Highlights
-
•
Deep serum N-glycomics of septic shock patients infected with different pathogens.
-
•
Unique serum N-glycome in patients with candidaemia versus other infection groups.
-
•
Fungal-infected sera differ in α2,6-sialylation, core fucose and IgG-type N-glycans.
Keywords: Pathogen, septic shock, glycomics, N-glycans, serum, bacteraemia, candidaemia
Abstract
The morbidity and mortality of sepsis remain high. Clinicians lack effective markers to rapidly diagnose sepsis and identify the underlying pathogen infection particularly for patients with candidaemia or cases of culture-negative sepsis where culture-based diagnostics are inadequate. In our search for new lines of potential sepsis biomarkers, we here explore the impact of various classes of infectious agents on the serum N-glycome in a septic shock cohort. Comparative N-glycomics was performed on sera collected from 49 septic shock patients infected with viral (n = 9), bacterial (n = 37) or fungal (n = 3) pathogens using an established PGC-LC-MS/MS method. Aberrant serum N-glycosylation features were observed in patients with fungal infection relative to the other infection sub-groups including i) altered expression of prominent α2,6-sialylated biantennary N-glycan isomers, ii) elevated levels of IgG-type N-glycosylation and iii) a global shift in the serum N-glycome involving altered glycan type distribution and considerable changes in core fucosylation and α2,6-sialylation. Septic shock patients infected with bacterial and viral pathogens exhibited similar global serum N-glycome features and therefore could not be stratified based on their serum N-glycosylation. Subtle and less consistent serum N-glycome differences were observed between septic shock patients infected with different bacterial pathogens. In conclusion, our study has tested the impact of different pathogen classes on the serum N-glycome in a septic shock cohort, and reports that fungal infection impacts the host serum N-glycome differently compared to bacterial or viral infections thus potentially opening avenues for glycan-based biomarkers to better diagnose patients with candidaemia.
1. Introduction
Sepsis is the excessive host immune response to pathogen infection and often escalates into life-threatening septic shock. These serious conditions pose a major and still growing health concern accounting globally for ∼20% of all reported deaths and consuming an equally significant proportion of health care resources [1,2]. Little progress has been achieved in recent decades in the treatment of individuals suffering from septic shock, leaving intensive care physicians with relatively few, and rather blunt, tools and untargeted intervention options to treat patients [3]. Accordingly, sepsis and septic shock sufferers experience dishearteningly high morbidity and mortality rates. Sepsis and septic shock require urgent clinical care due to the serious and time-sensitive nature of these conditions that can swiftly escalate and lead to rapid deterioration of otherwise healthy individuals [4]. Current culture-based identification methods to reveal causative pathogen(s) and conventional methods to establish disease severity are unacceptably slow and insensitive, hindering timely and effective treatment and patient care [5]. Patients with fungal infection (candidaemia) are particularly challenging to diagnose. There is therefore a clear need for more effective pathogen-specific sepsis markers.
Glycosylation of serum proteins is a dynamic process sensitive to the underlying pathophysiology [6,7]. Advances in high-throughput glycomics have resulted in significant progress towards identifying disease-specific serum glyco-signatures [8,9]. Glycans are integral to the interactions between pathogens and host in the establishment of infection, as well as the modulation of the immune response [[10], [11], [12]]. While a better understanding of the glycobiology underpinning host-pathogen interactions is important to unveil diagnostic and therapeutic targets for the clinical management of infection, many facets of host glycome response to infection remain unexplored.
Infection-driven aberrations of the IgG-glycome and serum N-glycome were found to correlate with infection activity, severity and the immune response [13,14] and alternative N-glycosylation of the Fc portion of IgG is known to critically modulate the antibody effector functions [15]. For example, N-glycans of the Fc domain of IgG can report on disease states and discriminates active from latent Mycobacterium tuberculosis infection [16]. In HIV infection, elevated sialylation levels of the Fc N-glycans of gp120-directed IgG is associated with HIV-non-progression and broadly neutralising antibody responses in children, offering insight into the importance of glycosylation in vaccine strategies [17]. Less is known about the serum N-glycome in the context of host infection, but, similar to the IgG glycosylation, specific infection-driven alterations have been identified. For example, glycome changes indicative of disease severity have been identified in severe SARS-CoV-2 infection with elevated sialylation of serum glycoproteins reportedly linked to complement activation [18] and in influenza infection where oligomannosidic N-glycans were associated with disease severity [19]. Just as serum glycome remodelling can act to promote inflammatory responses to infection, glycans also play key roles in host interactions with the microbiome. An example of this is the decreased complexity of serum N-glycans observed in association with successful faecal microbiota transplantation for severe Clostridioides difficile infection [20]. Exploration of the glycome in infection has provided insights into the host immune response to infection and has highlighted the potential of quantitative glycomics in biomarker discovery.
The pathogenesis of septic shock and links to glycobiology remain poorly understood. Glycoprotein remodelling involving increased fucosylation and branching of alpha-1-antichymotrypsin, an acute phase reactant, was found to occur upon sepsis independently of protein concentration, but with notable inter-individual variation of the glycoproteoform profile [21]. Global shifts in the serum glycome characterised by increased glycan complexity and branching have been identified as a conserved response to sepsis [13,22,23]. Glycoproteomic profiling of samples taken within six hours of patient admission to intensive care unit (ICU) found that serum glycosylation differ between sepsis survivors and non-survivors; alterations in glycoproteins related to cell adhesion, complement pathways and coagulation cascade suggested that early changes in glycoproteins impact detrimental inflammatory processes that may contribute to outcome [23]. Beyond these scattered observations, the structural and functional modulation of the host serum glycome in sepsis are yet to be elucidated.
The impact of bacteraemia on host glycosylation has allowed identification of glycome and glycoproteome signatures that vary according to the infecting pathogen [24,25]. We previously utilised comparative N-glycomics to demonstrate that the serum N-glycome can differentiate bacteraemic patients with relatively modest disease severity (without septic shock) from healthy controls and according to the infecting bacterial organism [26]. Unsupervised hierarchical cluster analysis of serum N-glycome data effectively separated four bacteraemic patient groups (Staphylococcus aureus, Streptococcus viridans, Pseudomonas aeruginosa, Escherichia coli) from healthy donors, suggesting the host glycome may vary according to the infecting pathogen type. Similarly, investigation of the host glycoproteome utilising site-specific glycopeptide analysis of serum from 91 patients, within 24 hours of presentation with infection, revealed 96 glycopeptides that differed between bacterial and viral infection [25]. Distinct glycoproteome signatures that could discriminate bacterial from viral infection were identified and were able to predict pathogen species in 65% of patients within the cohort. Collectively, these studies suggest that pathogen-driven alterations in the host-glycome responses may serve as a diagnostic infection marker.
