Abstract
For brain functions such as working memory and motor planning, neuronal circuits are able to sustain persistent activity after transient inputs. Theoretical studies have suggested that persistent activity can exist in recurrently connected networks as active reverberation. However, the actual cellular processes underlying such reverberation are not well understood. In this study, we investigated the basic synaptic mechanisms responsible for reverberatory activity in small networks of rat hippocampal neurons in vitro. We found that brief stimulation of one neuron in a network could evoke, in an all-or-none fashion, reverberatory activity lasting for seconds. The reverberation was likely to arise from recurrent excitation because it was eliminated by partial inhibition of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type glutamate receptors (but not by blockade of NMDA receptors). In contrast, blocking inhibitory transmission with bicuculline enhanced the reverberation. Furthermore, paired-pulse stimuli with interpulse intervals of 200–400 ms were more effective than single pulses in triggering reverberation, apparently by eliciting higher levels of asynchronous transmitter release. Suppressing asynchronous release by EGTA-AM abolished reverberation, whereas elevating asynchronous release by strontium substantially enhanced reverberation. Finally, manipulating calcium uptake into or release from intracellular stores also modulated the level of reverberation. Thus, the oft-overlooked asynchronous phase of synaptic transmission plays a central role in the emergent phenomenon of network reverberation.
Keywords: asynchronous release, neural network, persistent activity, cell assembly, hippocampal culture
Afundamental aspect of brain function is to act not only on immediate sensory stimuli, but also on prior information that is held as memory traces in the brain after the cessation of the external input. Whereas long-term memory traces are likely to be stored in the connection strengths of neuronal circuits (1–3), short-term memory traces (on the time scale of seconds) are believed to be held “on-line” by persistent neuronal activity (1, 4–7). Indeed, persistent activity has been observed in a wide variety of brain areas in vivo during such tasks as working memory and motor planning (4–7). However, the mechanism underlying such persistent activity has remained unclear. In principle, persistent activity may be maintained in a group of recurrently connected neurons or “cell assemblies” for extended periods of time through active reverberation, as proposed by Lorente de Nó (8) and Hebb (1). The computational implications of such reverberation were first demonstrated by McCulloch and Pitts (9) in their idealized few-cell networks and have been explored extensively in the past decades in various attractor models (5–7, 10–18). In most models, recurrent excitation as an internal positive feedback mechanism keeps the network or cell assembly in distinct “up-states” of persistent firing activity without external drive, thus providing short-term memory traces of input stimuli.
However, partly because of the enormous complexity of the native circuitry in the brain, it is difficult to directly investigate reverberatory activity in vivo. In fact, even in vitro studies of network dynamics have mostly focused on the collective activity that occurs spontaneously (19–25), and few experiments have reproduced (under different conditions) evoked persistent activity that resembles the stable persistent activity observed in vivo (22, 26, 27). Moreover, fundamental issues regarding the cellular mechanisms underlying reverberatory activity in neuronal networks have remained to be addressed (5–7). For example, what is the minimum requirement for cellular substrates to support persistent reverberation? Can recurrent synaptic excitation alone sufficiently sustain persistent activity, or is bistability of single neurons needed? What components of synaptic currents are responsible for sustaining stable reverberation?
We addressed these questions by using small networks of cultured rat hippocampal neurons. In this highly reduced system, we found that brief stimuli of one neuron in a network could evoke long-lasting reverberatory activity with moderately low firing rates similar to those observed in vivo. In addition, the reverberation depended critically on excitatory synaptic transmission, suggesting that it was indeed driven by recurrent excitation. Surprisingly, asynchronous release (28–35), a fundamental yet often ignored component of synaptic transmission, played a central role in the initiation and persistence of network reverberation.
Materials and Methods
Cell Culture. Low-density cultures of dissociated embryonic rat hippocampal neurons were prepared as described (36) with minor modifications. Hippocampi were removed from embryonic day 17–18 (E17–18) rats and were treated with trypsin for 15 min at 37°C, followed by washing and gentle trituration. The dissociated cells were plated on glass coverslips in 35-mm Petri dishes with 45,000–90,000 cells per dish. Coverslips were precoated with patterns of poly(l-lysine) spots of ≈1 mm diameter by using custom-made stamps. The culture medium was DMEM (BioWhittaker) supplemented with 10% heat-inactivated bovine calf serum (HyClone), 10% Ham's F-12 with glutamine (BioWhittaker), 50 units/ml penicillin/streptomycin (Sigma), and 1× B-27 supplement (Invitrogen/Gibco). Twenty-four hours after plating, one-third of the culture medium was replaced with the same medium supplemented with 20 mM KCl. Cytosine arabinoside (Sigma) was added to the culture dish (final concentration, 5 μM) around 7–10 days in vitro (div) to prevent overgrowth of glial cells. Cultures were used at 14–21 div when reverberatory activity was commonly observed. A typical network selected for the present study consisted of ≈20–100 neurons on a patch of glial cell monolayer on a poly(l-lysine) spot. In some cases, the number of neurons in a network could not be counted because isolation between adjacent islands was not complete. However, the network size could be roughly estimated because it generally correlated with the width of recorded polysynaptic current clusters. Larger networks often exhibited uncontrolled bursts of spontaneous reverberation episodes and were avoided in the present study.
