Abstract
Mutations in the human nuclear factor-κB2 gene (NFKB2) are associated with common variable immunodeficiency (CVID) or combined immunodeficiency diseases (CID), characterized by B-cell lymphopenia, hypogammaglobulinemia, and T-cell dysfunction. This study investigated whether B cells with NFKB2 mutations exhibit intrinsic impairments in activation, class-switch recombination, and differentiation. We analyzed five patients from four unrelated families with CVID, each carrying a heterozygous NFKB2 mutation: P1 (C.2595_2614del, p.A867Gfs*12), P2 (C.2597G > A, p.S866N), P3 (C.2540dupT, p.R848Efs*38), and P4 and P5 (C.2570_2571insCAGCACA, p.A860Qfs*28). The patients with frameshift mutations (P1, P3, P4, and P5) exhibited truncated proteins detectable in their peripheral blood mononuclear cells, while P2 had a missense mutation. All identified mutations disrupted the processing of p100 into the active p52 form, resulting in NF-κB2 loss of function and IκBδ gain of function. Clinically, P1, P2, and P3 exhibited B-cell lymphopenia, and all five patients presented with hypogammaglobulinemia. Notably, P2 exhibited a markedly low B-cell count, associated with increased proportions of memory B and IgD−CD27− double-negative B cells. In vitro experiments with naïve B cells from P1 and P4 demonstrated decreased survival, impaired activation, and reduced differentiation into CD27+IgD− cells and plasmablasts, while class-switch recombination was unaffected. These findings reveal novel B-cell intrinsic functional defects in patients with NFKB2 mutations.
Keywords: NFKB2, CVID, B-cell differentiation, class-switch recombination
B cells with NFKB2 mutations exhibit impaired activation (①) and differentiation into plasma cells (②) in response to T-I stimuli, such as CpG. Although they retain the ability to undergo Ig gene CSR (③), these mutant B cells show impaired activation and differentiation into plasma cells (④) and memory B-like cells (⑤) in response to both T-I and T-D stimuli, such as CMIL2 and CD40L + IL-4. T-I: T-independent; T-D: T-dependent; CSR: class-switch recombination; GC B: germinal center B cell; PC: plasma cell. Red arrows indicate an increase or decrease in a B-cell subset.
Graphical Abstract
Graphical Abstract.
Introduction
Common variable immunodeficiency (CVID), one of the most prevalent inborn errors of immunity (IEI) with an incidence of 1/10 000–1/25 000, is a clinically and genetically heterogeneous disease characterized by hypogammaglobulinemia and a poor response to antigens [1, 2]. Affected individuals frequently present with recurrent respiratory infections as well as immune dysregulation or autoimmune symptoms, with single-gene mutations accounting for 10–20% of cases [3, 4]. Mutations in the nuclear factor-κB2 gene (NFKB2) have been associated with CVID or combined immunodeficiency (CID) [5–7].
In mammals, the NF-κB family comprises five members, such as NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB, and c-REL [8]. NF-κB proteins typically bind to members of the inhibitor of κB (IκB) family and exist as components of inactive cytoplasmic complexes [9, 10]. NF-κB activation occurs through canonical and non-canonical pathways. The canonical pathway mediates the activation of NF-κB1 p50, RelA, and c-REL (canonical NF-κB family members), primarily triggering a broad inflammatory response. The non-canonical pathway selectively activates NF-κB2 p52 and RelB (non-canonical NF-κB family members), affecting B-cell maturation, peripheral lymphocyte development, germinal center reactions, bone metabolism, and thymus development [11]. NFKB1 and NFKB2 are initially translated into precursor proteins p105 and p100, respectively. Both contain a carboxyl (C)-terminal IκB homology region that functions as an IκB-like NF-κB inhibitor [12]. Due to their NF-κB inhibitory activity, p105 and p100 are sometimes referred to as IκBγ and IκBδ, respectively [12]. The proteasome mediates the selective degradation of the C-terminal region of p105 and p100, producing the mature NF-κB1 p50 and NF-κB2 p52 and interrupting the IκB-like function of these precursor proteins [11].
In contrast to the canonical NF-κB pathway, which exhibits a rapid but transient activation in response to stimulation from various immune receptors, the non-canonical NF-κB pathway is characterized by slow and persistent activation [13, 14]. Ligands involved in the non-canonical pathway include lymphotoxin β (LT-β), B-cell activation factor (BAFF), CD40 ligand (CD40L), and receptor activator of NF-κB ligand (RANKL). These extracellular signals activate NF-κB-inducing kinase (NIK; also known as MAP3K14), which phosphorylates and activates the IKKα complex. This, in turn, phosphorylates the p100 C-terminal residues [serine (S) 866 and 870] of the p100/RelB dimer, leading to the recruitment of the E3 ubiquitin ligase β-transducin repeat-containing protein (β-TRCP). This process results in the ubiquitination and subsequent proteasomal processing of the p100 into the active form of NF-κB2 p52. The transcriptionally competent p52/RelB dimer translocates to the nucleus and activates the transcription of relevant genes [11, 15].
The activation of the non-canonical NF-κB pathway relies on the processing of p100, which consists of four major structural domains. The N-terminal Rel homology domain (RHD) is responsible for the binding of homo- and heterodimers to DNA, while the ankyrin repeat domain (ARD) and the processing-inhibitory domain (PID) play crucial roles in regulating p100. The PID contains a death domain (DD), and the ARD masks the nuclear translocation sequence at the N-terminal RHD, inhibiting p100 nuclear translocation by the action of DD. The C-terminal NIK-responsive domain (NRD), which contains phosphorylation sites (S866 and S870) and a ubiquitination site [lysine (K) 855], is responsible for the processing of p100 [15, 16].
To date, more than 100 CVID/CID patients with NFKB2 mutations have been identified [5, 17, 18]. Based on their functional impact on transcriptional (p52) activity and regulatory (p100/IκBδ) activity, autosomal-dominant NFKB2 mutations can be classified into three types, namely, p52 loss of function (LOF)/IκBδ gain of function (GOF), p52LOF/IκBδLOF, and p52GOF/IκBδLOF [17]. Among these, p52LOF/IκBδGOF patients are the most common, typically presenting with CVID with early-onset hypogammaglobulinemia, T-cell dysfunction, and, in nearly half of the cases, accompanying endocrinopathy, ectodermal dysplasia, and autoimmunity [5]. Flow cytometry and cytometry by time of flight (CyTOF) analyses of peripheral B cells from p52LOF/IκBδGOF patients have revealed B lymphopenia and disturbed B-cell subsets [5, 17]. However, intrinsic functional abnormalities in B cells of p52LOF/IκBδGOF patients remain largely unknown.
In this study, we report five patients with CVID who carry heterozygous NFKB2 mutations at the C-terminus. These mutations impaired the processing of p100 into active p52, resulting in a p52LOF/IκBδGOF phenotype. Although three out of five patients exhibited B-cell lymphopenia and all presented with hypogammaglobulinemia, noticeable variations in T- and B-cell differentiation were observed among them. In vitro functional analyses of isolated naïve B cells revealed intrinsic defects in B-cell activation and in the differentiation into CD27+IgD− cells and plasma cells in p52LOF/IκBδGOF B cells, while class-switch recombination remained unaffected. Possible mechanisms accounting for both the distinct and overlapping immune phenotypes observed in these patients are discussed.
Materials and methods
Human subjects
Peripheral blood samples from patients, their parents, and healthy controls (HC) were obtained from the Children’s Hospital of Fudan University. All samples were collected with the informed consent of the parents or the patients themselves. All experiments were approved by the Ethics Committee of Fudan University and the Ethics Committee of the Children’s Hospital of Fudan University.
Routine evaluation of immunological function
A complete blood count was evaluated using a cell counter with anticoagulated whole blood. Serum IgG, IgA, IgM, and IgE levels were determined by an automated clinical chemistry analyzer (Erba, model XL-200).