Identification of biomarkers that exploit the host response to infection is of particular interest in cases where culture-based diagnostics are limited by poor sensitivity due to low inoculum and prolonged time to detection such as for septic shock patients with candidaemia and other invasive fungal infections; or in cases of culture-negative sepsis. As opposed to diagnosis of infection by pathogen identification using culture, antigen detection, or genomic testing, host-response biomarkers may be less liable to false-negative results because of empiric antimicrobial use and false-positive results due to commensal microbes or sample contaminants. The human serum N-glycome has not been previously studied in the context of candidaemia, nor has it been established if pathogen-specific glyco-signatures can be observed in the setting of septic shock. Here, the impact of different infecting pathogen classes on the serum N-glycome was explored in a septic shock patient cohort using comparative N-glycomics.
2. Materials and methods
2.1. Sample collection and ethics
This was a single centre, prospective observational cohort study investigating the relationship between serum N-glycome profiles in septic shock patients to the infecting pathogen subtype. Patients were recruited from the Royal Adelaide Hospital, Australia and were receiving noradrenaline for 6 to 48 h in the intensive care unit (ICU) for the management of septic shock. The study was conducted in accordance with the Declaration of Helsinki and approved by the Human Research Ethics Committee of Central Adelaide Local Health Network (R20160208 HREC/16/RAH/29). Participants or their legally authorised next-of-kin were provided with written informed consent. When death occurred after enrolment and prior to consent, participants were included due to the study's low-risk nature. Septic shock was diagnosed either clinically or microbiologically, with a sequential organ failure assessment (SOFA) score of ≥2 points. Exclusion criteria included pregnancy, expectation of death within 24 h, noradrenaline infusion for >48 h, known conditions that affect cortisol secretion including the use of corticosteroids for ≥3 months and disorders of the hypothalamic-pituitary-adrenal axis, or administration of steroids in the ICU for indications other than shock. Whole blood was collected and samples de-identified. Following centrifugation, sera were aliquoted and stored at -80°C until use. The patient samples were accompanied by demographic, clinical and outcome data out to 90 days. The sera samples were previously studied in another context [27].
2.2. Patient metadata
Patient data relevant to the hypotheses of this study were retrospectively collected by review of the existing research data collected using the web-based Research Electronic Data Capture (REDCap) software (v11.0.2, Nashville, TN, USA) and the patients’ electronic medical record. For each patient, microbiological investigations were reviewed to allow an estimate of the significance of cultured organisms, and categorisation according to major infecting pathogen, either fungal, bacterial or viral. Patients were excluded in the study cohort if they had clinically diagnosed infection without confirmatory microbiology (culture-negative sepsis), infection diagnosed by non-sterile site culture such as urine or wound swab without positive blood culture (inability to retrospectively determine significance of non-sterile site culture results), no serum sample available, or confirmed concurrent bacterial, fungal or viral infection.
2.3. Cohort and sample collection
The study cohort included 49 participants with baseline characteristics outlined in Table 1. There were three subgroups; bacterial infection as diagnosed by positive blood culture, which could be poly- or monomicrobial (n = 37), fungal infection as diagnosed by positive blood culture with growth of Candida species (n = 3), and respiratory viral infection diagnosed with positive nasopharyngeal respiratory viral PCR or serum viral PCR in absence of diagnosed fungal or bacterial infection (n = 9). There was significant heterogeneity within each group including the infection characteristics (different infecting organisms, anatomical focus of infection) and clinical factors (medications, comorbidities).
Table 1.
Characteristics of the septic shock cohort. IQR: interquartile range.
| Patient characteristic | Fungal infection (n = 3) |
Bacterial infection (n = 37) |
Viral infection (n = 9) |
|---|---|---|---|
| Patient age - median years (IQR) | 54 (49–55.5) | 66.5 (58.3–70.5) | 73 (68–77) |
| Female – cases (%) | 2 (67) | 22 (75.9) | 5 (55.5) |
| White cells - median (x109 cells/L) (IQR) | 5.65 (3.6–14.7) | 16.55 (9.5- 19.7) | 16.1 (9.6–17.3) |
| Sampling relative to ICU admission – median days (IQR) |
5 (5–6) | 1 (1–1) | 2 (1–2) |
| Sampling relative to pathogen identification – median days (IQR) |
0 (-1 - 3.5) | 1 (1–1) | 0 |
The bacterial cohort (n = 37) included monomicrobial bacteraemic patients (n = 35) with blood culture isolation of gram-positive type Staphylococcus aureus (n = 7), Coagulase-negative staphylococci (n = 3), Streptococcus spp (n = 8), Enterococcus spp. (n = 2) and Microaerophilic streptococcus (n = 1); and gram-negative type Pseudomonas aeruginosa (n = 3), E. coli (n = 8), Serratia marcescens (n = 1), Haemophilus influenzae (n = 1) and Klebsiella pneumoniae (n = 1). Additionally, there were two patients with polymicrobial bacteraemia (n = 2); one diagnosed with K. pneumoniae, P. aeruginosa and Enterococcus casseliflavus, the other with E. coli, E. gallinarum and K. pneumoniae. The fungal subgroup (n = 3) included candidaemia secondary to Candida glabrata (n = 2) or Candida dubliniensis (n = 1). The viral subgroup (n = 9) included respiratory syncytial virus (n = 3), rhinovirus (n = 2), human metapneumovirus (n = 1), cytomegalovirus (n = 2) and influenza B virus (n = 1).
