Abstract
Background
Previous research has established that chronic kidney disease (CKD) and heart failure with preserved ejection fraction (HFpEF) often coexist. Although we have a preliminary understanding of the potential correlation between HFpEF and CKD, the underlying pathophysiological mechanisms remain unclear. This study aimed to elucidate the molecular mechanisms associated with CKD and HFpEF through bioinformatics analysis.
Methods
Datasets for HFpEF and CKD were obtained from the Gene Expression Omnibus (GEO) database. The R software package “limma” was employed to conduct differential expression analysis. Functional annotation was performed using the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO). We conducted weighted gene co-expression network analysis (WGCNA), correlation analysis with autophagy, ferroptosis, and immune-related processes, as well as transcriptional regulation analysis, immune infiltration analysis, and diagnostic performance evaluation. Finally, the diagnostic potential of the identified hub genes for CKD and HFpEF was assessed using ROC curve analysis (GSE37171).
Results
Differential expression analysis revealed 58 overlapping genes, comprised of 40 up-regulated and 18 down-regulated genes. Both GO and KEGG analyses indicated enriched pathways relevant to both disorders. WGCNA identified 4086 genes associated with CKD. Further comparison with differentially expressed genes (DEGs) identified three hub genes (KLF4, SCD, and SEL1L3) that were linked to autophagy, ferroptosis, and immune processes in both conditions. Additionally, a miRNA-mRNA regulatory network involving 376 miRNAs and 12 transcription factors (TFs) was constructed. ROC curve analysis was performed to evaluate the diagnostic utility of the hub genes for CKD and HFpEF.
Conclusion
This study elucidated shared pathogenic mechanisms and identified diagnostic markers common to both HFpEF and CKD. The identified hub genes show promise as potential tools for early diagnosis and treatment strategies for these conditions.
Keywords: Heart failure with preserved ejection fraction (HFpEF), Chronic kidney disease (CKD), Bioinformatics, Immune infiltration
Highlights
-
•
We developed diagnosis of heart failure and chronic kidney disease by bioinformatics analysis.
-
•
We found that three hub genes in patients with chronic kidney disease and heart failure through functional enrichment analysis and immune infiltration.
-
•
We evaluated diagnostic potential of hub genes by using ROC curve, indicating their potential utility in the clinical diagnosis.
1. Background
Chronic kidney disease (CKD) and heart failure with preserved ejection fraction (HFpEF) frequently coexist [1]. CKD is commonly observed in patients with cardiopulmonary conditions and is associated with increased mortality and morbidity, especially in long-term dialysis patients [2]. Therefore, a comprehensive understanding of the relationship between CKD and HFpEF is crucial for early diagnosis and intervention. Heart failure (HF) represents a significant global cardiovascular health burden, with its prevalence rapidly increasing [3]. According to the 2016 European Society for Cardiology management guidelines, HF is characterized by symptoms and signs arising from structural and functional cardiac abnormalities, leading to elevated intracardiac pressure during rest or stress and a decrease in cardiac output [4,5]. Three subsets of HF have been defined based on ejection fraction: preserved ejection fraction (≥50 %), reduced ejection fraction (<40 %), and mid-range ejection fraction (40–49 %; HFmrEF) [6,7]. Among these, HFpEF has emerged as the most prevalent form of heart failure, particularly in the aging population [8]. Compared to patients with HF with reduced ejection fraction (HFrEF), those with HFpEF are typically older and more likely to be female [3].
CKD is a clinical condition characterized by permanent alterations in the structure or function of the kidneys [9]. It progresses gradually and irreversibly. In clinical practice, various kidney diseases such as glomerulonephritis, occult nephritis, pyelonephritis, Henoch-Schönlein purpura nephritis, lupus erythematosus nephritis, gouty kidney, IgA nephropathy, nephrotic syndrome, membranous nephropathy, diabetic nephropathy, hypertensive nephropathy, and polycystic kidney are diagnosed [5,10]. When the duration of these kidney diseases exceeds three months with difficult healing, abnormal urine and related blood indicators, abnormal renal pathology and imaging findings, or an effective glomerular filtration rate less than 60 %, they are collectively referred to as “chronic kidney disease” [1]. The incidence of CKD has been increasing rapidly due to rising rates of diabetes, hypertension, obesity, and an aging population [11,12]. Understanding the pathophysiology of CKD, particularly its cardiovascular consequences and impact on mortality, is a crucial aspect of the disease [13].
CKD often coexists with heart failure due to factors such as water and sodium retention, erythropoietin deficiency, electrolyte imbalances, and metabolite accumulation. In this study, we employed multiple integrative bioinformatics tools to identify differentially expressed genes (DEGs) in HFpEF and CKD. We collected HFpEF datasets and CKD datasets (GSE192886 and GSE175759) from the Gene Expression Omnibus (GEO) database. The weighted gene co-expression network analysis (WGCNA) method was utilized to analyze hub genes, and a regulatory network was constructed by integrating these hub genes with immune, autophagy, and ferroptosis-related genes. Furthermore, using the miRNet database, we predicted associated miRNAs, and we employed the xCell method to examine immune cell properties and investigate immune modulation in CKD and HFpEF. Finally, in the validation set GSE37171, we evaluated the diagnostic efficacy of our findings in CKD using ROC curve analysis.