Genetic analysis
Genomic DNA was extracted from the whole blood of the patients and their parents. Whole exome sequencing (WES) and analysis were performed by the Molecular Diagnostics Center of the Children’s Hospital of Fudan University, as described previously [19]. Mutations in NFKB2 were confirmed using Sanger sequencing with a forward primer (5ʹ-ACGCCTCTTGACCTCACTTG-3ʹ) and a reverse primer (5ʹ-CAGTGCACCTGAGGCTGG-3ʹ).
Construction of pFLAG-CMV-5a vectors for NIK and WT/mutant NF-κB2 expression
WT NFKB2 and NIK genes were amplified using high-fidelity KOD-plus polymerase (TOYOBO) to minimize PCR errors. NIK amplification was performed using a forward primer 5ʹ-CCGGAATTCCCACCATGGCAGTGATGGAAATGGC-3ʹ (including an EcoRI site) and a reverse primer 5ʹ-CGCGGATCCGGGCCTGTTCTCCAGCTGG-3ʹ (including a BamHI site and excluding the stop codon to allow the addition of a FLAG tag at the C-terminus of NIK). The amplification was carried out on HC cDNA with the following PCR conditions: initial denaturation at 95°C for 5 min, followed by 32 cycles of 95°C for 30 s, 64°C 10 s, and 68°C for 3 min. A final extension step was performed at 68°C for 7 min, followed by a hold at 4°C. A human WT NFKB2 cDNA fragment was amplified using 5ʹ-CCGGAATTCCCACCATGGAGAGTTGCTACAACCCAG-3ʹ (including an EcoRI site) and 5ʹ-CGCGGATCCTCAGTGCACCTGAGGCTGG-3ʹ (including a BamHI site) primers from HC cDNA with the following conditions: 95°C for 5 min, 32 cycles of 95°C for 30 s, 64°C for 10 s, and 68°C for 3 min, followed by 68°C for 7 min and hold at 4°C. The PCR products were verified by Sanger sequencing and inserted into pFlag-CMV-5a (MilliporeSigma) to generate the pFlag-CMV-5a-NIK and pFlag-CMV-5a-NFKB2 vectors. The pFlag-CMV-5a-NFKB2 vector was then subject to site-directed mutagenesis using PrimeSTAR Max DNA Polymerase 2 (TaKaRa) to generate pFlag-CMV-5a vectors for mutant NFKB2 expression. Following primers were used: P1-F, 5ʹ-GCACAGCAGAGGTGAAGGAAGACAGTGGAGCAGGAGGCAGA-3ʹ; P1-R, 5ʹ-CGTGTCGTCTCCACTTCCTTCTGTCACCTCGTCCTCCGTCT-3ʹ; P2-F, 5ʹ-GCACAGCAGAGGTGAAGGAAGACAATGCGTACGGGAGCCAGTCAGTGGAG-3ʹ; P2-R, 5ʹ-CGTGTCGTCTCCACTTCCTTCTGTTACGCATGCCCTCGGTCAGTCACCTC-3ʹ; P3-F, 5ʹ-TAGAGGAGGGAGTGAGGCTGCTTGAGGGGTCCAGAAACCCGAGACAAG-3ʹ; P3-R, 5ʹ-ATCTCCTCCCTCACTCCGACGAACTCCCCAGGTCTTTGGGCTCTGTTC-3ʹ. For the mutant NFKB2 of P4 and P5, Oligos around the mutation sites (P4/P5-F, 5ʹ-TGCTGAGGGGTCCAGAAACCCGAGACAAGCTGCCCAGCACACAGCACAGCAGAGGTGAAGGAAGACAATGCGTACGGGAGCC-3ʹ; P4/P5-R, 5ʹ-TGCCTCCTGCTCCACTGACTGGCTCCCGTACGCATTGTCTTCCTTCACCTCTGCTGTGCTGTGTGCTGGGCAGCTTGTCTCGGG-3ʹ) were annealed and assembled into the pFlag-CMV-5a-NFKB2 vector through homologous recombination (Vazyme).
Transfection of the pFLAG-CMV-5a vectors for NIK and WT/mutant NFKB2 expression
293T cells in 24-well plates were transfected with pFlag-CMV-5a-NIK (0.5 μg per well) and WT or mutant pFlag-CMV-5a-NFKB2 plasmid (0.5 μg per well) using the Lipofectamine 2000 transfection kit (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Luciferase assay
293T cells were seeded in 96-well plates and co-transfected with the pGL3-NF-κB response element reporter plasmid and the indicated pFlag-CMV-5a-NFKB2 plasmid. The following day, cells were treated with TNF-α (100 ng/mL) for 5 h, and luciferase activities were then measured using the luciferase assay kit (Promega), according to the manufacturer’s protocol.
Abs and flow cytometry
Single-cell suspensions from peripheral blood were first incubated with human Fc block (BD Biosciences) to block Fcγ receptors. Cells were then stained with the following anti-human antibodies (Abs): CD19-APC (clone HIB19, BioLegend), CD20-FITC (clone 2H7, BioLegend), CD20-BV650 (clone 2H7, BD Biosciences), CD27-APC/Cyanine7 (clone O323, BioLegend), IgD-PE (clone IA6–2, BD Biosciences), IgM-FITC (clone G20–127, BD Biosciences), IgM-APC/Cy7(clone MHM-88, BioLegend), IgM-BV510 (clone MHM-88, BD Biosciences), CD43-PE (clone CD43–10G7, BioLegend), CD38-PE/Cy7 (clone HIT2, BioLegend), IgG-PE (clone HP6017, BioLegend), IgG-PE/Cy7 (clone G18–145, BD Biosciences), CD80-FITC (clone L307.4, BD Biosciences), CD86-APC (clone IT2.2, BD Biosciences), HLADR-PE (clone G46–6, BD Biosciences), CD69-PE/Cy7 (clone FN50, BD Biosciences), CD3-BV605 (clone SK7, BD Biosciences), CD4-APC/Cy7 (clone RPA-T4, BD Biosciences), CD8-FITC (clone HIT8a, BD Biosciences), CD25-PE (clone M-A251, BD Biosciences), CD127-Alexa Fluro 647 (clone HIL-7R-M21, BD Biosciences), CD183-PE/Cy7 (clone 1C6/CXCR3, BD Biosciences), CD185-APC/R700 (clone RF8B2, BD Biosciences), CD194-BV786 (clone 1G1, BD Biosciences), CD196-BV421 (clone 11A9, BD Biosciences), CD197-BB700 (clone 3D12, BD Biosciences), CD45RA-BV605 (clone H100, BD Biosciences), CD11c-FITC (clone 3.9, eBioscience), CD14-APC/H7 (clone MφP9, BD Biosciences), CD16-APC (clone B73.1, BD Biosciences), CD56-BV421 (clone NCAM16.2, BD Biosciences), and CD123-PE/Cy7 (clone 6H6, BioLegend). 7-AAD Viability Staining Solution (eBioscience, Thermo Fisher Scientific) was used for live versus dead cell discrimination. Samples were analyzed with a FACSVerse flow cytometer (BD Biosciences) and Cytek Aurora (Cytek Biosciences). We collected 5 × 104–1 × 106 cells for FACS analysis. Data analysis was performed with FlowJo 10 software (Treestar).
Enrichment of human naïve B cells in peripheral blood
Peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood, and naïve B cells were then enriched using a MojoSort Human Naïve B-Cell Isolation Kit (BioLegend) with biotinylated anti-human IgG beads to remove IgG+ cells, as described previously [20].