2.4. Comparative serum N-Glycomics
Comparative serum N-glycomics was performed using an established porous graphitised carbon liquid chromatography tandem mass spectrometry (PGC-LC-MS/MS) method [28,29]. Briefly, proteins (50 g) from each serum sample were reduced using 10 mM dithiothreitol (DTT) for 45 min at 56°C, then alkylated using 25 mM iodoacetamide for 30 min in the dark at 20°C, and the reaction quenched using excess DTT. Proteins were then immobilised by dot blotting onto a primed polyvinylidene fluoride membrane. The dried spots were stained with Direct Blue and transferred to a polypropylene 96-well plate, blocked with 1% (w/v) polyvinylpyrrolidone in 50% (v/v) methanol and washed with water. N-glycans were released from serum proteins using 2.5 U peptide:N-glycosidase F (PNGase F) per 50 g protein in 25 L water for 16 h at 37°C. Free N-glycans were hydroxylated by addition of 100 mM ammonium acetate, pH 5 for 1 h at 20°C, reduced using 1 M sodium borohydride in 50 mM potassium hydroxide for 3 h at 50°C, and the reaction quenched using glacial acetic acid. The N-glycans were desalted with strong cationic exchange resin, and then porous graphitised carbon (PGC) resin packed as micro-columns on top of C18 discs in P10 solid-phase extraction (SPE) formats. The N-glycans were eluted from the SPE micro-columns using 0.05% trifluoroacetic acid: 40% acetonitrile (ACN): 59.95% water (v/v/v) and dried. Prior to LC-MS/MS, the desalted N-glycans were reconstituted in 20 L water, centrifuged at 14,000 x g for 10 min at 4°C, and transferred into high recovery glass vials.
The N-glycans were quantitatively profiled using PGC-LC-MS/MS-based glycomics as described [30]. In brief, N-glycans were injected onto a heated (50°C) HyperCarb KAPPA PGC-LC column (particle/pore size 3 µm/250 Å; column length, 30 mm; inner diameter, 0.18 mm) and separated over a 60 min linear gradient of 0–45% (v/v) of ACN (solvent B) in 10 mM ammonium bicarbonate (solvent A) on an Agilent 1260 Infinity Capillary HPLC system. The separated N-glycans were ionised using electrospray ionisation and detected over an m/z range of 570–2000 in negative ion polarity mode using a Thermo Velos Pro linear ion trap mass spectrometer. The source voltage was -2.75 kV. The automatic gain control (AGC) for the MS1 scans was 3 × 104 ions with a maximum accumulation time of 10 ms. For MS/MS, the AGC was 1 × 104 ions, and the maximum accumulation time was 250 ms. Employing data-dependent acquisition, the five most abundant precursors in each MS1 scan were selected for MS/MS using resonance-activation collision-induced dissociation (CID) at a fixed 33% normalised collision energy with an activation Q of 0.250, activation time of 10 ms and using precursor isolation windows of m/z 2.0. Dynamic exclusion was enabled with repeat counts set at a maximum of 1, repeat duration at 15 s, and exclusion duration at 15 s. Spectra were acquired in profile mode. To avoid potential systematic bias in the LC-MS/MS data collection, samples were injected in a random order.
Mass spectral data was interrogated using Xcalibur v2.2 (Thermo Fisher Scientific) and Glycoworkbench v2.1 [31]. Glycans were manually identified based on their monoisotopic precursor mass, MS/MS fragmentation pattern, and PGC-LC retention time as described [32,33]. Where possible, the fine structures of the N-glycan isomers were annotated including their monosaccharide compositions, topology/branch patterns (e.g. fucose position), and key glycosidic bonds (e.g. sialyl linkages). Glycan cartoons were drawn using SNFG nomenclature [34]. The relative abundance of individual glycans was determined by the area-under-the-curve of extracted ion chromatograms of each identified N-glycan as a proportion of the entire N-glycome using RawMeat v2.1 (Vast Scientific) and Skyline (64-bit) v23.1.0.455 [33].
The quantitative serum N-glycomics datasets were subjected to principal component analysis (PCA) using partial least squares discriminant analysis (PLS-DA) that discovers latent variables (labelled as components, in this case, glycans), which maximise the separation between different predefined groups in the data. The PCA was performed with MetaboAnalyst 5.0. Unsupervised hierarchical clustering analysis was performed with Perseus [35] using Euclidean distance with average linkages. Differential glycan abundance in the subgroups were identified using Wilcoxon rank-sum test and unpaired two-tailed Student's t-tests were performed using a confidence threshold of p < 0.05. Descriptive statistics of cohort characteristics were reported using median and interquartile ranges (IQR).
3. Results
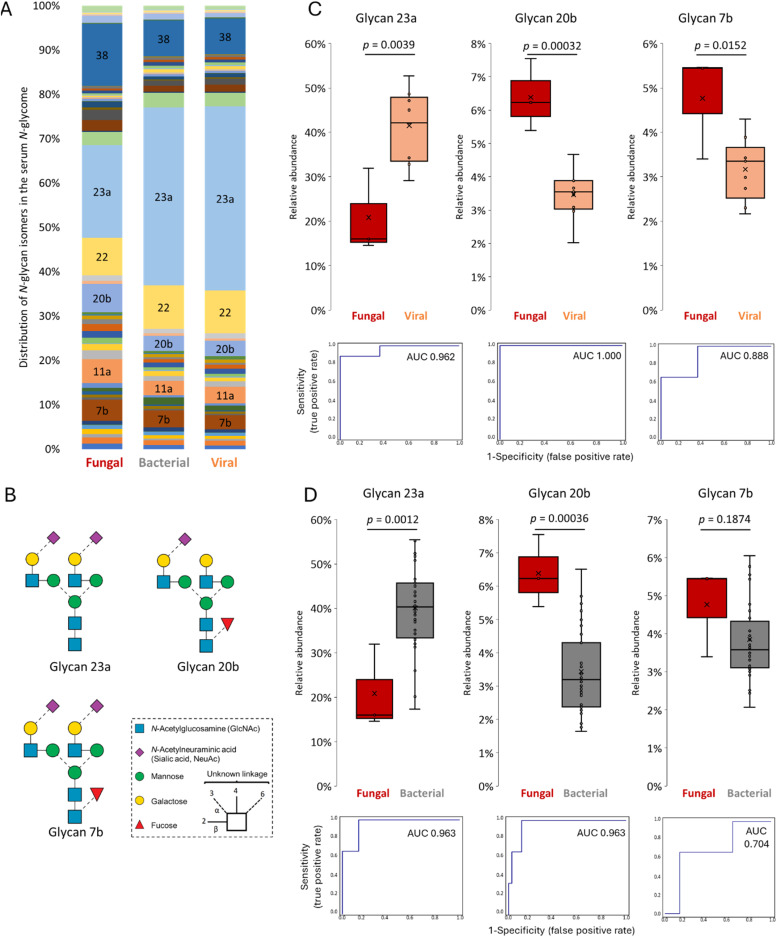
An established PGC-LC-MS/MS-based N-glycomics approach was applied to sera from a cohort of 49 septic shock patients spanning individuals infected with bacterial (n = 37), fungal (n = 3) and viral (n = 9) pathogens, Table 1. The N-glycomics analysis identified a total of 54 N-glycan isomers spanning 43 glycan compositions across the serum samples, Fig. 1. The strength of the applied PGC-LC-MS/MS approach is that glycan fine structural details such as glycan composition, topology/branch points and glycosidic linkages can be determined for each glycan structure in the mixture while simultaneously obtaining quantitative information of the individual glycan structures that therefore can be compared between samples [28]. In line with knowledge of the serum N-glycome [26,36], the complex N-glycans spanning mostly sialylated and fucosylated bi- and tri-antennary structures were abundant in the serum samples (∼93–96%), while the oligomannosidic- (∼3–5%) and hybrid-type (∼1–2%) N-glycans were less abundant. Also consistent with the serum N-glycome literature [7], the serum N-glycans predominantly carried α2,6-sialylation (∼60–70%) and core fucosylation (∼30–50%) while the corresponding isomers exhibiting α2,3-sialylation (∼10%) and antennary fucosylation (∼2–4%) were less abundant as were the bisecting GlcNAc features (∼5%), see Supplementary Table S1 for all tabulated serum N-glycome data.