Electrophysiology. Whole-cell perforated patch recordings were carried out with patch clamp amplifiers (Axopatch 700A, Axon Instruments) at room temperature. The pipette solution contained 136.5 mM potassium gluconate, 17.5 mM KCl, 9 mM NaCl, 1 mM MgCl2, 10 mM Hepes, 0.2 mM EGTA, and 200 μg/ml amphotericin B (pH 7.3). The external bath solution was a Hepes-buffered saline (HBS) containing 150 mM NaCl, 3 mM KCl, 3 mM CaCl2, 2 mM MgCl2, 10 mM Hepes, and 5 mM glucose (pH 7.3). Stock solutions of 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), bicuculline methiodide (BMI), 2-amino-5-phosphonopentanoic acid (AP5), cyclopiazonic acid (CPA), ryanodine, and EGTA-AM (all from Sigma/Research Biochemicals) were first prepared in water or DMSO and diluted (1:1,000) in HBS when being used. Throughout the recording, the culture was perfused with fresh bath solution at a constant rate of ≈1 ml/min. Signals filtered at 2 kHz were acquired at a sampling rate of 5 kHz by using a 16-bit digitizing board (DigiData 3200, Axon Instruments) and processed with the pclamp software (Axon Instruments). It was observed that, immediately after the cessation of a reverberation episode, the network could not be evoked to reverberate again until after a rest period. In this study, all test stimuli (single or paired pulses) were given to a single cell at a fixed interval of 30 s to allow for network recovery. Networks showing systematic run-up or run-down in reverberation duration and/or probability of occurrence (more than two times the standard deviation over 30 min control period) were excluded from further analysis.
Custom matlab programs were used to automate analysis of reverberation characteristics (e.g., duration and probability of occurrence). Reverberation duration of each evoked current trace was defined as the time period from the point when the rising phase of the first polysynaptic current (PSC) cluster crossed a threshold (at half of the average PSC amplitude) to the time point when falling phase of the last PSC cluster crossed the threshold (with no additional threshold crossing in the next 500 ms). Only a trace with duration >500 ms was considered a successful reverberation. Traces with shorter durations usually contained only one PSC cluster and were considered failures. For each experiment, at least 20 consecutive traces were acquired under a given condition (e.g., control, BMI, CNQX, etc.) to calculate the occurrence probability and mean duration of successful reverberation episodes (mean duration was zero by definition when no successful reverberation was evoked). Asynchronous transmission level was measured by averaging PSC amplitudes within a window of 50–100 ms after the end of the first synchronous PSC cluster and before the onset of the following PSC cluster when reverberation occurred (typically 100–300 ms after stimulation; see Fig. 3C). Traces with occasional synchronous polysynaptic inputs (>100 pA) within this measurement window were excluded from the measurement. A fixed measurement window was used for all traces from one experiment, although different windows were used for different networks. For the correlation analysis in Fig. 4I, changes in mean asynchronous transmission level (A) and the occurrence of reverberation (R) were normalized against the sum of the values in control and test conditions, so that ΔA* = (A_test - A_control)/(A_test + A_control), and ΔR* = (R_test - R_control)/(R_test + R_control).
Fig. 3.
Enhancement of network reverberation by paired-pulse stimulation (PPS). (A) Network responses to single-pulse stimulation and PPS. (Scale for example traces: 500 pA, 100 ms.) (B) Summary of enhanced activation of network reverberation by PPS. Enhancement was significant in reverberation occurrence (from 0.03 ± 0.02 to 0.32 ± 0.08, n = 5, P < 0.05) but not in duration (from 2.84 ± 2.70 s to 4.75 ± 0.80 s, P = 0.4). (C) Example traces showing enhancement of a slower “asynchronous” phase of synaptic transmission by paired-pulse stimulation. Black bars indicate the time window within which asynchronous transmission was quantified by averaging current amplitudes from the traces of corresponding colors (black, single-pulse-evoked response; red, PPS-evoked nonreverberatory response; blue, PPS-evoked reverberation). (D) Relationship between asynchronous transmission and reverberation. Each data point represents one recorded trace evoked by either a single pulse (open symbols) or paired pulses (filled symbols) from which reverberation duration and asynchronous transmission were measured (see Materials and Methods). The distribution of durations shows the all-or-none nature of reverberation. PPS evoked significantly higher levels of asynchronous PSC (1.37 ± 0.04, P < 0.001, normalized against corresponding current evoked by single pulses for each network). In addition, PPS-evoked asynchronous currents from reverberating traces were significantly higher than those from nonreverberating traces (1.54 ± 0.05, vs. 1.31 ± 0.05, P < 0.001).