Cell culture
293T cells were cultured in DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco, Thermo Fisher Scientific). Human PBMC and naïve B cells were cultured in RPMI 1640 (Gibco, Thermo Fisher Scientific) supplemented with 10% heat-inactivated FBS, antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin), and 5 × 10−5 M 2-mercaptoethanol. PBMC were stimulated with pre-coated anti-human CD3 (2 μg/mL, BD Biosciences) for 48 h. Naïve B cells were stimulated with CpG ODN 2006 (0.6 μM, Sangon Biotech), goat F(abʹ)2 anti-human IgM (2 μg/mL, SouthernBiotech), CpG ODN 2006 (0.6 μM) + F(abʹ)2 anti-human IgM (2 μg/mL, SouthernBiotech) + IL-2 (10 ng/mL, R&D Systems), CD40L (0.2V, house made) [21] + IL-4 (25 ng/mL, BD Biosciences), and CD40L (0.2V, house made) + IL-21 (50 ng/mL, R&D Systems). The cells were collected after 2 and 6 days for flow cytometry analysis. The culture supernatants were collected after 6 days and analyzed for IgM and IgG levels by enzyme-linked immunosorbent assay (ELISA).
ELISA
96-well ELISA plates (Costar, Corning) were coated with goat anti-human Ig (SouthernBiotech) at a dilution of 1:200 in PBS at 4°C overnight. The plates were then blocked with blocking buffer (PBS + 1% BSA) at room temperature for 1 h. The culture supernatants and serially diluted standards were then added and incubated at room temperature for 1 h. After washing with PBST (PBS + 0.05% Tween 20), biotin-anti-human IgG/M (SouthernBiotech) Ab (1:10 000 dilution) was added and incubated at room temperature for 1 h. After another round of washing with PBST, avidin-HRP (BioLegend) at a dilution of 1:5000 was added to the plates, incubated for 30 min, and visualized with TMB substrate reagents (Invitrogen, Thermo Fisher Scientific) for 5, 10, or 15 min. OD450–OD570 was measured by a spectrophotometer (Bio-Rad). The amount of Ig in duplicate wells was calculated based on a standard curve.
Immunoblot
Whole-cell lysates extracted using medium radioimmunoprecipitation assay (RIPA) lysis buffer (Cwbiotech) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a 0.45-μm PVDF membrane (Millipore). Membranes were blocked with 2% nonfat milk and incubated overnight at 4°C with specific antibodies against NF-κB2 (CST), NIK-FLAG (anti-DYKDDDDK, CST), and β-ACTIN (Sigma), followed by horseradish peroxidase (HRP)-conjugated secondary Abs for 1 h at room temperature. Detection was carried out using an enhanced chemiluminescence light (ECL) reagent (Millipore).
Statistical analysis
Data were analyzed using Graphpad Prism 8 (GraphPad Software LLC). Statistical analysis between independent samples was performed using Student’s t-test (two sided). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. P < 0.05 was considered statistically significant.
Results
Clinical manifestation of the patients
P1 was born to healthy, nonconsanguineous parents. At 6 months of age, he presented with pneumonia and persistent diarrhea for 1 month. Subsequently, he developed recurrent lower respiratory infections, tonsillitis, atopic dermatitis, and oral candida infection. P2, a 35-year-old male, presented with recurrent respiratory infections, sinusitis, facial pustules, and bilateral knee osteoarthritis at the age of 29. He also developed squamous epithelial papilloma of the supramaxilla. P3 was born to healthy, nonconsanguineous parents. He presented with cough, mumps, and Penicilliposis marneffei infection detected in blood and bone marrow at 3 years old. He later developed superficial gastritis, suspected viral encephalitis accompanied by drowsiness, vomiting, and gait instability, as well as adrenocorticotropic hormone (ACTH) deficiency. P4, a female patient, suffered from acute respiratory distress syndrome (ARDS) at 4 months of age due to a Pneumocystis jirovecii (PJP) infection and required mechanical ventilation. She had multiple episodes of pneumonia caused by parainfluenza virus, rhinovirus, and streptococcus pneumoniae infections before the age of 2, and suffered from PJP again at the age of 2. P5, the mother of P4, is susceptible to upper respiratory tract infections but has no other symptoms.
Identification of four NFKB2 heterozygous mutations from the five patients
To investigate the genetic defects in these patients, we performed WES and found that all patients had NFKB2 heterozygous mutations: P1 (C.2595_2614del, p.A867Gfs*12); P2 (C.2597G > A, p.S866N); P3 (C.2540dupT, p.R848Efs*38); P4 and P5 (C.2570_2571insCAGCACA, p.A860Qfs*28) (Fig. 1A and Supplementary Fig. S1). Among these variants, S866N (P2) and R848Efs*38 (P3) have been previously reported [17, 22], while A867Gfs*12 (P1) and A860Qfs*28 (P4/5) represent novel variants, although different mutations at the same amino acid positions have been reported [17, 23]. The identified mutations are indicated in Fig. 1B.
Figure 1:
genetics and pedigrees of patients with NFKB2 mutations and NF-κB2 protein expression. (A) Pedigrees of patients with NFKB2 mutations. (B) Schematic of NF-κB2 with the Rel homology domain (RHD), ankyrin repeat domain (ARD), processing-inhibitory domain (PID), and NIK-responsive domain (NRD). The amino acid sequence and the location of mutations in the affected individuals are shown with an arrow. (C) Alignment of the amino acids in the C-terminus of NF-κB2 of each patient. K in red, ubiquitination site (K855); S in red, phosphorylation sites (S866 and S870); Yellow highlights, mutant amino acids. (D) HEK293T cells were transiently transfected with the indicated constructs expressing WT or mutant NF-κB2 in the presence or absence of NIK coexpression. Cell lysates were analyzed for WT or mutant p100 and p52 expression by immunoblotting. β-ACTIN was used as a loading control.
The NFKB2 heterozygous mutation C.2595_2614del in P1 is a frameshift mutation that changes alanine (A) to glycine (G) at position 867 and introduces a premature stop codon 12 amino acids further downstream (Fig. 1C). This mutation affects key phosphorylation site S870. The missense mutation C.2597G > A in P2 changes serine (S) to asparagine (N) at position 866, disrupting the critical phosphorylation site S866 (Fig. 1C). The frameshift mutation C.2540dupT in P3 leads to a change from arginine (R) to glutamic acid (E) at position 848 and the introduction of a premature stop codon 38 amino acids downstream. This mutation affects the critical ubiquitination site (K855) as well as the phosphorylation sites (S866 and S870) (Fig. 1C). The frameshift mutation C. 2570_2571insCAGCACA in P4 and P5 results in a change of alanine (A) to glutamine (Q) at position 860 and the introduction of a premature stop codon 28 amino acids downstream. This mutation affects both phosphorylation sites (S866 and S870) (Fig. 1C). Based on these findings, the mutations in P1, P3, P4, and P5 likely truncate NF-κB2 and prevent phosphorylation at the critical S sites, ubiquitination, and subsequent active p52 processing. The mutation in P2 disrupts the critical phosphorylation site S866 at the C-terminus of p100 and may affect its phosphorylation and subsequent p100 processing and nuclear translocation. Since the ubiquitination of K855 and the subsequent degradation of the C-terminus of p100 depend on the phosphorylation of both S866 and S870, all these mutations likely prevent p100 processing, resulting in a p52LOF/IκBδGOF phenotype.
Impaired processing of the mutant NF-κB2 into active p52
To directly demonstrate the inability of mutant NF-κB2 to undergo processing into the active p52 form, we co-transfected 293T cells with an NIK expression vector and vectors expressing either wild-type or mutant NF-κB2 and analyzed p52 processing by immunoblot 24 h later. As shown in Fig. 1D, WT p100 protein was successfully processed into the active p52 following co-transfection with NIK. As expected, the two previously reported variants (p.S866N of P2 and p.R848Efs*38 of P3) showed impaired processing into p52, confirming earlier findings [17, 22]. Moreover, both the p.A867Gfs*12 (P1) and p.A860Qfs*28 (P4/P5) variants exhibited severely reduced, but not completely absent, processing into the active p52 form, extending previous findings. These mutants, along with WT, were able to efficiently suppress NF-κB activation (Supplementary Fig. S2), indicating that they indeed represent p52LOF/IκBδGOF mutants.