Fig. 1.
Map of the N-glycan isomers identified in septic shock sera. Identifiers used in this study (Glycan 1–43) have been assigned to each unique glycan compositions while a, b, c, d denote unique glycan isomers within each glycan composition. The glycan structural features including the branch patterns and glycosidic linkages were elucidated and annotated or left unassigned (brackets) in case of structural ambiguity. See key for symbol and linkage nomenclature [34].
The serum N-glycome data of the 49 patient samples were then explored for global differences. Unsupervised hierarchical clustering analysis of the entire serum N-glycome data partially separated patients with fungal infection from patients with bacterial or viral infection (Fig. 2A). In support, principal component analysis (PCA) of the same serum N-glycome data using partial least squares discriminant analysis (PLS-DA) fully separated patients with fungal infection from those with viral or bacterial infection (Fig. 2B). Being the principal contributor to the separation of the fungal group, component 1 of the PCA comprised 10 glycans including Glycan 8, 4, 29, 10, 20b, 17, 3a, 5, 16, and 3 (see Fig. 1 for structures) indicating that a subset of the serum N-glycome is altered in fungal-infected sera. Closer interrogation of the data revealed that the serum N-glycome of fungal-infected septic shock patients exhibited slightly higher relative levels of oligomannosidic- and concomitantly less complex-type N-glycans (both p < 0.01) than the two other infection sub-groups (Fig. 2C). On the global level, core fucosylation was found to be elevated (p < 0.01) and α2,6-sialylation reduced (p < 0.05) in the serum N-glycome of patients infected with fungal pathogens compared to those with bacterial and viral infections (Fig. 2D-E). Other glycan features (i.e. antennary fucosylation, α2,3-sialylation, bisecting GlcNAcylation) were not altered across infection groups.
Fig. 2.
The serum N-glycome of fungal-infected septic shock patients differ from patients with bacterial and viral infection. A) Unsupervised hierarchical clustering analysis of the serum N-glycomics data of the entire septic shock cohort (n = 49) including patients with bacterial infection (grey, n = 37), fungal infection (also referred to as candidaemia, red, n = 3), and viral infection (salmon, n = 9). B) PCA of the same serum N-glycome data using PLS-DA with default settings and 10 variables/component. Comparison of C) N-glycan type distribution, and the global level of D) core fucosylation and E) α2,6-sialylation between fungal, viral and bacterial infected septic shock patients. For all bar graphs, mean and SD are plotted. Statistical tests were performed using two-tailed t-tests. *, p < 0.05; ** p < 0.01.
To further detail the N-glycome shifts upon fungal infection, we surveyed the expression of the individual serum N-glycans across the infection sub-groups. The distribution of the serum N-glycan isomers appeared visibly different in the fungal subgroup relative to the bacterial and viral subgroups (Fig. 3A). Out of the 54 N-glycan isomers observed in septic shock sera, the relative abundance of 14 (∼25%) and 21 (∼40%) structures were found to be altered in patients with candidaemia compared to those with viral and bacterial infection, respectively (Supplementary Table S1). Amongst these were three abundant complex-type biantennary N-glycan isomers (Glycan 23a, 20b and 7b) all carrying α2,6-sialylation (Fig. 3B and Supplementary Figure S1-S3). Glycan 23a, a biantennary non-core fucosylated N-glycan capped with two α2,6-linked sialic acid residues was dramatically reduced while the related Glycan 20b carrying a single α2,6-linked sialic acid residue and core fucosylation was elevated in fungal-infected sera (all p < 0.01) (Fig. 3C-D). ROC analyses of Glycan 23a and Glycan 20b provided near-perfect stratification between patients with fungal and viral/bacterial infection (AUC: 0.962–1). Despite not consistently reaching significance, another biantennary glycan carrying two α2,6-linked sialic acid residues and core fucosylation (Glycan 7b) was also elevated in fungal-infected sera. The differential expression of these three high abundance serum N-glycans across the infection groups are therefore major contributors to the raised core fucosylation and reduced α2,6-sialylation observed in the serum N-glycome of patients with candidaemia (Fig. 2D-E).
Fig. 3.
Fungal infection alters the levels of prominent sialo-N-glycan isomers in septic shock. A) Distribution of the mean relative abundances of the 54 N-glycan isomers identified in septic shock sera across the three infection subgroups (fungal, bacterial, viral pathogens). Glycan identifiers are annotated for the prominent N-glycan isomers (see Fig. 1 for structures and Supplemental Table S1 for tabulated serum N-glycome data). B) Dominant sialo-N-glycan isomers displaying altered expression upon fungal infection. See insert for key [34] and Supplementary Figure S1-S3 for spectral evidence of the three N-glycan structures. Comparison of the relative abundance level of Glycan 23a, 20b and 7b in serum from fungal-infected versus C) viral- and D) bacterial-infected septic shock patients. For all graphs, mean and boxes indicating 1st and 3rd quartile range are plotted as are the individual data points. ROC plots and AUC values are provided. Statistical tests were performed using two-tailed t-tests. Significance (p) have been stated for each analysis.