Fig. 4.
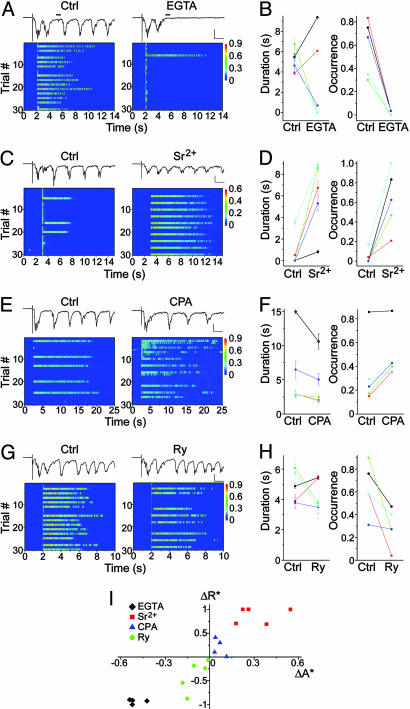
The role of asynchronous synaptic transmission and its modulation in network reverberation. (A) Suppression of reverberation by EGTA-AM (100 μM). Note that, in the sample traces, the asynchronous portion of PSC (measured by the mean current amplitude over the 50-ms period marked by the black bar) decreased dramatically (from 126 pA in control to 27 pA in EGTA-AM). (B) Summary of effects of EGTA-AM on reverberation duration (from 5.21 ± 0.48 s to 3.23 ± 1.09 s, n = 5, P = 0.4) and occurrence (from 0.58 ± 0.11 to 0.02 ± 0.01, P < 0.01). (C) Enhancement of reverberation by replacing half of the extracellular Ca2+ with Sr2+ (from 3 mM Ca2+ to 1.5 mM Ca2+ plus 1.5 mM Sr2+). (D) Summary of effects of Sr2+ on reverberation duration (from 0.81 ± 0.69 s to 6.06 ± 1.46 s, n = 5, P < 0.02) and occurrence (from 0.04 ± 0.03 to 0.63 ± 0.14, P < 0.01). (E) Enhancement of reverberation by CPA (50 μM). (F) Summary of effects of CPA on reverberation duration (from 5.66 ± 1.94 s to 4.26 ± 1.36 s, n = 6, P = 0.07) and occurrence (from 0.34 ± 0.13 to 0.48 ± 0.10, P < 0.05). (G) Suppression of reverberation by ryanodine (20 μM). (H) Summary of effects of ryanodine on reverberation duration (from 4.87 ± 0.46 s to 4.33 ± 0.45 s, n = 5, P = 0.51) and occurrence (from 0.63 ± 0.10 to 0.29 ± 0.07, P < 0.05). (I) Correlation between normalized changes in the occurrence of reverberation (R) and asynchronous release (A) caused by different pharmacological challenges (see Materials and Methods for definitions of ΔR* and ΔA*. Correlation coefficient ρ = 0.93, P < 0.001). (Scale in A, C, E, and G, 100 ms, 0.5 nA.)
Results
Evoked, All-or-None Reverberatory Activity in Cultured Networks. We selected networks of 20–100 rat hippocampal neurons grown on near confluent glial islands (Fig. 1A). Perforated whole-cell patch-clamp recordings were made from one or two neurons within the network. At the stage of our studies (2–3 weeks in vitro), spontaneous activity was rare in these networks. However, in most cases, a brief stimulation pulse (100 mV, 1 ms) applied to one cell (if excitatory) under voltage clamp could evoke a reproducible waveform of PSC recorded from the same cell. A PSC usually consisted of a cluster of many current components and lasted for ≈50–100 ms, as observed in smaller networks in culture (36). Such a PSC pattern reflected multiple polysynaptic pathways in the network that were activated in a particular temporal order (36). They were also reminiscent of the network activity motifs observed in other systems (19–25).
Fig. 1.