Expression of mutant NF-κB2 protein in PBMC of patients
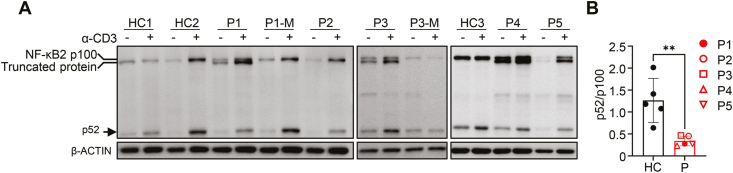
Previous reports have shown that T-cell receptor (TCR) signaling can activate the non-canonical NF-κB pathway [24]. To investigate the effect of the four mutations on NF-κB2 protein expression, we performed immunoblotting of whole-cell lysates from PBMC of patients cultured for 2 days with or without α-CD3. As expected, truncated NF-κB2 proteins were detected in PBMC from P1, P3, P4, and P5 but not P2 (Fig. 2A). After α-CD3 stimulation, the ratio of p52/p100 band intensities was significantly lower in patients compared to the mothers of P1 and P3 and three HC (Fig. 2A and B), indicating that all four mutations affected the processing of p100 into active p52.
Figure 2:
WT and mutant NF-κB2 protein expression in PBMC before and after anti-CD3 stimulation. (A) Total PBMC from individuals with NFKB2 mutations, their parents, and HC were stimulated with anti-CD3 (2 μg/mL) for 48 h. Cell lysates were prepared and analyzed for the expression of full-length (p100) and processed form (p52) NF-κB2 by immunoblot. β-ACTIN was used as a loading control. M, the patient mother. HC, healthy control. (B) The ratio of p52 and p100 band intensities after anti-CD3 stimulation. Mean ± SD is shown. **P < 0.01
Reduced B-cell numbers and altered B-cell differentiation in the three patients
Routine immune analysis of PBMC revealed that the patients exhibited normal to elevated CD3+ T-cell numbers compared with reference ranges (Table 1). However, substantial differences were observed in the proportion of T-cell subsets (Table 1). Specifically, P3 and P4 had greatly reduced proportions of CD4 and CD8 effector memory T cells (CD4 TEM and CD8 TEM), as well as CD8 effector memory re-expressing CD45RA (CD8TEMRA), while displaying relatively high proportions of naïve CD4 and CD8 T cells. These findings suggest potential impairments in T-cell activation and/or differentiation in these two patients. P1, P2, and P5 presented lower proportions of CD4 TEM while relatively normal in other T-cell subsets. Further analysis of various Th subsets in P2, P3, P4, and P5 showed that the proportions of Tfh and Th1 cells were significantly decreased in the patients compared to HC (Supplementary Fig. S3A and B). Th2, Th17, and Treg cells were also moderately reduced in the patients although the differences did not reach statistical significance.
Table 1:
immunological analysis of the patients
| P1 | P2 | P3 | P4 | P5 | Reference values (1–4 years/6–12 years/adults) | |
|---|---|---|---|---|---|---|
| Age# (years) | 11 | 31 | 7 | 2 | 31 | |
| Sex | M | M | M | M | F | |
| Lymphocytes | ||||||
| CD3+ (/μL) | 2171 | 1945↑ | 2622↑ | 7750↑ | 1367↑ | 1500–2900/1100–2200/700–1300 |
| CD3+ (%) | 81.5↑ | 78.2↑ | 84.1↑ | 71.0 | 74.1 | 53.9–72.9/57.1–73.4/56.8–75.0 |
| CD4+ (/μL) | 1357 | 1294↑ | 1661↑ | 6017↑ | 829↑ | 1000–2100/600–1600/400–700 |
| CD4+ (% of CD3+) | 50.9↑ | 52.0↑ | 53.3 | 55.1↑ | 45.0↑ | 24.1–42.5/24.0–38.7/22.3–40.9 |
| CD4 naïve T (% of CD4+) | 78.0↑ | 62.2 | 84.1↑ | 81.7 | 75.7↑ | 46.1–84.4/39.7–69.6/39.5–66.3 |
| CD4 TEM (% of CD4+) | 1.3↓ | 3.0↓ | 0.4↓ | 0.2↓ | 1.8↓ | 0.9–6.5/3.4–11.2/4.2–16.3 |
| CD4 TCM (% of CD4+) | 20.6↓ | 34.8 | 15.5↓ | 18.1 | 22.4↓ | 13.9–48.1/24.2–52.7/25.3–49.9 |
| CD4 TEMRA (% of CD4+) | 0.0↓ | 0.0 | 0.0↓ | 0.03↓ | 0.02 | 0.0–1.4/0.1–1.3/0.0–2.1 |
| CD8+ (/μL) | 777 | 643 | 905 | 1595↑ | 448 | 700–1100/500–1200/300–800 |
| CD8+ (% of CD3+) | 29.2 | 25.9 | 29.0 | 14.6↓ | 24.3 | 19.0–32.5/21.0–33.9/21.0–36.8 |
| CD8 Naïve T (% of CD8+) | 59.6 | 57.6 | 95.4↑ | 90.7↑ | 72.2 | 36.8–83.2/41.4–73.0/35.3–72.3 |
| CD8 TEM (% of CD8+) | 8.6 | 7.5 | 0.4↓ | 0.6↓ | 4.6 | 0.7–11.2/1.5–15.4/2.4–15.8 |
| CD8 TCM (% of CD8+) | 9.1↓ | 22.1 | 3.3↓ | 8.4 | 18.5 | 5.2–31.7/13.2–37.9/11.0–36.9 |
| CD8 TEMRA (% of CD8+) | 22.7↑ | 12.8 | 0.9↓ | 0.3↓ | 4.7 | 0.8–33.0/2.0–21.7/3.9–31.2 |
| DN T (% of CD3+) | 2.4 | 3.9↑ | 3.1↑ | 3.9↑ | 9.1↑ | 0.4–1.8/0.8–2.9/0.6–2.5 |
| γδ T (% of CD3+) | 1.3↓ | 2.0↓ | 3.2↓ | 2.0↓ | 7.3 | 4.9–18.0/8.1–20.8/6.4–20.3 |
| CD16+CD56+ NK (/μL) | 257↓ | 520↑ | 298↓ | 2229↑ | 349 | 300–600/300–600/100–400 |
| CD16+CD56+ NK (%) | 9.6↓ | 20.9 | 9.7↓ | 20.4 | 18.9 | 7.2–20.9/10.0–27.0/10.1–28.3 |
| CD19+ (/μL) | 174↓ | 6↓ | 110↓ | 832 | 109 | 500–1200/200–600/100–200 |
| CD19+ (%) | 6.5↓ | 0.2↓ | 3.5↓ | 7.6↓ | 5.9↓ | 13.2–26.4/9.2–19.5/7.7–17.8 |
| Transitional B (% of CD19+) | 10.1↑ | 0.0↓ | 11.1↑ | 6.4 | 6.9 | 5.2–17.2/2.5–9.1/1.4–9.4 |
| Naïve B (% of CD19+) | 95.1↑ | 27.5↓ | 81.9↑ | 85.5 | 88.0↑ | 65.5–86.6/51.8–77.6/48.4–78.6 |
| Memory B (% of CD19+) | 0.6↓ | 20.0 | 4.4↓ | 3.0 | 3.4↓ | 3.0–14.2/9.0–24.1/7.2–23.8 |
| Plasmablast (% of CD19+) | 0.7 | 17.5↑ | 4.9 | 0.08↓ | 0.05↓ | 0.5–7.1/0.7–5.7/0.5–7.1 |
| Serum antibodies | ||||||
| IgG (g/L) | 3.30↓ | 5.00*↓ | 9.80* | 2.00↓ | 3.80↓ | 5.52–11.46/6.09–12.85/7.60–16.60 |
| IgA (g/L) | 0.01↓ | UD↓ | 0.10↓ | 0.01↓ | 0.34↓ | 0.06–0.74/0.52–2.16/0.91–3.50 |
| IgM (g/L) | 0.16↓ | 0.19↓ | 0.15↓ | 0.51↓ | 0.43↓ | 0.60–2.12/0.67–2.01/0.48–2.12 |
| IgE (KU/L) | 2.06 | UD | 19.84 | 6.81 | 4.82 | <100 KU/L |
#: Age at evaluation, *: under IVIG, Bold: values out of reference range, ↑: values higher than reference range, ↓: values below reference range, NA: not available, UD: undetectable, Naïve T, CD45RA+CD27+, TEM: effector memory T, CD45RA−CD27−, TCM: central memory T, CD45RA−CD27+, TEMRA: effector memory T re-expressing CD45RA, CD45RA+CD27−, Transitional B, CD19+CD24hiCD38hi, naïve B, CD19+IgD+CD27−, Memory B, CD19+IgD−CD27+, Plasmablast, CD19+CD24−CD38hi. We analyzed 5 × 105 PBMC by FACS. Note that the sum of the B-cell subsets may exceed 100% since the naïve B-cell subset (CD19+IgD+CD27−) includes the transitional B cells (CD19+CD27−CD24hiCD38hi). Additionally, the sum may fall below 100% as certain subsets within the CD19+ population, such as the IgD−CD27− double-negative B cells and IgD+CD27+ marginal zone-like B cells, were not included in the table.