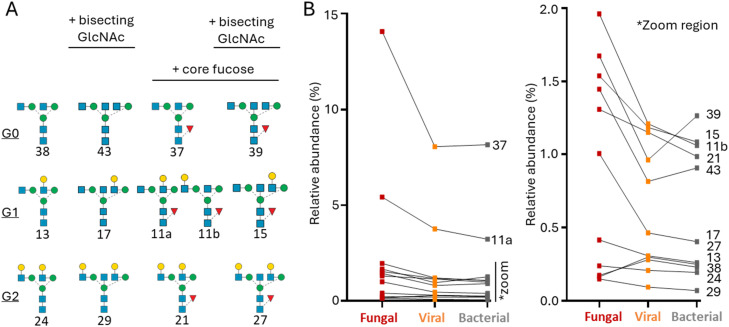
A subset of the serum N-glycans exhibited features suggesting an immunoglobulin G (IgG) origin including the so-called G0, G1, G2 structures and their corresponding isomers with core fucosylation and bisecting GlcNAcylation (Fig. 4A). With the exception of Glycan 13 and 38, the entire set of these IgG-type N-glycan isomers (11/13, ∼85%) was more abundant in fungal-infected sera than in viral- and bacterial-infected sera that overall appeared similar (Fig. 4B). Collectively, this analysis indicate that IgG-type N-glycans are generally elevated in septic shock patients with fungal infection compared to the other infection classes.
Fig. 4.
Elevated IgG-type N-glycans in serum of fungal-infected septic shock patients. A) Overview of the IgG-type N-glycans surveyed in this analysis spanning G0, G1 and G2-type structures with and without bisecting GlcNAc and core fucosylation. See Fig. 1 for symbol and linkage nomenclature [34]. B) Relative abundance (mean) of each IgG-type N-glycan observed in the serum N-glycome of septic shock patients infected with fungal (n = 3), viral (n = 9) and bacterial (n = 37) pathogens. Glycan identifiers have been provided, see structures in panel A. *zoom region enabling a closer look at the lower abundance IgG-type N-glycans.
Finally, we focused on the large subset of septic shock patients infected by a single bacterial pathogen. Recapitulating findings from our previous study showing that the serum N-glycome differ in bacteraemic patients infected with different bacterial pathogens [26], the serum N-glycome data partially separated the septic shock patients according to infecting bacterial sub-groups (Supplementary Figure S4). While not achieving complete stratification, particularly P. aeruginosa (n = 3), E. coli (n = 8) and S. aureus (n = 7) showed tendencies to group when using an unsupervised hierarchical clustering analysis of the entire serum N-glycome data demonstrating that different types of bacterial classes impact the host serum N-glycome differently.
4. Discussion
This study has found that the serum N-glycome of patients with fungal infection (candidaemia) differ compared to those with bacteraemia and viral infection both at a whole serum N-glycome level, and for specific N-glycan features (core fucosylation, α2,6-sialylation) and specific N-glycan isomers (e.g. biantennary sialo-N-glycans and IgG-type N-glycans). Amongst the septic shock patients infected by a single bacterial pathogen, minor and less consistent serum N-glycome differences were observed between sub-groups infected with different types of bacterial pathogens.
The mechanism(s) underlying the remodelling of the host serum N-glycome observed in patients with candidaemia is unknown, but may be directly due to alternative glycosylation of serum proteins resulting from host-candida interactions or indirectly due to alterations in the serum proteome resulting from the host response to infection e.g. synthesis of new serum glycoproteins from alternative tissue origins or selective removal/degradation of specific serum glycoproteoforms. Supporting that alterations occur in the serum proteome, a recent study performed transcriptomics of the host response to candidaemia by RNA sequencing of circulating leukocytes and demonstrated a conserved and unique response that could distinguish patients with candidaemia from those with viral, bacterial, or non-infective aetiologies of systemic inflammation [37]. Most upregulated genes in the setting of candidaemia were those involved in the immune response to fungal infection including T cell signalling, heme biosynthesis and neutrophil activation. Whilst the host glycome response to fungal infection has not been previously investigated, C. albicans cell wall mannoprotein-derived N-glycans were found to contribute to immune evasion in a sepsis mouse model through host dectin-2 recognition and IL-10 induction with resultant downregulation of proinflammatory cytokines [38]. The recognition of pathogen-derived N-glycans by the host immune system exemplifies the significance of glycans in host-pathogen interactions which are likely to influence the serum glycome in the setting of fungaemia.
The raised IgG-type N-glycans in fungal-infected serum suggest either a relatively higher level of serum IgG protein or a higher glycosylation site occupancy of serum IgG in patients with candidaemia relative to levels in other infection classes. Clinical measurements of IgG levels are not usually measured in patients with septic shock leaving us unable to assess the relative total IgG levels across the patient subgroups from the accompanying pathology data. The literature exploring IgG levels and the humoral immune response in sepsis is limited, but suggests that IgG levels are heterogenous in septic shock cohorts [39]. Related to the host immunoglobulin response, anti-mannan antibody detection has been used for the detection of invasive candidiasis [40], albeit such tests suffer from variable performance and low sensitivity and specificity [41]. Combining the anti-mannan antibody detection with measurements of beta-D-glucan and mannan levels were reported to improve the performance of the detection method [42]. IgG against Candida enolase and fructose-bisphosphate aldolase has also been found to distinguish fungal infection from other pathogens and healthy controls [43]. These antibody responses may be detectable early sometimes before positive blood culture. In contrast, organism-specific antibody responses are not used in the diagnosis of acute bacteraemia and organism-specific IgG may be lower in patients with bacteraemia who progress to sepsis. Streptococcus pyogenes produces endoglycosidases that deglycosylate IgG Fc as an immune evasion mechanism [44,45]. Low S. aureus-directed IgG at diagnosis of S. aureus-induced bacteraemia has been correlated with progression to sepsis [46]. Low S. aureus glycan-specific IgM (not IgG) was found in patients with S. aureus-induced bacteraemia and correlated with mortality and impaired opsonisation [47]. Taken together, a body of literature is available supporting a host IgG response to candidiasis that differ from the response to other types of infection, and therefore may contribute to the elevated IgG-type N-glycosylation observed in fungal-infected sera. The observed suppression in the relative levels of α2,6-sialylation in the serum N-glycome of patients with candidaemia may simply be a consequence of an elevation in serum IgG known to carry low levels of sialylation [48]. At this stage, it also cannot be ruled out that candida is able to produce fungal sialidases that may directly lower the sialylation level of serum glycoproteins similar to sialidases produced by P. aeruginosa [49].