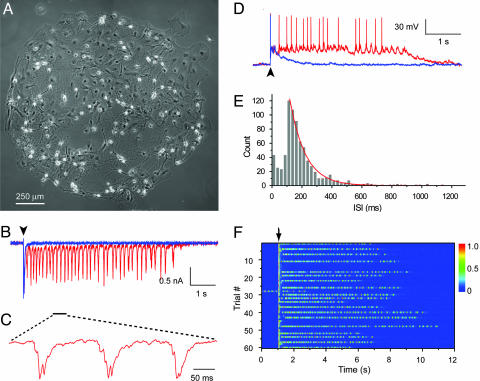
Evoked reverberatory activity in cultured neuronal networks. (A) A network of cultured hippocampal neurons on an island of glial cell monolayer. (B) Two current traces of evoked responses (blue, short polysynaptic current; red, persistent reverberation) recorded from a single neuron under voltage clamp. A single 1-ms stimulus pulse applied to the same neuron was delivered at the beginning of each trace (arrowhead). (C) A 0.5-s segment of the trace shown in B is expanded to show the details of repeated PSC profiles. (D) Two traces of evoked potentials showing spike patterns of a single neuron during short response (blue) and persistent reverberation (red). (E) Distribution of interspike interval (ISI) during reverberation from the same experiment as shown in D. Results for ISI > 100 ms were fit with a single exponential curve (y = A × e-t/τ, A = 382.6, τ = 103.5). (F) “All-or-none” activation of reverberation. Shown are 60 consecutive trials of current traces, each triggered by a single stimulus (arrow) delivered at 0.03 Hz, from the same experiment as shown in B. Color codes current amplitude (in nA). A segment of spontaneous activity was recorded in trace 28.
In a fraction of networks (≈20%), a single stimulus could evoke a long-lasting polysynaptic activity episode persisting for seconds (Fig. 1B). Such an episode consisted of many PSC clusters with similar current waveforms (Fig. 1C), suggesting the repetition of similar firing patterns in the network. When recorded under current clamp, a neuron in the reverberating network usually fired zero to two spikes during the time period of each PSC cluster. The firing of the neuron had a moderate overall rate of 5–15 Hz and a Poisson-like interspike interval distribution (Fig. 1 D and E). It should be noted that the polysynaptic pattern was better resolved by voltage clamp recordings than by current clamp recordings because the latter were limited by the membrane time constant. The multiple components within each PSC cluster were due to inputs from different neurons in the network with different firing timings. During the long-lasting response, such firing patterns within the local neuronal circuit or cell assembly appeared to regenerate themselves, resulting in reverberatory activity reflected by the repeated PSC clusters. Here, repetition is used in a statistical or population sense, because the firing of an individual neuron during the period of a PSC cluster exhibited a degree of stochasticity (Fig. 1D). At a low frequency (e.g., 0.03 Hz), a test stimulus usually could trigger, in a largely all-or-none manner, either a short PSC response lasting no more than a few hundred milliseconds or reverberation lasting for seconds (Fig. 1F, see also Fig. 3D).
Roles of Inhibitory and Excitatory Synaptic Transmission in Reverberation. A large portion of networks we examined did not exhibit evoked reverberation under normal conditions. We hypothesized that this could be at least in part due to unbalanced inhibition in the cultured networks because a significant portion (≈10–20%) of these neurons were GABAergic. As expected, in the presence of 10 μM bicuculline that fully blocks GABAA receptors, similar test stimuli could elicit reverberation in previously nonreverberating networks (Fig. 2A). Even in normally reverberating networks, bicuculline often increased the duration and occurrence probability of the evoked reverberation (Fig. 2B). Therefore, GABAA receptor-mediated inhibitory transmission played an important role in suppressing reverberation. Interestingly, blockade of inhibition did not lead to spontaneous epileptiform activity as observed in other systems (37). This might be due to the relatively small network size used in this study because epileptiform high-frequency firing was indeed observed in some larger networks. In later experiments, we often added bicuculline to enhance network reverberation before adding other pharmacological reagents to evaluate their impact. Still, reverberation properties (duration and occurrence) varied greatly from one network to another, perhaps because of the inherent heterogeneity of these randomly formed circuits. However, changes in these properties in response to pharmacological challenges were consistent and thus could provide valuable insights into the underlying mechanisms.
Fig. 2.