Serum IgG, IgA, and IgM levels were below the reference range in all five patients (Table 1). All patients except P5 received regular intravenous immunoglobulin (IVIG) replacement. The proportion and absolute number of CD19+ B cells were significantly reduced in P1, P2, and P3, with P2 showing an almost complete absence. P4 and P5 had normal B-cell counts but reduced proportions of B cells (Table 1). While P1, P2, and P3 displayed significantly reduced B-cell numbers in the peripheral blood, notable differences were observed in B-cell differentiation. Specifically, P1 and P3 had elevated proportions of naïve B (CD19+CD27−IgD+) and transitional B (CD19+CD38hiCD24hi) cells, while the proportions of memory B (CD19+CD27+IgD−) cells were considerably lower compared to normal reference ranges. These findings indicate impaired B-cell differentiation in these patients. In contrast to P1 and P3, P2 demonstrated a more pronounced reduction in total B-cell numbers, with a reduced proportion of naïve B cells and the absence of transitional B cells. However, the proportion of plasmablasts was significantly increased, suggesting that the limited B-cell population in P2 underwent enhanced differentiation.
In addition, P1 and P3 showed severely reduced IgM and IgG memory B cells, as well as reduced class-switched B cells, compared with HC (Fig. 3A-D). In contrast, the proportions of IgM and IgG memory B cells were normal or even increased in P2 (Fig. 3B, C). Notably, the proportion of class-switched B cells in P2 was nearly three times that of HC (Fig. 3D). Moreover, P2 exhibited a striking increase in IgD−CD27− DN B cells (Fig. 3E). Further analysis revealed that the proportions of the DN B cells within the class-switched B cells were 18.2% in P1, 35.4% in P2, 16.2% in P3, and 17.9 ± 11.7 in HC. Routine immune analysis also revealed reduced memory B cells and plasmablasts in P4 and P5 (Table 1). Myeloid and NK cell evaluations showed no gross abnormalities in P2 (Supplementary Fig. S4).
Figure 3:
analysis of IgM and IgG memory B cells, class-switched B cells, and DN B in patients. (A) Gating strategy used to identify CD19+ B cells. (B-E) Left, representative flow cytometric profiles showing the specific B-cell subsets within the gated CD19+ cells; Right, summary of percentages of the indicated B-cell subsets among total CD19+ B cells from P1, P2, and P3, along with their age-matched HC. HC1 and P1 were analyzed concurrently and used identical gates, while HC2, P2, and P3 were analyzed on a separate day and used the same gate. Experiments for P2 were conducted twice, and the average values were used for analysis. The data presented are derived from ex vivo analysis. *P < 0.05
Intrinsic defects in activation and differentiation of P1 and P4 B cells
To investigate whether the diminished antibody production in the patients is due to an intrinsic defect in B cells or impaired T-cell function, we conducted in vitro culture experiments using naïve B cells purified from P1. Following the confirmation of high B-cell purity (Supplementary Fig. S5A, upper panels) and little contamination of memory B cells (Supplementary Fig. S5A, lower panels), we subjected these naïve B cells to different culture conditions. These included CpG 2006 alone (CpG), F(abʹ)2 anti-human IgM alone (α-IgM), CpG 2006 + F(abʹ)2 anti-human IgM + IL-2 (CMIL2), or CD40L + IL-4. We selected these stimuli since signaling through BCR, TLR9, or CD40 can activate NF-κB2 and also induce B-cell activation and/or differentiation [11, 25, 26]. After 2 days, we assessed B-cell survival and activation. After 6 days, we analyzed B-cell differentiation into memory B cells or plasmablasts, as well as antibody secretion and Ig gene class-switching.
As shown in Fig. 4A, P1 B cells appear to have slightly reduced survival in response to all stimuli, including CpG, α-IgM, and CMIL2, except for CD40L + IL-4, compared to his mother and two HC. Additionally, P1 B cells showed a general reduction in the upregulation of CD80 (Fig. 4B) and HLADR (Fig. 4C) in response to various stimuli except for CD40L + IL-4.
Figure 4:
intrinsic defects in B-cell activation in patients. Naïve B cells from P1, his mother, and two HC were cultured with medium alone, CpG 2006, α-IgM, CpG 2006+α-IgM + IL-2 (CMIL2), or CD40L + IL-4 for 48 h, and then analyzed for (A) survival, (B) CD80 expression, and (C) HLADR expression. M, the patient’s mother; HC: healthy control; MFI: mean fluorescence intensity. Mean ± SD of duplicate wells of P1 are shown. Naïve B cells from P4 were cultured with CpG 2006, α-IgM, and CD40L alone for 48 h, and subsequently analyzed for their CD80 (D) and HLADR (E) expression. MFI of CD80 and HLADR was shown as fold change compared with mean values of the HC group at day 0.
We also purified naïve B cells from frozen PBMC of P4, achieving a purity greater than 94.5%, as shown in Supplementary Fig. S5B (upper panels), with the successful removal of memory B cells (lower panels). Similar to P1, P4 B cells also showed reduced CD80 upregulation (Fig. 4D) in response to CpG, α-IgM, and CD40L alone, although HLADR expression was upregulated comparably to that in HC B cells (Fig. 4E). Collectively, these results suggest that naïve B cells from both P1 and P4 exhibit intrinsic defects in activation in response to both T-independent (CpG, α-IgM, and CMIL2) and T-dependent (CD40L) stimuli.
We have recently shown that CMIL2 stimulation can efficiently induce naïve B cells to differentiate into IgD−CD27+ cells and plasmablasts [20, 27]. Consistently, while memory B cells were barely detectable in purified naïve B cells (Supplementary Fig. S5A, lower panels), CMIL2 induced 10–20% CD27+IgD− cells in P1’s mother and two HC (Fig. 5A). However, B cells from P1 only produced 0.8% CD27+IgD− cells (Fig. 5A). We further assessed plasmablast differentiation under these in vitro culture conditions. As depicted in Fig. 5B, under CpG 2006 stimulation, only 0.03% of P1 naïve B cells differentiated into plasmablasts (CD20loCD38hi), in contrast to 0.4–0.8% of plasmablasts generated from B cells of P1’s mother and 2 HC (Fig. 5B). With CMIL2 stimulation, only 0.6% of P1 naïve B cells differentiated into CD20loCD38hi plasmablasts, compared to 6.9–10.6% from P1’s mother and two HC (Fig. 5C). Consistently, P1 B cells produced much lower levels of both IgM (Fig. 5D, left) and IgG (Fig. 5D, right) antibodies compared to his mother and two HC.