Our previous study reporting that the serum N-glycome patterns differ according to the bacterial pathogen sub-class in bacteraemic patients pre-progression to septic shock [26] were partially recapitulated in this cohort of septic shock patients. Alternative glycosylation of serum proteins resulting from the inflammatory milieu of septic shock may overwhelm the more subtle glycome differences that occur as a result of specific pathogen-host interactions ahead of progression to septic shock. Unique serum N-glycome patterns were not seen when comparing the viral and bacterial infection subgroups at a whole glycome level. Undiagnosed bacterial infection in the cohort of patients classified with viral infection is probable given their clinical diagnosis of septic shock and may have contributed to the lack of observable differences across these two infection sub-groups.
Our study has several limitations. There was considerable heterogeneity within the study cohort including patient demographics, clinical characteristics (comorbidities, medications), and features of infection (anatomical focus of infection) all which may have influenced the serum N-glycome and confounded the results of this study. The small candidaemia subgroup reflects the challenge of obtaining such clinical samples. Potential confounders in the candidaemia subgroup include lower baseline white cell count, the timing of sample collection following ICU admission and poor correlation between timing of serum sample collection and blood culture positivity (candidaemia group: day +7, -2 and 0 from blood culture positivity). Investigations in larger cohorts of patients with candidaemia and serially collected sera over time are required to validate if, how and when the serum glycome is differentially affected by the infecting pathogen class and to determine if the observed changes are unique to candidaemia. Finally, pathogen identification was performed at the time of patient admission and reviewed daily during ICU stay and retrospectively based on clinical findings, cultured pathogen data, ICU and ID physician notes/opinions. While false pathogen identification cannot be ruled out for some cases, the risk of incorrect assignment was mitigated by exclusion of patients with septic shock where infection was diagnosed by a non-sterile site culture.
Despite providing deep and quantitative insight into the serum N-glycome, PGC-LC-MS/MS-based N-glycomics remains a laborious approach with manual annotation of data hence currently limiting population-wide profiling efforts. We were therefore restricted to relatively small numbers of patients within each pathogen infection group resulting in a low statistical power preventing us to observe aberrations in the serum N-glycome correlating to patient characteristics and adjust for confounding factors. Powered by the emergence of (semi-)automated glycan annotation software (e.g. Byos, Protein Metrics) future efforts employing larger patient cohorts are warranted to confirm observations reported herein. Parallel glycoproteomic analysis [50] could provide important orthogonal insights into the protein carriers and glycosylation sites of the serum N-glycans yet was outside the scope of this study.
5. Conclusions
Aberrant expression of specific N-glycan isomers as well as a global shift in the serum N-glycome was observed in septic shock patients with candidaemia, as compared to those with bacterial or viral infection. This observation may aid the investigation of timely pathogen-specific immunopathogenesis in sepsis and in the development of host response-based diagnostic assays for candidaemia or other invasive fungal infections where existing diagnostic methods lack sensitivity and pathogen identification from culture is often prolonged.
CRediT authorship contribution statement
Helena Torpy: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. The Huong Chau: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Sayantani Chatterjee: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. Anastasia Chernykh: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – review & editing. David J. Torpy: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – review & editing. Emily J. Meyer: Data curation, Formal analysis, Project administration, Resources, Supervision, Writing – review & editing. Morten Thaysen-Andersen: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Morten Thaysen-Andersen reports financial support was provided by Australian Research Council. The Huong Chau reports financial support was provided by Macquarie University. Emily J. Meyer reports financial support was provided by Royal Adelaide Hospital Research Fund. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Data availability statement
In line with community standards, all glycomics PGC-LC-MS/MS raw data is freely available through the GlycoPOST repository (identifier GPST000503). The files can be downloaded from the link https://glycopost.glycosmos.org/preview/1569444274673d22a9c253c (PIN 9656).
Acknowledgements
We thank A/Prof Daniel Bojar for fruitful discussions relevant to glycome quantitation. T.H.C. is supported by an International Research Training Program Scholarship (20224231) funded by the Australian Government. A.C. is supported by a Research Training Program Scholarship funded by the Australian Government. E.J.M. is supported by the Royal Adelaide Hospital Research Fund 2023 Early Career Research Fellowship (MYIP 17236). M.T.-A. is the recipient of an ARC Future Fellowship (FT210100455).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.bbadva.2025.100138.