Differential contributions of excitatory and inhibitory synaptic transmission to evoked reverberation. (A) Enhanced reverberation in the presence of GABAA receptor antagonist bicuculline methiodide (BMI, 10 μM). Expanded views of example traces (1-s segments) are shown above the color graphs (scale, 0.5 nA, 100 ms). Arrowheads and arrows mark the time points when stimuli were applied to another neuron in the network. (B) Summary of effects of BMI on the occurrence probability and duration of reverberation. Each data point is the mean value of reverberation duration (error bars are SEM) or occurrence probability measured from a single experiment that included at least 20 trials of stimulation at 0.03 Hz under a given condition (see Materials and Methods). BMI resulted in significant increase in both the average reverberation duration (from 0.66 ± 0.25 s to 4.08 ± 0.62 s, n = 24, P < 0.001, t test for paired samples) and occurrence probability (from 0.06 ± 0.02 to 0.52 ± 0.06, P < 0.001). (C) Suppression of reverberation by partial blockade of AMPA receptor with CNQX (0.25 μM). In the example traces, the peaks of first PSC clusters (peak amplitude, 1,306 pA in control, 1,120 pA in CNQX) were cut off to better show the reverberatory PSC waveforms. (Scale, 300 pA, 100 ms.) (D) Summary of effects of CNQX (0.05 to 0.25 μM, as indicated) on reverberation. Significant reduction in both duration (from 1.67 ± 0.26 s to 0.88 ± 0.38 s, n = 7, P < 0.05) and occurrence probability (from 0.43 ± 0.03 to 0.18 ± 0.08, P < 0.01) was observed. (E) Suppression of reverberation with full blockade of NMDA receptor with d-AP5 (25 μM). (Scale, 1 nA, 100 ms.) (F) Summary of effects of d-AP5 (25 μM) on reverberation. No significant reduction in duration (from 2.45 ± 0.96 s to 2.03 ± 0.99 s, n = 7, P = 0.14) was observed, but the reduction in occurrence probability (from 0.38 ± 0.08 to 0.17 ± 0.06, P < 0.001) was significant.
To evaluate the role of excitatory synaptic transmission in network reverberation, we examined the effects of both CNQX, a specific antagonist of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA)-type glutamate receptors, and d-AP5, a specific blocker of the NMDA-type glumatate receptors. Interestingly, partial inhibition of AMPA receptors with low concentrations of CNQX (e.g., 0.2 μM that reduced EPSC amplitude by ≈20%) resulted in substantial reduction or elimination of reverberation (Fig. 2 C and D). Therefore, the reverberation depended critically on synaptic weights, consistent with predictions by attractor models of recurrently connected cell assemblies (5, 7). In contrast, full blockade of NMDA receptors with 25 μM d-AP5 reduced the occurrence and duration of reverberation, but did not completely eliminate it (Fig. 2 E and F). Therefore, although NMDA currents contributed to the reverberation, their contribution was limited, perhaps because of a low NMDA/AMPA current ratio in the hippocampal cells.
Enhanced Activation of Reverberation by Paired-Pulse Stimulation. When a network was stimulated with different input patterns, we found that paired-pulse stimuli (PPS) with 200- to 400-ms interpulse intervals were more likely to elicit network reverberation than single pulses (Fig. 3 A and B). This indicated that a network could be more sensitive to the physiologically relevant repetitive input stimuli. Notably, PPS resulted in an increase in a slow, asynchronous phase of the polysynaptic currents after the more synchronized PSC cluster (Fig. 3C). Moreover, the probability of evoked reverberation appeared to be associated with the size of this slow phase of PSC: for a given network, reverberatory traces tended to have higher levels of the slow phase of PSC (Fig. 3D). This observation suggests that the slow phase of PSC was important in reactivating the network after a burst of semisynchronous activity, thereby maintaining the network in the up-state. One possible source of the slow phase was neuronal firing in the network (at a lower rate). However, large evoked synaptic current components (>100 pA) were rare in the gap between the PSC clusters, suggesting that the network was rather quiet during the asynchronous periods. Therefore, we hypothesize that the slow phase of PSC was due to asynchronous synaptic transmitter release.