Figure 5:
patients’ B cells have an intrinsic defect in B-cell differentiation but normal in class-switch recombination. Analysis of P1 B-cell differentiation under in vitro culture conditions. (A) Freshly isolated naïve B cells from P1 were stimulated with CpG 2006+α-IgM + IL-2 (CMIL2) for 6 days and then analyzed for the proportion of IgD−CD27+ cells. Mean ± S.D. of duplicate wells of P1 are shown. Freshly isolated naïve B cells from P1 were stimulated with CpG2006 alone (B) or CMIL2 (C) for 6 days and then analyzed for the proportion of CD20loCD38hi plasmablasts. (D) The concentration of IgM (left) and IgG (right) in the supernatant of P1 naïve B cells cultured for 6 days with CMIL2, as measured by ELISA. Analysis of plasmablast differentiation of P4 naïve B cells. (E) Freshly isolated naïve B cells of P4 were stimulated with CD40L + IL-21 for 6 days and then analyzed for the generation of CD20loCD38hi or CD27+CD38+ plasmablasts. (F) The concentration of IgM (left) and IgG (right) in the supernatant of P4 naïve B cells cultured for 6 days with CD40L + IL-21. (G) Analysis of in vitro class-switching. Naïve B cells isolated from HC1, P1, HC2, P4, HC3, and P1-M were stimulated with CD40L + IL-4 for 6 days and analyzed for the proportion of IgG+ cells. HC1, P1, HC3, and P1-M were analyzed concurrently and used identical gates. HC2 and P4 were analyzed on a different day and used the same gate.
To further validate the defect in plasmablast differentiation in the patient, we cultured naïve B cells from P4 and an HC in the presence of CD40L + IL-21, which represents a T-dependent condition that can induce plasmablast differentiation [28]. We analyzed the induction of CD20loCD38hi and CD27+CD38+ cells, both recognized markers for plasmablast. Consistently, P4 generated significantly fewer plasmablasts compared to the HC, as revealed by reduced proportions of both CD20loCD38hi (Fig. 5E, upper panels) and CD27+CD38+ (Fig. 5E, lower panels) cells. Additionally, P4 produced much less IgM and IgG than the HC (Fig. 5F). These results underscore an intrinsic defect in the differentiation of both P1 and P4 B cells into CD27+IgD− cells and plasmablasts. Additionally, we examined Ig gene class-switching in B cells following stimulation with CD40L + IL-4 for 6 days. As depicted in Fig. 5G, naïve B cells from both P1 and P4 exhibited normal class-switching to IgG, similar to observations in P1’s mother and the HC, suggesting that B cells of p52LOF/IκBδGOF patients do not have an intrinsic defect in class-switching ability.
Discussion
The canonical (NF-κB1) and non-canonical (NF-κB2) signaling pathways play a key role in the differentiation and functions of B cells [29, 30]. In 1995, researchers found that p50 (NF-κB1) knockout mice exhibit defective B-cell immunity and impaired responses to bacterial infections [31]. In 1998, another study showed that p52 (NFKB2) knockout mice have defective humoral immune response and spleen microarchitecture [32]. Subsequently, several studies reported the discovery of NFKB1 and NFKB2 mutations in CVID patients [5, 6, 33–39]. In this study, we reported four NFKB2 mutations from five patients. The clinical and immunological presentations of these patients are consistent with those of previously reported cases.
B-cell dysregulation in heterozygous NFKB2 mutation patients has been well documented, primarily using flow cytometry analysis of peripheral B cells or bulk PBMC culture in vitro. Typical features include reduced total B-cell numbers, increased proportion of naïve B cells, and reduced memory B cells and marginal zone B cells [5, 17]. Overall, our findings align with previous studies and further demonstrate B-cell-intrinsic defects through the assessment of naïve B-cell function, as well as switching and differentiation potential. However, profiling B-cell subsets in P2 revealed reduced naïve B cells, normal proportions of memory B cells, and increased DN B, class-switched B cells, and plasmablasts (Table 1 and Fig. 3D and E). Similarly, two patients heterozygous for the R853* variants in NFKB2 displayed decreased B-cell numbers but normal memory B-cell proportions [5]. Although not mentioned in their results, we observed an increase in DN B cells in these patients based on their FACS data. Another patient heterozygous for the K855Sfs*7 variant showed decreased B-cell numbers, reduced naïve B cells, and increased DN B cells [34]. We recently analyzed 52 patients with IEI and found that in those with severely reduced B-cell numbers, compensatory homeostatic proliferation leads to an increased proportion of memory B and DN B cells in the periphery [40]. This phenomenon was also observed in P2, who displayed extremely low B-cell numbers in the periphery. It should be noted that homeostatic proliferation partially depends on BAFFR-mediated signaling, which activates the non-canonical NF-κB2 pathway [40]. Thus, defects in NFKB2 may also restrict the extent of homeostatic B-cell expansion under B-lymphopenic conditions.
Using in vitro culture of PBMC, previous studies have observed reduced activation and impaired differentiation into plasma cells and memory B cells in patients with heterozygous NFKB2 mutations [6, 41]. Our study, using purified naïve B cells of P1 and P4 for in vitro functional analysis, further demonstrated intrinsic defects in B-cell activation and differentiation. To our surprise, class-switching in patient B cells was normal when supplied with adequate T-cell-derived stimuli. This finding suggests that the reduced number of class-switched B cells in patients with heterozygous NFKB2 mutations might be due to impaired T-cell function or other cellular influences within the microenvironment, rather than an intrinsic defect in the B cells themselves. Consistently, Lee et al. reported that three related individuals carrying the NF-κB2 pD865G variant exhibited a severely reduced frequency of Tfh cells in peripheral blood [6]. Other studies have similarly documented a decrease in Tfh cells among patients with NFKB2 mutations [5, 33, 42–44]. In our study, we also observed a significantly reduced Tfh population, indicating that the decrease in switched B cells in patients with NFKB2 mutations is likely due to impaired T-cell interactions and reduced production of cytokines, such as IL-4 and IL-21, by Tfh cells due to NFKB2 mutations. Nonetheless, several studies have reported that some patients exhibit normal antigen-specific IgG responses following vaccination [5, 6, 33, 45–47], indicating that under certain conditions, B cells with NFKB2 mutations are capable of normal class-switching in vivo.
P3 and P4 suffered from Penicilliposis marneffei and PJP infections, respectively. PJP is a clinical feature of IEI due to mutations in genes that significantly affect the function of CD4+ T cells. Consistently, these patients exhibited severely reduced proportions of CD4 effector memory T cells (TEM, Table 1) and reduced Tfh, Th1, Th2, and Th17 cell populations (Supplementary Fig. S3B). Previous studies have documented similar decreases in various Th cell subsets in patients with NFKB2 mutations [5, 42, 43]. Although these reductions suggest potential impairments in T-cell proliferation and differentiation, it has been reported that T-cell activation and proliferation were normal in most patients under α-CD3/α-CD28 stimulation but were reduced under antigen-specific stimulation with tetanus, candida, CMV, and adenovirus antigens [5, 23, 35, 36, 48]. Thus, further research is needed to elucidate the mechanisms underlying these potential impairments in T-cell function. Additionally, a recent study has highlighted a high prevalence of autoantibodies against type 1 interferons in patients with p52LOF/IκBδGOF mutations [17]. It remains to be determined whether the patients in this study, particularly P3 and P4 who have experienced viral infections, produce type 1 interferon autoantibodies. In conclusion, the present study revealed intrinsic defects in B-cell activation and differentiation into CD27+IgD− cells and plasma cells in patients with heterozygous NFKB2 mutations.