2. Methods
2.1. Data acquisition and processing
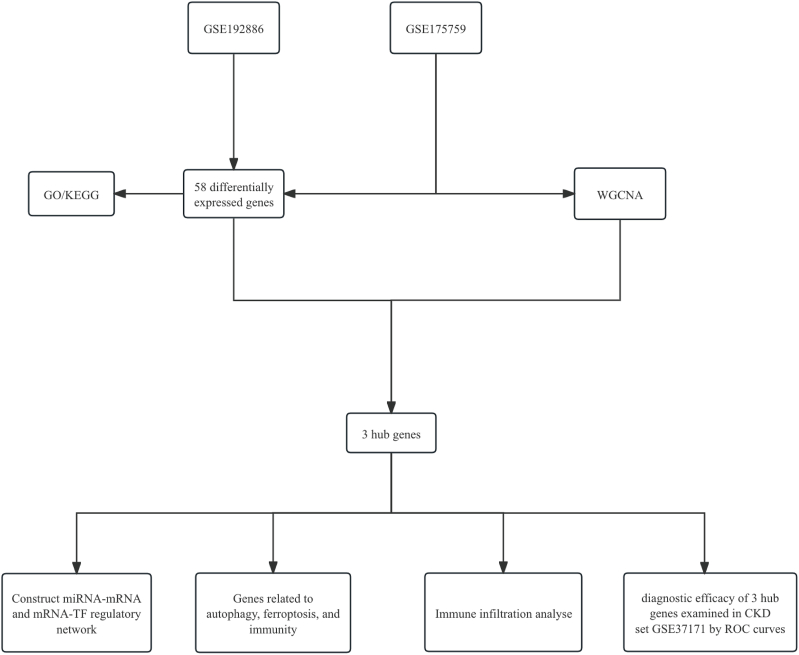
We conducted a search in the GEO database using the MeSH terms “heart failure with preserved ejection fraction” and “chronic kidney disease”. The dataset GSE192886 was downloaded from the NCBI GEO public database (https://www.ncbi.nlm.nih.gov/geo/), with the annotation platform being GPL524676. This dataset comprises transcriptome data obtained from epicardial adipose samples collected from patients with HFpEF (n = 5) and without heart failure (n = 5), resulting in a total of 10 transcriptome profiles. Similarly, we downloaded the GSE175759 dataset, which is annotated under GPL16791. It includes a total of 90 transcriptome profiles derived from various kidney diseases, including 46 IgA nephropathy, 3 diabetes mellitus nephropathy, 3 focal segmental glomerulosclerosis, 3 lupus nephritis, 4 membranous nephropathy, 9 minimal change disease biopsy cores, and 22 nephrectomy controls. RNA sequencing was performed for these samples. GSE37171, including 60 patients with CKD and 20 healthy control subjects, was used for validation. The descriptive statistics of the GEO datasets can be found in the supplementary materials. The workflow chart adopted in this study is shown in Fig. 1.
Fig. 1.
Flow chart depicting the multistep screening strategy used to analyze the bioinformatics data.
2.2. Identification of differentially expressed genes
Gene expression levels were estimated by calculating fragments per kilobase of transcript per million (FPKM) [14]. To identify the DEGs between CKD and HFpEF, we utilized the R package “limma (version 3.60.6)” [15,16]. The criteria for defining DEGs were set as |log2Fold change| > 0.5 and P < 0.05 [8,17]. Heatmaps were generated to visualize the expression levels of the top 50 highly expressed genes, while volcano plots were used to depict the DEGs.
2.3. Functional enrichment analysis
In this study, we employed two methods to perform gene expression enrichment analysis: Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and Gene Ontology (GO) enrichment analysis. These analyses aimed to uncover enriched gene sets associated with the DEGs, as well as important biological pathways and functional annotations [[18], [19], [20]]. For KEGG pathway and GO enrichment analyses of the common DEGs, we utilized Oebiotech (https://cloud.oebiotech.com/). This platform allowed us to explore the Kyoto Encyclopedia of KEGG and GO databases to identify relevant biological pathways and functional annotations.
2.4. WGCNA for co-expression network construction
To identify co-expressed gene modules and investigate the core genes within the network and their connection to the phenotype, we utilized weighted gene co-expression network analysis (WGCNA) (version 1.73) [21]. WGCNA facilitated the construction of co-expression networks for all genes in the dataset. First, we constructed a co-expression network for all genes using the WGCNA R package, selecting the top 10,000 genes with higher variance for further investigation. Second, we determined an appropriate “soft” threshold power to compute intergenic adjacency, defining the strength of network connections. Third, a hierarchical clustering technique was employed to build a clustering tree structure based on the topological overlap matrix (TOM), derived from the weighted adjacency matrix. This process enabled the identification of distinct gene modules represented by branches in the clustering tree, with each module assigned a specific color. Genes with similar expression patterns were grouped into the same module, and the weighted correlation coefficients of thousands of genes were used to categorize them into multiple modules based on their expression profiles.
2.5. MiRNA and transcription factors (TFs) analysis
TFs play a crucial role in the regulation of gene expression. In this study, we employed miRNet (www.mirnet.ca) to explore the correlation between transcription factors and essential binding motifs associated with hub genes [22,23]. By utilizing miRNet, we aimed to identify potential regulatory interactions between transcription factors and the hub genes identified in our analysis.
2.6. Immunity, autophagy and ferroptosis
To investigate the involvement of immunity, autophagy, and ferroptosis in our study, we obtained disease gene sets related to these processes from multiple databases. The immunity-related gene set was acquired from the Immport database (https://www.immport.org/shared/genelists), while the FerrDb database provided the ferroptosis-related gene set. Additionally, the Human Autophagy Database (http://www.autophagy.lu/index.html) supplied the autophagy-related gene set.
2.7. Immune infiltration analysis
To investigate the composition and abundance of immune cells in CKD and HFpEF datasets (GSE175759 and GSE192886), we utilized the “xCell (version 1.1.0)” package. This novel method integrates gene set enrichment with deconvolution approaches to estimate the presence of 64 different types of immune cells. By applying the “xCell (version 1.1.0)” package, we quantified the immune cell infiltration based on the gene expression profiles of CKD and HFpEF samples [24]. This analysis allowed us to gain insights into the immune cell landscape and assess potential differences in immune cell populations between CKD and HFpEF conditions.
2.8. Verification of hub gene expression in CKD datasets and receiver-operating characteristic (ROC) curve analysis for diagnostic efficacy
We utilized the CKD dataset (GSE37171) to identify the hub genes associated with CKD. Subsequently, we employed additional CKD validation datasets to assess the diagnostic utility of these hub genes [28]. To evaluate the diagnostic efficiency of the hub genes, we constructed receiver-operating characteristic (ROC) curves. The ROC curves provided a graphical representation of the sensitivity and specificity of the hub genes in distinguishing CKD cases from controls. The area under the curve (AUC) was calculated to quantify the diagnostic efficacy of each hub gene. A higher AUC value indicates a higher efficiency in diagnosing the disease using the respective gene.