Asynchronous Synaptic Transmission Plays a Central Role in Reverberation. Asynchronous transmitter release is a fundamental property of synaptic transmission, caused by increased probability of synaptic vesicle exocytosis in response to residual calcium elevation after action potentials that trigger the “synchronous” phase of synaptic transmission (28–35). It typically has a time constant of a few hundred milliseconds and is controlled to a large extent by calcium clearance (31, 32, 38). Could this “noise” of synaptic transmission, by filling the gap between PSC clusters, maintain the network in a reverberating state? To evaluate this possibility, we took advantage of a slow calcium chelator, EGTA-AM, that buffers residual calcium elevation and thereby suppresses asynchronous release while allowing normal synchronous transmission (32, 38). After 10-min treatment with EGTA-AM, we observed significant reduction in the asynchronous phase of PSC profiles (Fig. 4 A and I). Meanwhile, the long-lasting reverberation was virtually abolished (Fig. 4 A and B). It should be noted that several of these experiments (four of five) were performed in the presence of BMI to block GABAA receptors. Thus, the effects of EGTA-AM were not due to changes in inhibitory transmissions. Because EGTA-AM also affected, although to a much less extent, the synchronous phase of synaptic transmission (38), in some experiments we first lowered extracellular calcium concentration (e.g., from 3 mM to 2 mM) and then tested the effects of adding EGTA-AM together with increased extracellular calcium (e.g., from 2 mM to 3 mM). Reverberation was again eliminated, although there was no reduction of the synchronous PSC amplitudes (see Fig. 5, which is published as supporting information on the PNAS web site). Therefore, the effect of EGTA-AM on reverberation was likely due to inhibition of the asynchronous phase of evoked synaptic transmission. In a converse experiment, we replaced part of the extracellular calcium with strontium, a divalent cation that partially supports synaptic transmission and has been shown to enhance asynchronous transmitter release at various nerve terminals (29, 31, 39). Both the duration and the occurrence probability of reverberation were substantially enhanced (Fig. 4 C and D). Similar results were also obtained when extra Sr2+ was added to the bath solution without reducing Ca2+ (see Fig. 6, which is published as supporting information on the PNAS web site). In some cases, we also observed elevated spontaneous reverberatory activity in Sr2+. It should also be noted that, with strontium replacing calcium, synchronous transmission was reduced (Fig. 4C). Thus, asynchronous release, instead of being an insignificant “noise” in synaptic transmission, played a central role in supporting the persistent state of reverberation in these networks.
What might regulate asynchronous transmission under physiological conditions? One candidate was the presynaptic endoplasmic reticulum (ER), an intracellular calcium store that plays a key role in calcium clearance and may also facilitate synaptic vesicle exocytosis by releasing calcium (40–42). Uptake into and release from calcium stores are modulated by intricate inter- and intracellular signaling systems (43). As a demonstration of principle, we interfered with the store function by using two commonly used reagents: CPA, which blocks calcium uptake through calcium ATPase on the presynaptic ER (43), and ryanodine, which, at high concentrations, blocks calcium store release through ryanodine receptors (40–42). Reverberation was markedly enhanced within the first 20 min of CPA application (Fig. 4 E and F). In contrast, 20 μM ryanodine reduced reverberating activity in the network (Fig. 4 G and H). Therefore, through regulating asynchronous synaptic transmission, intracellular calcium stores at the presynaptic terminal might play a role in modulating the dynamics of functional neuronal circuits. As shown in Fig. 4I, a tight correlation was revealed between the effects of different reagents on asynchronous release and their effects on reverberation, further strengthening the causal link between the synaptic process and the network phenomenon.
Discussion
Since its conceptualization by Lorente de Nó (8) and Hebb (1), reverberating activity in neural networks has been explored in a variety of theoretical frameworks (5–7). Using a simplified culture system, our observations demonstrate that reverberating activity can emerge in relatively simple generic networks of mammalian central neurons without predefined anatomical specializations. The reverberation depends critically on the strength of excitatory synaptic transmission, and can be abolished by partial inhibition of excitatory synapses with low doses of CNQX. In addition, reverberation occurs more frequently with the blockade of synaptic inhibition. Thus, the reverberation we observed is likely to be driven by recurrent synaptic excitation, similar to that observed in simpler two-cell networks of invertebrate neurons (44). In general, persistent activity can also result from intrinsic membrane bistability (or multistability) that by itself can serve as a short-term memory trace (26, 45). Under our experimental conditions, none of the neurons we have recorded exhibited intrinsic bistability that was capable of driving persistent firing. Nevertheless, it is conceivable that the combination of recurrent excitation and intrinsic bistability (perhaps in a small fraction of cells in the population) could lead to more robust reverberation in the network.
During the persistent state of reverberation, current clamp recording shows that a neuron in the network fires spikes at moderately low rates, reminiscent of the persistent activity observed in vivo during working memory tasks (5, 7, 46, 47). The stochastic firing of a neuron above a fluctuating background of synaptic inputs also to some extent resembles the spontaneous and evoked “up-states” observed in vivo and in slices (27, 48). However, it should be noted that there is a substantial component of oscillation in the reverberation as revealed more clearly by voltage clamp recording. Thus, during the reverberatory episode, firing of different cells in the network is partially synchronized, with alternating “on” and “off” periods. This finding appears to differ from the asynchronous persistent activity in vivo recorded by extracellular electrodes (5, 7, 46, 47). However, oscillation in the theta band (5–15 Hz, similar to that observed here), often involved in spatial exploration and memory formation (49), has also been found to be prominent in human cortex during certain working memory tasks (50). In addition, visual stimuli could evoke synaptically driven oscillatory activity at slightly higher frequencies in the cat visual cortex (51). Furthermore, the reverberation episodes in culture consist of repeating polysynaptic current motifs (PSC clusters) that reflect ordered network activity patterns, reminiscent of the “synchronous bursting” and activity motifs observed in other in vitro and in vivo systems (19–25). Whether and how the temporal structure of such activity patterns might carry physiologically relevant information remains to be investigated.