Appendix. Supplementary materials
References
- 1.Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta Y, Paul R, Rabbani R, Acharya SP, Withanaarachchi UK. Sepsis management in southeast asia: a review and clinical experience. J. Clin. Med. 2022;11(13) doi: 10.3390/jcm11133635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamath S, Hammad Altaq H, Abdo T. Management of sepsis and septic shock: what have we learned in the last two decades? Microorganisms. 2023;11(9) doi: 10.3390/microorganisms11092231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guarino M, Perna B, Cesaro AE, Maritati M, Spampinato MD, Contini C, et al. 2023 update on sepsis and septic shock in adult patients: management in the emergency department. J. Clin. Med. 2023;12(9) doi: 10.3390/jcm12093188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chun K, Syndergaard C, Damas C, Trubey R, Mukindaraj A, Qian S, et al. Sepsis pathogen identification. J. Lab. Autom. 2015;20(5):539–561. doi: 10.1177/2211068214567345. [DOI] [PubMed] [Google Scholar]
- 6.Lauc G, Pezer M, Rudan I, Campbell H. Mechanisms of disease: the human N-glycome. Biochimica. et. Biophysica. Acta. -. General. Subjects. 2016;1860(8):1574–1582. doi: 10.1016/j.bbagen.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Clerc F, Reiding KR, Jansen BC, Kammeijer GSM, Bondt A, Wuhrer M. Human plasma protein N-glycosylation. Glycoconjugate. Journal. 2016;33(3):309–343. doi: 10.1007/s10719-015-9626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dotz V, Wuhrer M. N-glycome signatures in human plasma: associations with physiology and major diseases. FEBS. Letters. 2019;593(21):2966–2976. doi: 10.1002/1873-3468.13598. [DOI] [PubMed] [Google Scholar]
- 9.Trbojevic-Akmacic I, Lageveen-Kammeijer GSM, Heijs B, Petrovic T, Deris H, Wuhrer M, et al. High-throughput glycomic methods. Chem. Rev. 2022;122(20):15865–15913. doi: 10.1021/acs.chemrev.1c01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin R, Mahal LK. Current Opinion in Structural Biology. Elsevier Ltd; 2021. The host glycomic response to pathogens; pp. 149–156. [DOI] [PubMed] [Google Scholar]
- 11.Lin B, Qing X, Liao J, Zhuo K. Role of protein glycosylation in host-pathogen interaction. Cells:. NLM. (Medline); 2020 doi: 10.3390/cells9041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabinovich GA, van Kooyk Y, Cobb BA. Annals of the New York Academy of Sciences. Blackwell Publishing Inc.; 2012. Glycobiology of immune responses; pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gornik O, Royle L, Harvey DJ, Radcliffe CM, Saldova R, Dwek RA, et al. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology. 2007;17(12):1321–1332. doi: 10.1093/glycob/cwm106. [DOI] [PubMed] [Google Scholar]
- 14.Gornik O, Gornik I, Kolednjak IZ, Lauc G. Change of transferrin sialylation differs between mild sepsis and severe sepsis and septic shock. Intern. Med. 2011;50(8):861–869. doi: 10.2169/internalmedicine.50.4704. [DOI] [PubMed] [Google Scholar]
- 15.de Haan N, Falck D, Wuhrer M. Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology. 2020;30(4):226–240. doi: 10.1093/glycob/cwz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu LL, Das J, Grace PS, Fortune SM, Restrepo BI, Alter G. Antibody Fc glycosylation discriminates between latent and active tuberculosis. Journal. of. Infectious. Diseases. 2020;222(12):2093–2102. doi: 10.1093/infdis/jiz643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muenchhoff M, Chung AW, Roider J, Dugast A-S, Richardson S, Kløverpris H, et al. Distinct immunoglobulin Fc glycosylation patterns are associated with disease nonprogression and broadly neutralizing antibody responses in children with HIV infection. mSphere. 2020;5(6) doi: 10.1128/mSphere.00880-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin R, Kurz E, Chen S, Zeck B, Chiribogas L, Jackson D, et al. α2,6-Sialylation is upregulated in severe COVID-19, implicating the complement cascade. ACS. Infect. Diseases. 2022;8(11):2348–2361. doi: 10.1021/acsinfecdis.2c00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heindel DW, Koppolu S, Zhang Y, Kasper B, Meche L, Vaiana CA, et al. Glycomic analysis of host response reveals high mannose as a key mediator of influenza severity. Proc. Nation. Acad. Sci. 2020;117(43):26926–26935. doi: 10.1073/pnas.2008203117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaghan TM, Pučić-Baković M, Vučković F, Lee C, Kao D, Wójcik I, et al. Decreased complexity of serum N-glycan structures associates with successful fecal microbiota transplantation for recurrent clostridioides difficile infection. Gastroenterology. 2019;157(6) doi: 10.1053/j.gastro.2019.08.034. 1676-8.e3. [DOI] [PubMed] [Google Scholar]
- 21.Čaval T, Lin YH, Varkila M, Reiding KR, Bonten MJM, Cremer OL, et al. Glycoproteoform profiles of individual patients’ plasma alpha-1-Antichymotrypsin are unique and extensively remodeled following a septic episode. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.608466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heindel DW, Chen S, Aziz PV, Chung JY, Marth JD, Mahal LK. Glycomic analysis reveals a conserved response to bacterial sepsis induced by different bacterial pathogens. ACS. Infect. Diseases. 2022;8(5):1075–1085. doi: 10.1021/acsinfecdis.2c00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Coux A, Tian Y, Deleon-Pennell KY, Nguyen NT, De Castro Brás LE, Flynn ER, et al. Plasma glycoproteomics reveals sepsis outcomes linked to distinct proteins in common pathways. Critic. Care Med. 2015;43(10):2049–2058. doi: 10.1097/CCM.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joenvaara S, Saraswat M, Kuusela P, Saraswat S, Agarwal R, Kaartinen J, et al. Quantitative N-glycoproteomics reveals altered glycosylation levels of various plasma proteins in bloodstream infected patients. PLOS ONE. 2018;13(3) doi: 10.1371/journal.pone.0195006. e0195006-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willems E, Gloerich J, Suppers A, van der Flier M, van den Heuvel LP, van de Kar N, et al. Impact of infection on proteome-wide glycosylation revealed by distinct signatures for bacterial and viral pathogens. iScience. 2023;26(8) doi: 10.1016/j.isci.2023.107257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chatterjee S, Kawahara R, Tjondro HC, Shaw DR, Nenke MA, Torpy DJ, et al. Serum N-glycomics stratifies bacteremic patients infected with different pathogens. J. Clin. Med. 2021;10(3):1–18. doi: 10.3390/jcm10030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer EJ, Nenke MA, Davies ML, Chapman M, Rankin W, Rushworth RL, et al. Corticosteroid-binding globulin deficiency independently predicts mortality in septic shock. J. Clin. Endocrinol. Metabol. 2022;107(6):1636–1646. doi: 10.1210/clinem/dgac035. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, Lee LY, Kawahara R, Abrahams JL, Adamczyk B, Anugraham M, et al. Protein paucimannosylation is an enriched N-Glycosylation signature of human cancers. Proteomics. 2019;19(21-22) doi: 10.1002/pmic.201900010. [DOI] [PubMed] [Google Scholar]