3. Results
3.1. Identification of differentially expressed genes
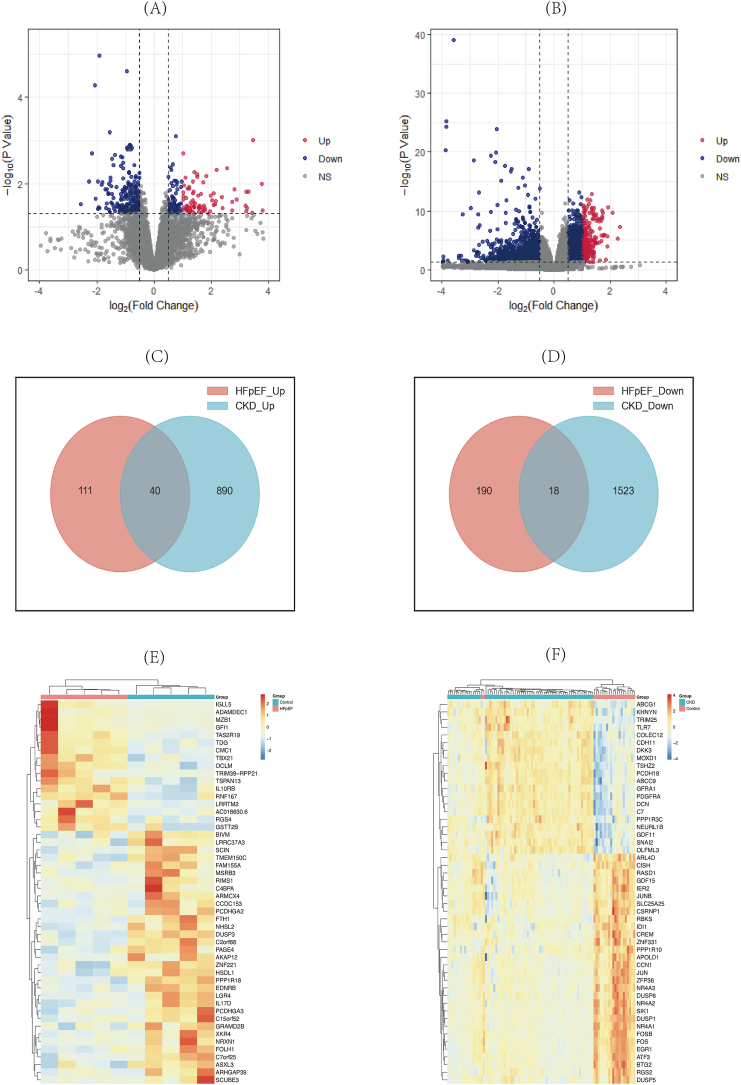
In the HFpEF and CKD groups, a total of 359 differentially expressed genes (DEGs) were identified, with 151 up-regulated and 208 down-regulated in HFpEF, and 2471 DEGs found in CKD, including 930 up-regulated and 1541 down-regulated (Fig. 2A–B). Heatmap analysis displayed the top 50 genes (Fig. 2C–D). Furthermore, 58 DEGs were commonly shared between HFpEF and CKD, with 40 up-regulated and 18 down-regulated (Fig. 2E–F).
Fig. 2.
Identification of differentially expressed genes (DEGs). (A) The volcano plot of GSE192886. (B) The volcano plot of GSE175759. (C, D) Venn plots showing common up-regulated and down-regulated DEGs shared by GSE192886 and GSE175759. (E) The top 50 genes with the most remarkable expression changes of GSE192886. (F) The top 50 genes with the most remarkable expression changes of GSE175759.
3.2. Functional enrichment analysis
To investigate the biological roles and pathways associated with the 58 DEGs, we conducted GO and KEGG enrichment analyses. A total of 546 significantly related biological functions were identified, along with 84 enriched KEGG signaling pathways. Based on the top 30 significant terms in GO (Fig. 3A), the analyses revealed that the DEGs were primarily involved in processes such as adaptive immune response, B cell activation, external side of the plasma membrane, immunological synapse, SH2 domain binding, MHC class I protein binding, and viral protein interactions with cytokines and their receptors. The top 20 signaling pathways in KEGG analysis was presented (Fig. 3B). The common genes are mainly focused on the pathways related to cytokine-cytokine receptor interaction, NF-kappa B signaling pathway, primary immunodeficiency, and B cell receptor signaling pathway.
Fig. 3.
Functional enrichment analysis. (A) Gene Ontology (GO) function analysis results on common differentially expressed genes (DEGs). (B)The bubble plots showing the KEGG enrichment analysis results, including biological process, cellular component and molecular function of genes.
3.3. Construction of weighted gene co-expression network
WGCNA was performed to identify the most relevant gene modules and investigate important genes associated with CKD in more detail. A soft-thresholding power of 21 was selected based on average connectivity and scale independence (Fig. 4A) [8]. Using this power, a total of 15 modules were generated, and their cluster dendrogram is shown in Fig. 4B. Among these modules, the turquoise module displayed the highest positive correlation with CKD (3770 genes, r = 0.46, p = 4e-06), while the grey module showed the most negative relation to CKD (316 genes, r = −0.57, p = 5e-09). Thus, the turquoise and grey modules were considered key modules for further analysis. Furthermore, a strong association between module membership and gene significance was observed in the turquoise (r = 0.42, p = 5e-161) and grey modules (r = −0.81, p = 9.2e-75), respectively (Fig. 4D–E). Consequently, a total of 4086 significant genes strongly linked to CKD were identified within the turquoise and grey modules. Additionally, by intersecting the crucial genes from WGCNA with the DEGs, three genes were identified as critical: SEL1L Family Member 3 (SEL1L3), KLF Transcription Factor 4 (KLF4), and Stearoyl-CoA Desaturase (SCD) (Fig. 4F).