From a mechanistic point of view, the finding that persistent reverberation requires the asynchronous phase of synaptic transmission is of particular interest. At low activity levels, the contribution of asynchronous transmitter release to synaptic currents often appears to be insignificant compared to “synchronous” release (35). However, it also has much slower kinetics (decay time constant ≈100–200 ms after single stimulus and longer after repeated stimuli) (31, 32, 38); this allows asynchronous transmission to bridge the gap between adjacent network bursting periods, thus supporting persistent reverberation. Furthermore, repeated synaptic activation can result in buildup of residual cytosolic calcium, which in turn enhances asynchronous release (32, 38). Thus, asynchronous transmission is in effect facilitating during reverberation. This facilitation provides another level of positive feedback that stabilizes all-or-none reverberation. At a functional level, the facilitation may also enable a network to detect physiologically relevant repetitive input stimuli. Several attractor models predict that a slow component of excitatory synaptic transmission is needed for the stability of various forms of network activity such as desynchronized persistent firing and robust graded persistent activity (15, 16, 18). In these models, NMDA receptor-mediated excitatory synaptic current has been proposed to play this role by virtue of its slow time constant. It is possible that asynchronous transmitter release could also play a similar role in these other forms of functionally relevant persistent activities, especially in systems such as hippocampal neurons where excitatory transmission is mainly mediated by AMPA currents.
Because asynchronous release is a fundamental component of synaptic transmission (28–35), it is likely to exist in various synapses in the brain and may play similar roles in the persistent activity in vivo. If this is the case, regulation of asynchronous release by cellular processes (e.g., calcium uptake into and release from intracellular stores) may be involved in modulating persistent activity relevant to system behaviors such as attention. Meanwhile, defects in the regulation could lead to system dysfunctions such as epilepsy. Although neurons in culture do not capture all of the complexity of network behavior in vivo, the simplicity of this reduced system allows us to directly evaluate fundamental principles and uncover basic mechanisms relevant to the complex behavior in an experimentally accessible setting.
Conclusion
This study shows that reverberatory activity can be evoked in cultured neuronal networks that have no preexisting anatomical specialization. The reverberation is primarily driven by recurrent synaptic excitation rather than cellular bistability. Furthermore, the persistence of reverberation depends critically on the oft-overlooked asynchronous transmitter release. Finally, asynchronous release, as well as its regulation by intracellular calcium stores, may act as functional nodes in the modulation of network reverberation.
Supplementary Material
Acknowledgments
We thank H. Wang and X. Zhao for their help with culture preparations and E. Aizenman, R. Gerkin, Y. Goda, J. Horn, and D. Nauen for discussions and comments on the manuscript. This work was partially supported by Burroughs Wellcome Fund Career Award in the Biomedical Sciences and National Institutes of Health/National Institute of Mental Health Grant R01 MH066962 (to G.-Q.B.). P.-M.L. was supported by National Institute of Neurological Diseases and Stroke Training Grant T32 NS07391.
Author contributions: P.-M.L. and G.-Q.B. designed research; P.-M.L. and G.-Q.B. performed research; P.-M.L. and G.-Q.B. analyzed data; and P.-M.L. and G.-Q.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; AP5, 2-amino-5-phosphopentanoic acid; BMI, bicuculline methiodide; CPA, cyclopiazonic acid; PSC, polysynaptic current; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid.
References
- 1.Hebb, D. O. (1949) The Organization of Behavior (Wiley, New York). [DOI] [PubMed]
- 2.Stevens, C. F. (1996) Nature 381, 471-472. [DOI] [PubMed] [Google Scholar]
- 3.Milner, B., Squire, L. R. & Kandel, E. R. (1998) Neuron 20, 445-468. [DOI] [PubMed] [Google Scholar]
- 4.Fuster, J. (1995) Memory in the Cerebral Cortex (MIT Press, Cambridge, MA).