Supplementary data
Supplementary data is available at Clinical and Experimental Immunology online.
Acknowledgements
We thank the clinicians in the Department of Clinical Immunology, Children’s Hospital of Fudan University, and the members in Wang’s lab at the Department of Immunology of School of Basic Medical Sciences for their helpful discussions.
Contributor Information
Qing Min, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China; Shanghai Sci-Tech Inno Center for Infection & Immunity, Shanghai, China.
Yaxuan Li, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Xuzhe Wu, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Meiping Yu, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Wenjing Ying, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Qinhua Zhou, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Jia Hou, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Bijun Sun, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Xiaoying Hui, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Lulu Dong, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Xin Meng, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Hai Zhang, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Ziying Hu, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Xiaoqian Feng, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China.
Jinqiao Sun, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Wenjie Wang, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Xiaochuan Wang, Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China; Shanghai Institute of Infectious Disease and Biosecurity, Shanghai, China.
Ji-Yang Wang, Department of Immunology, School of Basic Medical Sciences, Fudan University, Shanghai, China; Shanghai Sci-Tech Inno Center for Infection & Immunity, Shanghai, China; Department of Clinical Immunology, Children’s Hospital of Fudan University, National Children’s Medical Center, Shanghai, China.
Ethical approval
This study was carried out in accordance with the recommendations of the Ethics Committee of the Children’s Hospital of Fudan University (approval number: 2019-048). Written informed consent was obtained from the parents or the patients themselves, and the child’s assent was secured before any study-related procedures were conducted.
Conflict of interest: All the authors declare that they have no relevant financial or non-financial interests to disclose.
Funding
This work was supported by the Major Research Plan of the National Natural Science Foundation of China (grant no. 32330033 to J.-Y.W.), the National Natural Science Foundation of China (grant no. 32270932 to J.-Y.W.), Shanghai Municipal Science and Technology Major Project (grant no. ZD2021CY001 to X.W.), National Natural Science Foundation for Young Scholar (grant no. 82202013 to Q.M.), and China Postdoctoral Science Foundation Grant (grant no. 2022M720782 to Q.M.).
Data availability
Additional data related to this study, as well as specific materials and protocols, can be obtained from the corresponding authors upon reasonable request.
Author contributions
Q.M., Y.L., and X.W. performed the experiments and analyzed the data. W.W. collected and analyzed the clinical data. Y.L. and Q.M. provided a draft of the manuscript. M.Y., L.D., X.M., H.Z, Z.H., and X.F. participated in data collection and analysis. J.S. provided healthy control samples. W.Y., Q.Z., J.H., B.S., X.H., J.S., W.W., and X.W. diagnosed and treated patients and collected the clinical samples. X.W. and J.Y.W. supervised the study. J.Y.W. reviewed and revised the manuscript.
References
- 1.McCusker C, Upton J, Warrington R.. Primary immunodeficiency. Allergy Asthma Clin Immunol 2018, 14, 61. doi: 10.1186/s13223-018-0290-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaya-Uribe L, Rojas M, Azizi G, Anaya JM, Gershwin ME.. Primary immunodeficiency and autoimmunity: A comprehensive review. J Autoimmun 2019, 99, 52–72. doi: 10.1016/j.jaut.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 3.Mitsui-Sekinaka K, Sekinaka Y, Endo A, Imai K, Nonoyama S.. The primary immunodeficiency database in Japan. Front Immunol 2021, 12, 805766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bogaert DJ, Dullaers M, Lambrecht BN, Vermaelen KY, De Baere E, Haerynck F.. Genes associated with common variable immunodeficiency: One diagnosis to rule them all? J Med Genet 2016, 53, 575–90. [DOI] [PubMed] [Google Scholar]
- 5.Klemann C, Camacho-Ordonez N, Yang L, Eskandarian Z, Rojas-Restrepo JL, Frede N, et al. Clinical and immunological phenotype of patients with primary immunodeficiency due to damaging mutations in NFKB2. Front Immunol 2019, 10, 297. doi: 10.3389/fimmu.2019.00297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CE, Fulcher DA, Whittle B, Chand R, Fewings N, Field M, et al. Autosomal-dominant B-cell deficiency with alopecia due to a mutation in NFKB2 that results in nonprocessable p100. Blood 2014, 124, 2964–72. doi: 10.1182/blood-2014-06-578542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuehn HS, Niemela JE, Sreedhara K, Stoddard JL, Grossman J, Wysocki CA, et al. Novel nonsense gain-of-function NFKB2 mutations associated with a combined immunodeficiency phenotype. Blood 2017, 130, 1553–64. doi: 10.1182/blood-2017-05-782177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden MS, Ghosh S.. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–62. doi: 10.1016/j.cell.2008.01.020 [DOI] [PubMed] [Google Scholar]
- 9.Sun SC, Ley SC.. New insights into NF-kappaB regulation and function. Trends Immunol 2008, 29, 469–78. doi: 10.1016/j.it.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reus JB, Rex EA, Gammon DB.. How to inhibit nuclear factor-Kappa B signaling: Lessons from poxviruses. Pathogens 2022, 11, 1061. doi: 10.3390/pathogens11091061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun SC. The non-canonical NF-κB pathway in immunity and inflammation. Nat Rev Immunol 2017, 17, 545–58. doi: 10.1038/nri.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulero MC, Huxford T, Ghosh G.. NF-κB, IκB, and IKK: Integral components of immune system signaling. Adv Exp Med Biol 2019, 1172, 207–26. [DOI] [PubMed] [Google Scholar]
- 13.Hu H, Sun SC.. Ubiquitin signaling in immune responses. Cell Res 2016, 26, 457–83. doi: 10.1038/cr.2016.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallabhapurapu S, Karin M.. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 2009, 27, 693–733. doi: 10.1146/annurev.immunol.021908.132641 [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Lin L, Zhang Z, Zhang H, Hu H.. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct Target Ther 2020, 5, 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun SC. Non-canonical NF-κB signaling pathway. Cell Res 2011, 21, 71–85. doi: 10.1038/cr.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Voyer T, Parent AV, Liu X, Cederholm A, Gervais A, Rosain J, et al. Autoantibodies against type I IFNs in humans with alternative NF-κB pathway deficiency. Nature 2023, 623, 803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirasinha RC, Davies AR, Srivastava M, Sheridan JM, Sng XYX, Delmonte OM, et al. Nfkb2 variants reveal a p100-degradation threshold that defines autoimmune susceptibility. J Exp Med 2021, 218, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Min Q, Meng X, Zhou Q, Wang Y, Li Y, Lai N, et al. RAG1 splicing mutation causes enhanced B cell differentiation and autoantibody production. JCI insight 2021, 6, e148887. doi: 10.1172/jci.insight.148887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fornes O, Jia A, Kuehn HS, Min Q, Pannicke U, Schleussner N, et al. ; IRF4 International Consortium. A multimorphic mutation in IRF4 causes human autosomal dominant combined immunodeficiency. Sci Immunol 2023, 8, eade7953. doi: 10.1126/sciimmunol.ade7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai N, Min Q, Xiong E, Liu J, Zhang L, Yasuda S, et al. A tetrameric form of CD40 ligand with potent biological activities in both mouse and human primary B cells. Mol Immunol 2019, 105, 173–80. doi: 10.1016/j.molimm.2018.11.018 [DOI] [PubMed] [Google Scholar]