Fig. 4.
Screening of key module genes in the integrated CKD dataset via WGCNA and identification of CKD key genes through the intersection of key module genes and DEGs. (A) The best β value was found using the scale-free topology model; β = 21 was selected as the soft threshold based on scale independence and average connectedness. (B) A dendrogram of the grouping of CKD genes, where various colors correspond to different modules. (C) The heatmap that shows how the state of CKD and module eigengenes relate to another. The state of CKD and the correlation between the module eigengenes (upper) and p-value (bottom) were displayed. (D) The gene significance of genes in the turquoise module and the membership in the module are correlated. (E) The plot showing correlation between a gene's significance and its participation in the grey module. (F) By using the Venn diagram to show where critical module genes and DEGs overlap, a total of three key genes in CKD were found.
3.4. Transcriptional regulation analysis
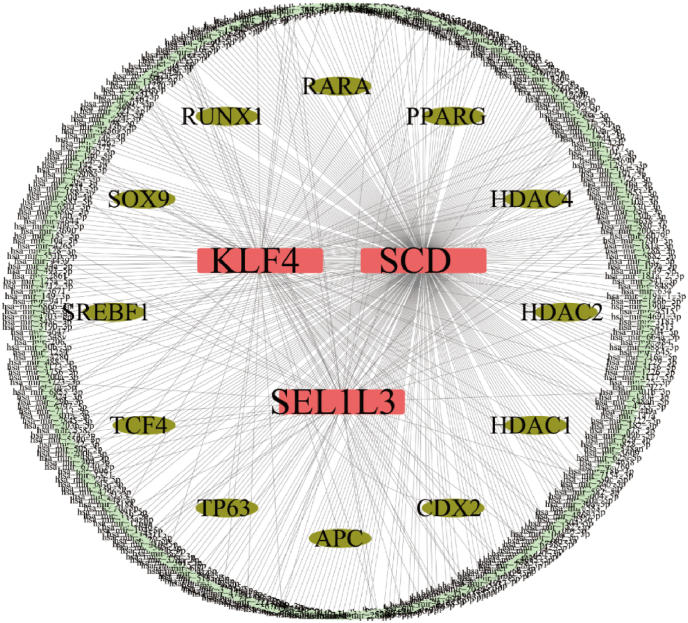
The miRNA-mRNA network and mRNA-TF network for KLF4, SCD, and SEL1L3 were established using the miRNet database (Fig. 5). A total of 376 miRNAs and 12 transcription factors associated with these genes were identified, including regulators of the WNT signaling pathway such as Adenomatous Polyposis Coli (APC), Caudal Type Homeobox 2 (CDX2), Histone Deacetylase 1 (HDAC1), Histone Deacetylase 2 (HDAC2), Histone Deacetylase 4 (HDAC4), Peroxisome Proliferator Activated Receptor Gamma (PPARG), Retinoic Acid Receptor Alpha (RARA), RUNX Family Transcription Factor 1 (RUNX1), SRY-Box Transcription Factor 9 (SOX9), Sterol Regulatory Element Binding Transcription Factor 1 (SREBF1), Transcription Factor 4 (TCF4), and Tumor Protein P63 (TP63).
Fig. 5.
miRNA-mRNA-TFs network. miRNA networks of hub genes, pink for mRNA, pale green for miRNA and bottle green for TFs (transcription factors annotated to motifs).
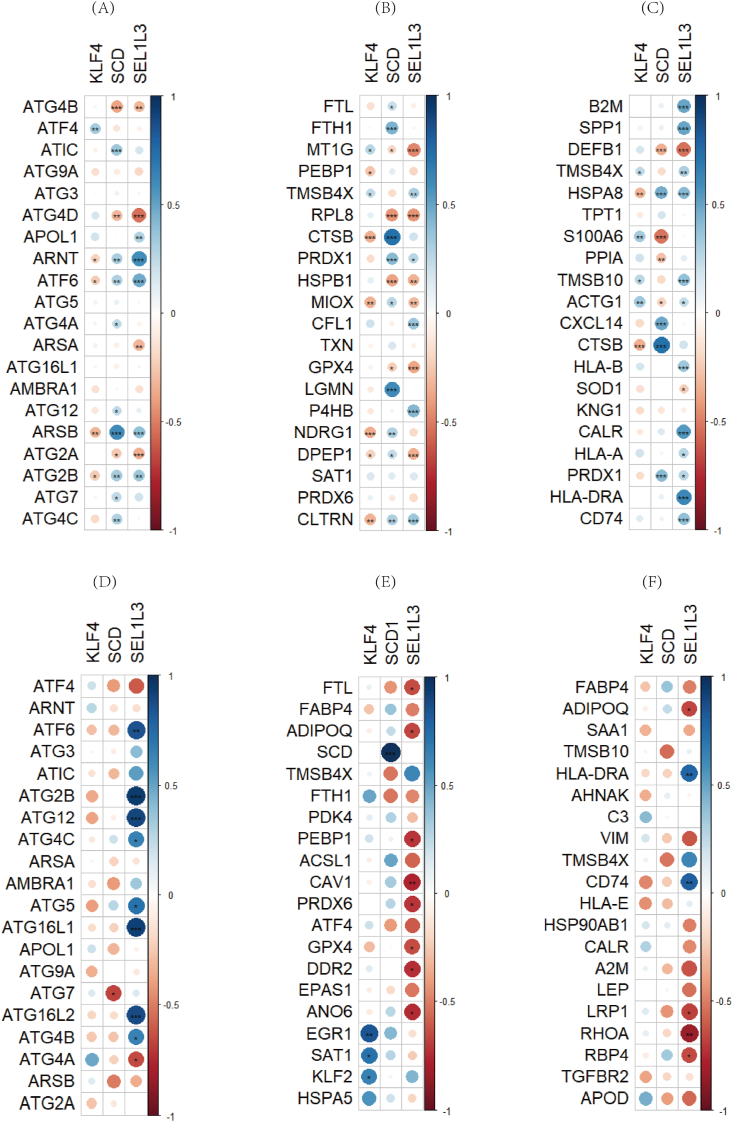
3.5. Relationship of hub genes to autophagy, ferroptosis, and immunological factors
The expression of hub genes in CKD and HFpEF correlated with autophagy, ferroptosis, and immunological factors to varying degrees. TMSB4X (thymosin beta 4 X-linked gene) was identified as both a ferroptosis-related gene and an immune-related gene (Fig. 6). It exhibited significant positive correlation with SCD and negative correlation with KLF4 and SEL1L3 in both diseases [25].
