. S., WILLIAMS K. R., BOYER M. H., KIRBY C. K. delta-aminolaevulate dehydratase from Rhodopseudomonas spheroides. Biochem J. 1963 Jun;87:462–472. doi: 10.1042/bj0870462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond E. J., De Matteis F. Biochemical changes in rat liver after administration of carbon disulphide, with particular reference to microsomal changes. Biochem Pharmacol. 1969 Oct;18(10):2531–2549. doi: 10.1016/0006-2952(69)90368-2. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- FOUTS J. R., BRODIE B. B. Inhibition of drug metabolic pathways by the potentiating agent, 2, 4-dichloro-6-phenyl-phenoxyethyl diethylamine. J Pharmacol Exp Ther. 1955 Sep;115(1):68–73. [PubMed] [Google Scholar]
- GIBSON K. D., LAVER W. G., NEUBERGER A. Initial stages in the biosynthesis of porphyrins. 2. The formation of delta-aminolaevulic acid from glycine and succinyl-coenzyme A by particles from chicken erythrocytes. Biochem J. 1958 Sep;70(1):71–81. doi: 10.1042/bj0700071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- KROL S., RIMINGTON C., TOOTH B. Detection and determination of porphobilinogen in urine. Scand J Clin Lab Invest. 1956;8(3):251–262. doi: 10.3109/00365515609049281. [DOI] [PubMed] [Google Scholar]
- Kaufman L., Swanson A. L., Marver H. S. Chemically induced porphyria: prevention by prior treatment with phenobarbital. Science. 1970 Oct 16;170(3955):320–322. doi: 10.1126/science.170.3955.320. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., NISHIDA G. A new method of hemin isolation. Biochim Biophys Acta. 1957 Nov;26(2):437–437. doi: 10.1016/0006-3002(57)90033-1. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of enzymes concerned in bacteriochlorophyll formation in growing cultures of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Dec;23:487–498. doi: 10.1099/00221287-23-3-487. [DOI] [PubMed] [Google Scholar]
- Landaw S. A., Callahan E. W., Jr, Schmid R. Catabolism of heme in vivo: comparison of the simultaneous production of bilirubin and carbon monoxide. J Clin Invest. 1970 May;49(5):914–925. doi: 10.1172/JCI106311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDY J., NIZET A., BENSON A. Identification of a plasma material stimulating haemoglobin synthesis in vitro. Biochim Biophys Acta. 1961 Dec 23;54:414–423. doi: 10.1016/0006-3002(61)90080-4. [DOI] [PubMed] [Google Scholar]
- Wada O., Yano Y., Urata G., Nakao K. Behavior of hepatic microsomal cytochromes after treatment of mice with drugs known to disturb porphyrin metabolism in liver. Biochem Pharmacol. 1968 Apr;17(4):595–603. doi: 10.1016/0006-2952(68)90275-x. [DOI] [PubMed] [Google Scholar]
- Waterfield M. D., Del Favero A., Gray C. H. Effect of 1,4-dihydro-3,5-dicarbethoxycollidine on hepatic microsomal haem, cytochrome b5 and cytochrome P450 in rabbits and mice. Biochim Biophys Acta. 1969 Jul 30;184(2):470–473. doi: 10.1016/0304-4165(69)90054-3. [DOI] [PubMed] [Google Scholar]
- Waxman A. D., Collins A., Tschudy D. P. Oscillations of hepatic delta-aminolevulinic acid synthetase produced in vivo by heme. Biochem Biophys Res Commun. 1966 Sep 8;24(5):675–683. doi: 10.1016/0006-291x(66)90377-9. [DOI] [PubMed] [Google Scholar]
- de Matteis F. Disturbances of liver porphyrin metabolism caused by drugs. Pharmacol Rev. 1967 Dec;19(4):523–557. [PubMed] [Google Scholar]