- 22.Abraham RS, Marshall JM, Kuehn HS, Rueda CM, Gibbs A, Guider W, et al. Severe SARS-CoV-2 disease in the context of a NF-κB2 loss-of-function pathogenic variant. J Allergy Clin Immunol 2021, 147, 532–44.e1. doi: 10.1016/j.jaci.2020.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindsley AW, Qian Y, Valencia CA, Shah K, Zhang K, Assa’ad A.. Combined immune deficiency in a patient with a novel NFKB2 mutation. J Clin Immunol 2014, 34, 910–5. doi: 10.1007/s10875-014-0095-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu J, Zhou X, Nakaya M, Jin W, Cheng X, Sun SC.. T cell-intrinsic function of the noncanonical NF-κB pathway in the regulation of GM-CSF expression and experimental autoimmune encephalomyelitis pathogenesis. J Immunol 2014, 193, 422–30. doi: 10.4049/jimmunol.1303237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, et al. BCR-signalling synergizes with TLR-signalling for induction of AID and immunoglobulin class-switching through the non-canonical NF-κB pathway. Nat Commun 2012, 3, 767. doi: 10.1038/ncomms1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpi C, Fallarino F, Pallotta MT, Bianchi R, Vacca C, Belladonna ML, et al. High doses of CpG oligodeoxynucleotides stimulate a tolerogenic TLR9-TRIF pathway. Nat Commun 2013, 4, 1852. doi: 10.1038/ncomms2874. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Min Q, Lai N, Csomos K, Wang Y, Liu L, et al. Cellular mechanisms underlying B cell abnormalities in patients with gain-of-function mutations in the PIK3CD gene. Front Immunol 2022, 13, 890073. doi: 10.3389/fimmu.2022.890073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tangye SG, Ma CS.. Regulation of the germinal center and humoral immunity by interleukin-21. J Exp Med 2020, 217, e20191638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bendall HH, Sikes ML, Ballard DW, Oltz EM.. An intact NF-kappa B signaling pathway is required for maintenance of mature B cell subsets. Mol Immunol 1999, 36, 187–95. doi: 10.1016/s0161-5890(99)00031-0 [DOI] [PubMed] [Google Scholar]
- 30.Miraghazadeh B, Cook MC.. Nuclear factor-kappaB in autoimmunity: Man and mouse. Front Immunol 2018, 9, 613. doi: 10.3389/fimmu.2018.00613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sha WC, Liou HC, Tuomanen EI, Baltimore D.. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 1995, 80, 321–30. doi: 10.1016/0092-8674(95)90415-8 [DOI] [PubMed] [Google Scholar]
- 32.Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, et al. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med 1998, 187, 147–59. doi: 10.1084/jem.187.2.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Hanson S, Gurugama P, Jones A, Clark B, Ibrahim MA.. Novel NFKB2 mutation in early-onset CVID. J Clin Immunol 2014, 34, 686–90. doi: 10.1007/s10875-014-0064-x. [DOI] [PubMed] [Google Scholar]
- 34.Chen K, Coonrod EM, Kumánovics A, Franks ZF, Durtschi JD, Margraf RL, et al. Germline mutations in NFKB2 implicate the noncanonical NF-κB pathway in the pathogenesis of common variable immunodeficiency. Am J Hum Genet 2013, 93, 812–24. doi: 10.1016/j.ajhg.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brue T, Quentien MH, Khetchoumian K, Bensa M, Capo-Chichi JM, Delemer B, et al. Mutations in NFKB2 and potential genetic heterogeneity in patients with DAVID syndrome, having variable endocrine and immune deficiencies. BMC Med Genet 2014, 15, 139. doi: 10.1186/s12881-014-0139-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lougaris V, Tabellini G, Vitali M, Baronio M, Patrizi O, Tampella G, et al. Defective natural killer-cell cytotoxic activity in NFKB2-mutated CVID-like disease. J Allergy Clin Immunol 2015, 135, 1641–3. doi: 10.1016/j.jaci.2014.11.038. [DOI] [PubMed] [Google Scholar]
- 37.Shi C, Wang F, Tong A, Zhang XQ, Song HM, Liu ZY, et al. NFKB2 mutation in common variable immunodeficiency and isolated adrenocorticotropic hormone deficiency: A case report and review of literature. Medicine (Baltimore) 2016, 95, e5081. doi: 10.1097/MD.0000000000005081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aird A, Lagos M, Vargas-Hernández A, Posey JE, Coban-Akdemir Z, Jhangiani S, et al. Novel heterozygous mutation in NFKB2 is associated with early onset CVID and a functional defect in NK cells complicated by disseminated CMV infection and severe nephrotic syndrome. Front Pediatr 2019, 7, 303. doi: 10.3389/fped.2019.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzini T, Fliegauf M, Klammer N, Frede N, Proietti M, Bulashevska A, et al. ; NIHR BioResource. Characterization of the clinical and immunologic phenotype and management of 157 individuals with 56 distinct heterozygous NFKB1 mutations. J Allergy Clin Immunol 2020, 146, 901–11. doi: 10.1016/j.jaci.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min Q, Csomos K, Li Y, Dong L, Hu Z, Meng X, et al. B cell abnormalities and autoantibody production in patients with partial RAG deficiency. Front Immunol 2023, 14, 1155380. doi: 10.3389/fimmu.2023.1155380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuijnenburg P, Allen HL, de Bree GJ, Savic S, Jansen MH, Stockdale C, et al. ; NIHR BioResource. Pathogenic NFKB2 variant in the ankyrin repeat domain (R635X) causes a variable antibody deficiency. Clin Immunol 2019, 203, 23–7. doi: 10.1016/j.clim.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 42.Slade CA, McLean C, Scerri T, Giang TB, Megaloudis S, Strathmore A, et al. Fatal enteroviral encephalitis in a patient with common variable immunodeficiency harbouring a novel mutation in NFKB2. J Clin Immunol 2019, 39, 324–35. doi: 10.1007/s10875-019-00602-x [DOI] [PubMed] [Google Scholar]
- 43.Maccari ME, Scarselli A, Di Cesare S, Floris M, Angius A, Deodati A, et al. Severe Toxoplasma gondii infection in a member of a NFKB2-deficient family with T and B cell dysfunction. Clin Immunol 2017, 183, 273–7. doi: 10.1016/j.clim.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 44.Montin D, Licciardi F, Giorgio E, Ciolfi A, Pizzi S, Mussa A, et al. Functional evaluation of natural killer cell cytotoxic activity in NFKB2-mutated patients. Immunol Lett 2018, 194, 40–3. doi: 10.1016/j.imlet.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Lal RA, Bachrach LK, Hoffman AR, Inlora J, Rego S, Snyder MP, et al. A case report of hypoglycemia and hypogammaglobulinemia: DAVID syndrome in a patient with a novel NFKB2 mutation. J Clin Endocrinol Metab 2017, 102, 2127–30. doi: 10.1210/jc.2017-00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mac TT, Castinetti F, Bar C, Julia S, Pasquet M, Romanet P, et al. Deficient anterior pituitary with common variable immune deficiency (DAVID syndrome): A new case and literature reports. J Neuroendocrinol 2023, 35, e13287. doi: 10.1111/jne.13287. [DOI] [PubMed] [Google Scholar]
- 47.Ramakrishnan KA, Rae W, Barcenas-Morales G, Gao Y, Pengelly RJ, Patel SV, et al. Anticytokine autoantibodies in a patient with a heterozygous NFKB2 mutation. J Allergy Clin Immunol 2018, 141, 1479–82.e6. doi: 10.1016/j.jaci.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Bienias M, Gabrielyan A, Geberzahn L, Rösen-Wolff A, Huebner A, Jacobsen EM, et al. More severe than CVID: Combined immunodeficiency due to a novel NFKB2 mutation. Pediatr Allergy Immunol 2021, 32, 793–7. doi: 10.1111/pai.13441. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data related to this study, as well as specific materials and protocols, can be obtained from the corresponding authors upon reasonable request.