Fig. 6.
Relationship of hub genes to other genes. (A–C) Correlation of hub genes in CKD with autophagy, ferroptosis and immune factors. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. (D–F) Correlation of hub genes in HFpEF with autophagy, ferroptosis and immune factors∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.6. Immune cell infiltration in CKD and HFpEF
The xCell algorithm was employed to characterize immune cell subsets and explore immune regulation in CKD and HFpEF. Analysis revealed the presence of 64 different types of immune cells in each sample (Fig. 7A–C), with significant differences observed in 20 immune cell subpopulations. In comparison to the control group, CKD displayed higher proportions of aDCs and smooth muscle cells, while exhibiting lower proportions of basophils, pro B-cells, neurons, mast cells, and class-switched memory B-cells (Fig. 7B) [26]. Moreover, the composition of immune cells differed between the HFpEF group and the control group, with HFpEF showing higher proportions of CD8+ Effector Memory T Cells (CD8+ Tem) and memory B-cells compared to the control group (Fig. 7D).
Fig. 7.
Immune cell infiltration analysis. (A) The heatmap displaying the immune cell proportions of CKD. (B)Violin plot showing immune cells between CKD and control groups. (C) The heatmap displaying the immune cell proportions between HFpEF and control groups. (D)Violin plot showing the comparison of CD8+ Tem and memory B-cell between HFpEF and control groups. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ∗∗∗∗P < 0.0001.
3.7. Validation of hub genes
We used CKD datasets to identify hub genes. In another CKD dataset, GSE37171, KLF4 and SCD were significantly down-regulated (Fig. 8A), consistent with the findings from GSE192886 and GSE175759. However, SEL1L3 did not show consistent down-regulation in GSE37171.
Fig. 8.
(A)Violin plot showing the comparison of 3 hub genes levels between CKD(GSE37171) and control groups. (B ∼ D)The ROC curve for the diagnostic performance of each candidate biomarker including KLF4(B), SCD(C) and SEL1L3(D),including AUC (area under the curve).
3.8. ROC curve analysis for diagnostic efficacy in CKD and HFpEF
ROC curves were constructed to evaluate the diagnostic efficacy of the three hub genes (KLF4, SCD, SEL1L3) in the validation set, GSE37171. The AUC values for KLF4, SCD, and SEL1L3 were 0.917, 0.662, and 0.907, respectively (Fig. 8B–D). These results indicate that the three hub genes can serve as valuable indicators for the diagnosis of chronic kidney disease [27].
4. Discussion
Prior research has established that chronic kidney disease (CKD) and heart failure with preserved ejection fraction (HFpEF) frequently coexist, which can significantly impact patient outcomes and complicate management strategies. Although there has been a slight decline in the incidence of heart failure over time, its prevalence persists in rising due to advancements in heart failure treatments and increased life expectancy in the population [28]. This trend poses ongoing challenges for public health and healthcare systems.
The widespread application of microarray and sequencing technologies has empowered researchers to investigate the molecular patterns and underlying mechanisms of various diseases with unprecedented ease, facilitating deeper insights into complex biological processes. Furthermore, the increasing use of bioinformatics analysis and machine learning tools enhances our ability to explore novel genes, potential diagnostic and prognostic biomarkers, disease mechanisms, and prospective therapeutic strategies [29]. These tools enable more comprehensive data analysis and interpretation. These instruments not only enhance our understanding of the nature of diseases but also offer significant support for developing innovative medical approaches, which could transform clinical practice and patient management. By employing a wide range of comprehensive bioinformatics analysis techniques, this research pioneers the investigation of core genes associated with CKD and HFpEF, thereby elucidating the intricate relationship between these two conditions.
In this research, we identified a total of 58 common DEGs (40 up-regulated and 18 down-regulated) in HFpEF and CKD. Results from the GO and KEGG suggested that inflammatory and immune mechanisms are vital in both conditions. The DEGs were predominantly enriched in immunological and inflammatory pathways, including “Natural killer cell mediated cytotoxicity”, “Cytokine-cytokine receptor interaction”, “Viral protein interaction with cytokine and cytokine receptor”, “B cell receptor signaling pathway”, and “Primary immunodeficiency”, as revealed by KEGG enrichment and GO biological process annotation analyses. This suggests that inflammatory-immune pathways could underlie the connection between HFpEF and CKD [30].
Additionally, by constructing a Weighted Gene Co-Expression Network Analysis (WGCNA) network, we identified 15 gene modules associated with CKD. WGCNA is particularly advantageous for elucidating relationships between clinical traits and co-expression modules. By merging the turquoise and grey modules, we identified a total of 4086 genes, which may play significant roles in the pathophysiology of CKD. After intersecting these WGCNA intersection genes with the 58 DEGs, we identified three key genes: KLF4, SCD, and SEL1L3, which may serve as critical biomarkers for CKD and HFpEF. Unlike other bioinformatics methodologies, WGCNA specifically focuses on the correlation between clinical traits and co-expression modules, yielding results that are more comprehensive, reliable, and biologically relevant. Lastly, these three biomarkers demonstrated remarkable diagnostic performance for CKD in the GSE37171 dataset, suggesting their potential as critical targets for developing therapeutic strategies in HFpEF patients [27].