- 29.Hinneburg H, Chatterjee S, Schirmeister F, Nguyen-Khuong T, Packer NH, Rapp E, et al. Post-column make-up flow (PCMF) enhances the performance of capillary-flow PGC-LC-MS/MS-based glycomics. Analytical. Chemistry. 2019;91(7):4559–4567. doi: 10.1021/acs.analchem.8b05720. [DOI] [PubMed] [Google Scholar]
- 30.Jensen PH, NG Karlsson, Kolarich D, Packer NH. Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protocols. 2012;7(7):1299–1310. doi: 10.1038/nprot.2012.063. [DOI] [PubMed] [Google Scholar]
- 31.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome. Res. 2008;7(4):1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 32.Everest-Dass AV, Abrahams JL, Kolarich D, Packer NH, Campbell MP. Structural feature ions for distinguishing N- and O-Linked glycan isomers by LC-ESI-IT MS/MS. J. Am. Soc. Mass Spectrom. 2013;24(6):895–906. doi: 10.1007/s13361-013-0610-4. [DOI] [PubMed] [Google Scholar]
- 33.Ashwood C, Lin C-H, Thaysen-Andersen M, Packer NH. Discrimination of isomers of released N-and O- glycans using diagnostic product ions in negative ion PGC-LC-ESI-MS/MS. J. Am. Soc. Mass Spectrom. 2018;29(6):1194–1209. doi: 10.1007/s13361-018-1932-z. [DOI] [PubMed] [Google Scholar]
- 34.Neelamegham S, Aoki-Kinoshita K, Bolton E, Frank M, Lisacek F, Lütteke T, et al. Updates to the symbol nomenclature for glycans guidelines. Glycobiology. 2019;29(9):620–624. doi: 10.1093/glycob/cwz045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods. 2016;13(9):731–740. doi: 10.1038/nmeth.3901. [DOI] [PubMed] [Google Scholar]
- 36.Gudelj I, Baciarello M, Ugrina I, De Gregori M, Napolioni V, Ingelmo PM, et al. Changes in total plasma and serum N-glycome composition and patient-controlled analgesia after major abdominal surgery. Sci. Rep. 2016;6:31234. doi: 10.1038/srep31234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinbrink JM, Myers RA, Hua K, Johnson MD, Seidelman JL, Tsalik EL, et al. The host transcriptional response to Candidaemia is dominated by neutrophil activation and heme biosynthesis and supports novel diagnostic approaches. Genome. Med. 2021;13(1) doi: 10.1186/s13073-021-00924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakita M, Oyama T, Shirai I, Tanaka S, Akaki K, Abe S, et al. Cell wall N-glycan of Candida albicans ameliorates early hyper- and late hypo-immunoreactivity in sepsis. Commun. Biol. 2021;4(1) doi: 10.1038/s42003-021-01870-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shankar-Hari M, Culshaw N, Post B, Tamayo E, Andaluz-Ojeda D, Bermejo-Martin JF, et al. Endogenous IgG hypogammaglobulinaemia in critically ill adults with sepsis: systematic review and meta-analysis. Inten. Care. Med. 2015;41(8):1393–1401. doi: 10.1007/s00134-015-3845-7. [DOI] [PubMed] [Google Scholar]
- 40.Mikulska M, Calandra T, Sanguinetti M, Poulain D, Viscoli C. Third European Conference on Infections in Leukemia G. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: recommendations from the Third European Conference on Infections in Leukemia. Crit. Care. 2010;14(6):R222. doi: 10.1186/cc9365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat. Rev. Dis. Primers. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- 42.Li F, Yu X, Ye L, Zhou G, Wang L, Luo Y. Clinical value of (1,3)-beta-D-glucan, mannan, antimannan IgG and IgM antibodies in diagnosis of invasive candidiasis. Med. Mycol. 2019;57(8):976–986. doi: 10.1093/mmy/myy158. [DOI] [PubMed] [Google Scholar]
- 43.Li FQ, Ma CF, Shi LN, Lu JF, Wang Y, Huang M, et al. Diagnostic value of immunoglobulin G antibodies against Candida enolase and fructose-bisphosphate aldolase for candidaemia. BMC. Infect. Dis. 2013;13:253. doi: 10.1186/1471-2334-13-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trastoy B, Du JJ, Cifuente JO, Rudolph L, Garcia-Alija M, Klontz EH, et al. Mechanism of antibody-specific deglycosylation and immune evasion by Streptococcal IgG-specific endoglycosidases. Nat. Commun. 2023;14(1):1705. doi: 10.1038/s41467-023-37215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toledo AG, Bratanis E, Velasquez E, Chowdhury S, Olofsson B, Sorrentino JT, et al. Pathogen-driven degradation of endogenous and therapeutic antibodies during streptococcal infections. Nat. Commun. 2023;14(1):6693. doi: 10.1038/s41467-023-42572-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stentzel S, Sundaramoorthy N, Michalik S, Nordengrun M, Schulz S, Kolata J, et al. Specific serum IgG at diagnosis of Staphylococcus aureus bloodstream invasion is correlated with disease progression. J. Proteomics. 2015;128:1–7. doi: 10.1016/j.jprot.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Hendriks A, Kerkman PF, Varkila MRJ, Haitsma Mulier JLG, Ali S, Ten Doesschate T, et al. Glycan-specific IgM is critical for human immunity to Staphylococcus aureus. Cell. Rep. Med. 2024;5(9) doi: 10.1016/j.xcrm.2024.101734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kristic J, Lauc G. The importance of IgG glycosylation-What did we learn after analyzing over 100,000 individuals. Immunol. Rev. 2024 doi: 10.1111/imr.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastoriza Gallego M, Hulen C. Influence of sialic acid and bacterial sialidase on differential adhesion of Pseudomonas aeruginosa to epithelial cells. Colloids. Surf. B. Biointerfaces. 2006;52(2):154–156. doi: 10.1016/j.colsurfb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 50.Chau TH, Chernykh A, Ugonotti J, Parker BL, Kawahara R, Thaysen-Andersen M. Glycomics-Assisted Glycoproteomics Enables Deep and Unbiased N-Glycoproteome Profiling of Complex Biological Specimens. Methods. Mol. Biol. 2023;2628:235–263. doi: 10.1007/978-1-0716-2978-9_16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
In line with community standards, all glycomics PGC-LC-MS/MS raw data is freely available through the GlycoPOST repository (identifier GPST000503). The files can be downloaded from the link https://glycopost.glycosmos.org/preview/1569444274673d22a9c253c (PIN 9656).