KLF4, Kruppel Like Factor 4, is a member of the evolutionarily conserved family of zinc finger transcription factors [31]. KLF4 plays a role in regulating mitochondrial biogenesis, metabolic dynamics, and autophagic clearance. In myeloid cells, KLF4 could provide protection against inflammatory CKD by inhibiting M1 polarization [32]. Furthermore, KLF4 contributes to the regulation of renal physiological functions and the progression of fibrosis [33]. Hence, KLF4 deletion intensifies the expression of M1 cytokines and chemokines such as TNFα, CCL2, and CCL5, leading to exacerbated glomerular and tubular damage and increased kidney fibrosis, which accelerates the progression of CKD [34]. In addition to its role in kidney disease, KLF4 also serves as a crucial regulatory factor for cardiac mitochondrial homeostasis. Reduced expression of KLF4 results in a significant decrease in ATP levels and an increase in reactive oxygen species (ROS) production in the myocardium after TAC, ultimately leading to an increase in cardiac metabolic demand and an elevated risk of heart failure [35]. Simultaneously, KLF4 influences the process of autophagic clearance. Diminished KLF4 expression can impair autophagic flux, causing potential myocarditis and leading to cardiac dysfunction [36].
SCD, a rate-limiting enzyme in the biosynthesis of monounsaturated fatty acids (MUFAs), is closely colocalized with diacylglycerol acyltransferase-2 in the endoplasmic reticulum membrane [37,38]. SCD can increase endogenous MUFA synthesis, thereby elevating the risk of metabolic diseases [39]. According to previous research, SCD could potentially be used to enhance heart performance by reprogramming myocardial metabolism [40]. The overexpression of SCD is associated with increased expression of genes involved in fatty acid transport and lipid synthesis in the heart. This overexpression also leads to elevated levels of free fatty acids, diacylglycerols (DAGs), triglycerides, and ceramides in the heart. It has the potential to reduce glucose transport and metabolism, induce metabolic changes, promote myocardial cell necrosis and apoptosis, and increase the risk of HF [40]. Previous studies have indicated that SCD overexpression can lead to hypertriglyceridemia, and serum MUFA levels are positively correlated with inflammation in CKD patients. Furthermore, lipid accumulation in renal parenchyma is detrimental to renal function. Free fatty acids (FFAs), in particular, are harmful to the kidneys. Excessive intake of FFAs can damage proximal tubular epithelial cells, podocytes, and the tubulointerstitial tissue through various mechanisms. One such mechanism involves FFAs increasing the production of ROS and lipid peroxidation, leading to mitochondrial damage and tissue inflammation, eventually resulting in glomerular and tubular lesions [41]. Therefore, upregulation of SCD can exacerbate heart failure complicated with CKD.
SEL1L3, a member of the Sel-1 Suppressor of Lin-12-Like (SEL1L) family, is located within the endoplasmic reticulum (ER) and plays a crucial role in facilitating ER-associated degradation. This degradation process is initiated by ER stress, which encourages the breakdown of misfolded proteins. The ER stress response is activated by the Bip/PERK/eIF2α axis, which is regulated by SEL1L3. ER-associated degradation (ERAD), a downstream mechanism to maintain ER homeostasis, gets stimulated by SEL1L [42]. SEL1L3 has been associated with the development of several types of cancer. According to prior studies, SEL1L3 is a promising prognostic marker for lung adenocarcinoma, melanoma, and colorectal cancer. ER stress promotes cardiomyocyte apoptosis, leading to cardiovascular dysfunction and involvement in the pathogenesis of HF [43]. Simultaneously, increasing evidence has stressed the important role of ER stress in the pathogenesis of various monogenic glomerular and tubular diseases. ER stress has been reported to be associated with some pathogenic effects in the kidney, particularly concerning the podocyte slit diaphragm and tubulointerstitium. ER stress can harm podocytes and intensify kidney damage by elevating the expression of Monocyte Chemoattractant Protein-1 (MCP-1), a key player in the inflammation associated with diabetic nephropathy. Furthermore, the unfolded protein response (UPR) pathway, a stress signal of ER stress, contributes to tubular cell apoptosis and resulting fibrosis [44].
We conducted a correlation analysis of the three hub genes using information from the Immport database, FerrDb database, and Human Autophagy Database to understand the underlying mechanisms of autophagy, ferroptosis, and immune factors. The correlation between Thymosin Beta 4 X-Linked (TMSB4X) and the hub genes is consistent in both diseases. Previous studies have demonstrated that TMSB4X has a protective effect in CKD, as it preserves the actin cytoskeleton of podocytes and maintains the integrity of the glomerular filtration barrier. Simultaneously, TMSB4X inhibits macrophage accumulation in the renal cortex, exerting anti-inflammatory effects that slow down disease progression [45].
Transcription factors greatly aid in the regulation of gene expression. In this research, we utilized miRNet to identify hub gene-corresponding transcription factors and significant binding motifs [46]. We constructed an mRNA‒miRNA regulatory network and identified 376 miRNAs and 12 TFs. In the immune cell infiltration analysis, we observed that CKD exhibited higher proportions of activated Dendritic Cells (aDC), Smooth Muscle cells and CD8+ Central Memory T cell (CD8+ Tcm), whereas lower proportions of Basophils, Pro B-cells, Neurons, Mast cells, and Class-switched memory B-cells. Previous studies have shown that CD8+ T cells can be activated through the cGAS-STING mediated signaling cascade and initiate cardiomyocyte apoptosis, thereby promoting the development of HF. Kurt Brassington et al. found that the interaction between cardiac cytotoxic memory CD8+T cells and over stressed cardiomyocytes is crucial for the development of non-ischemic hypertensive cardiac fibrosis in a hypertensive mouse model. Specifically, CD8+T cells activate T cells by expressing the innate stress sensing receptor NKG2D and interacting with the RAE-1 ligand on the surface of cardiomyocytes, leading to the release of perforin and triggering cardiomyocyte apoptosis. This process increases the number of macrophages expressing TGF-b1, thereby promoting fibrosis. Kyoko Komai et al. found that depletion of CD8+T cells can induce cardioprotective hypertrophy in a heart failure model caused by cardiac stress overload, characterized by increased expression of mitochondrial genes and growth factor receptor genes. In addition, CD8+T cells play a crucial role in myocardial adaptive response by regulating the transformation of cardiac resident and infiltrating macrophages into cardioprotective macrophages expressing growth factor genes such as Areg, Osm, and Igf1 [47,48]. In our results, CD8+ Tem T cells and Memory B-cells were significantly upregulated. Furthermore, the expression of three diagnostic markers was closely related to the infiltration of multiple immune cells. This finding underscores the crucial role of immune mechanisms in the development of inflammation and fibrosis in HFpEF and CKD patients.
Our study does have certain limitations. First, chronic kidney disease (CKD) is a broad term encompassing significant heterogeneity, and we were unable to analyze specific types of CKD. This variability presents a challenge, as our analysis did not differentiate between the various forms of CKD, which could affect the generalizability of our results. Second, while we identified three hub genes (KLF4, SCD, and SEL1L3), the functional roles of these genes in the context of CKD and heart failure with preserved ejection fraction (HFpEF) require further validation through experimental studies. Notably, SEL1L3 was significantly down-regulated in the GSE37171 dataset, which is inconsistent with observations from the GSE192886 and GSE175759 datasets. This discrepancy underscores the need for additional experimental and clinical research to validate our findings. Furthermore, the applicability of our results to diverse populations and clinical settings needs to be explored. Future studies should aim to replicate our findings in larger, more heterogeneous cohorts to assess the robustness of the identified biomarkers. Additionally, investigating the mechanistic pathways involving these hub genes through targeted in vitro experiments would provide deeper insights into their contributions to the pathogenesis of CKD and HFpEF. Overall, we recognize that further experimental validation is essential to confirm the clinical relevance of our findings and to elucidate the underlying biological mechanisms. Addressing these limitations will enhance the understanding of the complex interplay between CKD and HFpEF and inform future research directions.
5. Conclusions
In conclusion, we utilized transcriptome data in our study to three hub genes (KLF4, SCD, SEL1L3) in patients with CKD and HFpEF. Our findings enhance the understanding of the molecular mechanisms at play in CKD and HFpEF and propose potential therapeutic targets for HFpEF and CKD. Further validation and functional studies are required to confirm the clinical utility of these markers and their role in disease progression and treatment.
Author contributions
As the principal investigator, Fang Jia and Hui Wang conceptualized the study. Can Hou took the initial steps in drafting the manuscript and conducting the experimental procedures. Jiayi Xu, Min Zhou and Wanying Jiang were responsible for the data analysis. Xiaofei Wang, Junyu Huo and Tong Su prepared figures.
Data availability statement
The datasets presented in this study can be found in online repositories.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Funding
This work was Funding from Major Technology Projects of Changzhou Health Commission (ZD202212), Changzhou Health Talents Overseas Training Program (Chang Health Science Education❲2023❳252), Changzhou “Longcheng Talent Program"(CQ20220127), Changzhou Key Medical Discipline (CZXK202202), Changzhou Sci&Tech Program (CJ20235085), National Natural Science Foundation of China (81700351), Post-Doctoral Foundation of Jiangsu Province (2018K074B), Changzhou High-Level Medical Talents Training Project and Medical Youth Talent Project of Changzhou (QN201707), National Natural Science Foundation of China (82405245)
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge Gene Expression Omnibus (GEO) database for providing public data on Chronic Kidney Disease (CKD) patients and Heart Failure with preserved ejection fraction (HFpEF) patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2024.101911.
Contributor Information
Hui Wang, Email: missjasmine@126.com.
Fang Jia, Email: jiafangsjs@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
The data that has been used is confidential.
References
- 1.Zannad F., Rossignol P. Cardiorenal syndrome revisited. Circulation. 2018;138(9):929–944. doi: 10.1161/CIRCULATIONAHA.117.028814. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee D., Rosano G., Herzog C.A. Management of heart failure patient with CKD. Clin. J. Am. Soc. Nephrol. 2021;16(7):1131–1139. doi: 10.2215/CJN.14180920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmonds S.J., et al. Cellular and molecular differences between HFpEF and HFrEF: a step ahead in an improved pathological understanding. Cells. 2020;9(1) doi: 10.3390/cells9010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich P.A., et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2022;145(18) doi: 10.1161/CIR.0000000000001063. 1032. [DOI] [PubMed] [Google Scholar]
- 5.Eknoyan G. Chronic kidney disease definition and classification: the quest for refinements. Kidney Int. 2007;72(10):1183–1185. doi: 10.1038/sj.ki.5002576. [DOI] [PubMed] [Google Scholar]
- 6.House A.A., et al. Heart failure in chronic kidney disease: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–1317. doi: 10.1016/j.kint.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Roger V.L. Epidemiology of heart failure: a contemporary perspective. Circ. Res. 2021;128(10):1421–1434. doi: 10.1161/CIRCRESAHA.121.318172. [DOI] [PubMed] [Google Scholar]
- 8.Wang A., et al. Heart failure with preserved ejection fraction and non-alcoholic fatty liver disease: new insights from bioinformatics. ESC Heart Fail. 2023;10(1):416–431. doi: 10.1002/ehf2.14211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akchurin O.M. Chronic kidney disease and dietary measures to improve outcomes. Pediatr Clin North Am. 2019;66(1):247–267. doi: 10.1016/j.pcl.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levey A.S., et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80(1):17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 11.Vijay K., Neuen B.L., Lerma E.V. Heart failure in patients with diabetes and chronic kidney disease: challenges and opportunities. Cardiorenal Med. 2022;12(1):1–10. doi: 10.1159/000520909. [DOI] [PubMed] [Google Scholar]
- 12.Haynes R., et al. Chronic kidney disease, heart failure and neprilysin inhibition. Nephrol. Dial. Transplant. 2020;35(4):558–564. doi: 10.1093/ndt/gfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebert T., et al. Inflammation and premature ageing in chronic kidney disease. Toxins. 2020;12(4) doi: 10.3390/toxins12040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao L., et al. Identification of the susceptibility genes for COVID-19 in lung adenocarcinoma with global data and biological computation methods. Comput. Struct. Biotechnol. J. 2021;19:6229–6239. doi: 10.1016/j.csbj.2021.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritchie M.E., et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa-Silva J., Domingues D., Lopes F.M. RNA-Seq differential expression analysis: an extended review and a software tool. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0190152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akita K., et al. Comprehensive proteomics profiling identifies circulating biomarkers to distinguish hypertrophic cardiomyopathy from other cardiomyopathies with left ventricular hypertrophy. Circ Heart Fail. 2024 doi: 10.1161/CIRCHEARTFAILURE.124.012434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian A., et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 2021;49(D1):D325–d334. doi: 10.1093/nar/gkaa1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanehisa M., et al. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–d592. doi: 10.1093/nar/gkac963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M., et al. Construction of potential miRNA-mRNA regulatory network in COPD plasma by bioinformatics analysis. Int J Chron Obstruct Pulmon Dis. 2020;15:2135–2145. doi: 10.2147/COPD.S255262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang L., et al. miRNet 2.0: network-based visual analytics for miRNA functional analysis and systems biology. Nucleic Acids Res. 2020;48(W1):W244–w251. doi: 10.1093/nar/gkaa467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aran D., Hu Z., Butte A.J. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. doi: 10.1186/s13059-017-1349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He Y.J., et al. Discovery and validation of Ferroptosis-related molecular patterns and immune characteristics in Alzheimer's disease. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.1056312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W., et al. Identification of biomarkers and immune infiltration in acute myocardial infarction and heart failure by integrated analysis. Biosci. Rep. 2023;43(7) doi: 10.1042/BSR20222552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y., et al. Identification of biomarkers for the diagnosis of chronic kidney disease (CKD) with non-alcoholic fatty liver disease (NAFLD) by bioinformatics analysis and machine learning. Front. Endocrinol. 2023;14 doi: 10.3389/fendo.2023.1125829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savarese G., et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023;118(17):3272–3287. doi: 10.1093/cvr/cvac013. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y., et al. Exploring the common mechanism of vascular dementia and inflammatory bowel disease: a bioinformatics-based study. Front. Immunol. 2024;15 doi: 10.3389/fimmu.2024.1347415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu E., et al. Screening of immune-related secretory proteins linking chronic kidney disease with calcific aortic valve disease based on comprehensive bioinformatics analysis and machine learning. J. Transl. Med. 2023;21(1):359. doi: 10.1186/s12967-023-04171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Z., He J., Xie K. KLF4 transcription factor in tumorigenesis. Cell Death Discov. 2023;9(1):118. doi: 10.1038/s41420-023-01416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghaleb A.M., Yang V.W. Krüppel-like factor 4 (KLF4): what we currently know. Gene. 2017;611:27–37. doi: 10.1016/j.gene.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H., et al. KLF4 regulates TERT expression in alveolar epithelial cells in pulmonary fibrosis. Cell Death Dis. 2022;13(5):435. doi: 10.1038/s41419-022-04886-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen Y., et al. KLF4 in macrophages attenuates tnfα-mediated kidney injury and fibrosis. J. Am. Soc. Nephrol. 2019;30(10):1925–1938. doi: 10.1681/ASN.2019020111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., et al. Klf4 deficiency exacerbates myocardial ischemia/reperfusion injury in mice via enhancing ROCK1/DRP1 pathway-dependent mitochondrial fission. J. Mol. Cell. Cardiol. 2023;174:115–132. doi: 10.1016/j.yjmcc.2022.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Liao X., et al. Kruppel-like factor 4 is critical for transcriptional control of cardiac mitochondrial homeostasis. J. Clin. Invest. 2015;125(9):3461–3476. doi: 10.1172/JCI79964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin C.I., et al. A high-fructose-high-coconut oil diet induces dysregulating expressions of hippocampal leptin and stearoyl-CoA desaturase, and spatial memory deficits in rats. Nutrients. 2017;9(6) doi: 10.3390/nu9060619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velliquette R.A., et al. Regulation of human stearoyl-CoA desaturase by omega-3 and omega-6 fatty acids: implications for the dietary management of elevated serum triglycerides. J Clin Lipidol. 2009;3(4):281–288. doi: 10.1016/j.jacl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Balatskyi V.V., Dobrzyn P. Role of stearoyl-CoA desaturase 1 in cardiovascular physiology. Int. J. Mol. Sci. 2023;24(6) doi: 10.3390/ijms24065531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobrzyn P., Bednarski T., Dobrzyn A. Metabolic reprogramming of the heart through stearoyl-CoA desaturase. Prog. Lipid Res. 2015;57:1–12. doi: 10.1016/j.plipres.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Gai Z., et al. Lipid accumulation and chronic kidney disease. Nutrients. 2019;11(4) doi: 10.3390/nu11040722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H., et al. SEL1L3 as a link molecular between renal cell carcinoma and atherosclerosis based on bioinformatics analysis and experimental verification. Aging (Albany NY) 2023;15(22):13150–13162. doi: 10.18632/aging.205227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren J., et al. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021;18(7):499–521. doi: 10.1038/s41569-021-00511-w. [DOI] [PubMed] [Google Scholar]
- 44.Maekawa H., Inagi R. Stress signal network between hypoxia and ER stress in chronic kidney disease. Front. Physiol. 2017;8:74. doi: 10.3389/fphys.2017.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mason W.J., Vasilopoulou E. The pathophysiological role of thymosin β4 in the kidney glomerulus. Int. J. Mol. Sci. 2023;24(9) doi: 10.3390/ijms24097684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J., et al. Exploring the molecular mechanism of comorbidity of autism spectrum disorder and inflammatory bowel disease by combining multiple data sets. J. Transl. Med. 2023;21(1):372. doi: 10.1186/s12967-023-04218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brassington K., et al. Crosstalk between cytotoxic CD8+ T cells and stressed cardiomyocytes triggers development of interstitial cardiac fibrosis in hypertensive mouse hearts. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komai K., et al. Single-cell analysis revealed the role of CD8(+) effector T cells in preventing cardioprotective macrophage differentiation in the early phase of heart failure. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.763647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories.
The data that has been used is confidential.