- 5.Durstewitz, D., Seamans, J. K. & Sejnowski, T. J. (2000) Nat. Neurosci. 3, Suppl., 1184-1191. [DOI] [PubMed] [Google Scholar]
- 6.Seung, H. S. (2000) Nat. Neurosci. 3, Suppl., 1166. [DOI] [PubMed] [Google Scholar]
- 7.Wang, X. J. (2001) Trends Neurosci. 24, 455-463. [DOI] [PubMed] [Google Scholar]
- 8.Lorente de Nó, R. (1933) Arch. Neurol. Psychiatry 30, 245-291. [Google Scholar]
- 9.McCulloch, W. S. & Pitts, W. (1943) Bull. Math. Biophys. 5, 115-133. [DOI] [PubMed] [Google Scholar]
- 10.Hopfield, J. J. (1982) Proc. Natl. Acad. Sci. USA 79, 2554-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amit, D. J., Gutfreund, H. & Sompolinsky, H. (1985) Phys. Rev. A 32, 1007-1018. [DOI] [PubMed] [Google Scholar]
- 12.Kleinfeld, D. & Sompolinsky, H. (1988) Biophys. J. 54, 1039-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zipser, D., Kehoe, B., Littlewort, G. & Fuster, J. (1993) J. Neurosci. 13, 3406-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amit, D. J., Brunel, N. & Tsodyks, M. V. (1994) J. Neurosci. 14, 6435-6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, X. J. (1999) J. Neurosci. 19, 9587-9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seung, H. S., Lee, D. D., Reis, B. Y. & Tank, D. W. (2000) Neuron 26, 259-271. [DOI] [PubMed] [Google Scholar]
- 17.Laing, C. R. & Chow, C. C. (2001) Neural. Comput. 13, 1473-1494. [DOI] [PubMed] [Google Scholar]
- 18.Tegner, J., Compte, A. & Wang, X. J. (2002) Biol. Cybern. 87, 471-481. [DOI] [PubMed] [Google Scholar]
- 19.Maeda, E., Robinson, H. P. & Kawana, A. (1995) J. Neurosci. 15, 6834-6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Segev, R., Shapira, Y., Benveniste, M. & Ben-Jacob, E. (2001) Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 64, 011920. [DOI] [PubMed] [Google Scholar]
- 21.Beggs, J. M. & Plenz, D. (2003) J. Neurosci. 23, 11167-11177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cossart, R., Aronov, D. & Yuste, R. (2003) Nature 423, 283-288. [DOI] [PubMed] [Google Scholar]
- 23.Eytan, D., Brenner, N. & Marom, S. (2003) J. Neurosci. 23, 9349-9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beggs, J. M. & Plenz, D. (2004) J. Neurosci. 24, 5216-5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikegaya, Y., Aaron, G., Cossart, R., Aronov, D., Lampl, I., Ferster, D. & Yuste, R. (2004) Science 304, 559-564. [DOI] [PubMed] [Google Scholar]
- 26.Egorov, A. V., Hamam, B. N., Fransen, E., Hasselmo, M. E. & Alonso, A. A. (2002) Nature 420, 173-178. [DOI] [PubMed] [Google Scholar]
- 27.Shu, Y., Hasenstaub, A. & McCormick, D. A. (2003) Nature 423, 288-293. [DOI] [PubMed] [Google Scholar]
- 28.Del Castillo, J. & Katz, B. (1954) J. Physiol. 124, 574-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miledi, R. (1966) Nature 212, 1233-1234. [DOI] [PubMed] [Google Scholar]
- 30.Barrett, E. F. & Stevens, C. F. (1972) J. Physiol. 227, 691-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goda, Y. & Stevens, C. F. (1994) Proc. Natl. Acad. Sci. USA 91, 12942-12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cummings, D. D., Wilcox, K. S. & Dichter, M. A. (1996) J. Neurosci. 16, 5312-5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Atluri, P. P. & Regehr, W. G. (1998) J. Neurosci. 18, 8214-8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, T. & Trussell, L. O. (2000) Neuron 26, 683-694. [DOI] [PubMed] [Google Scholar]
- 35.Nelson, S. (2000) Neuron 26, 545-546. [DOI] [PubMed] [Google Scholar]
- 36.Bi, G.-Q. & Poo, M.-M. (1999) Nature 401, 792-796. [DOI] [PubMed] [Google Scholar]
- 37.McCormick, D. A. & Contreras, D. (2001) Annu. Rev. Physiol. 63, 815-846. [DOI] [PubMed] [Google Scholar]
- 38.Hagler, D. J., Jr. & Goda, Y. (2001) J. Neurophysiol. 85, 2324-2334. [DOI] [PubMed] [Google Scholar]
- 39.Xu-Friedman, M. A. & Regehr, W. G. (2000) J. Neurosci. 20, 4414-4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng, Y. (1996) J. Neurosci. 16, 6703-6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llano, I., Gonzalez, J., Caputo, C., Lai, F. A., Blayney, L. M., Tan, Y. P. & Marty, A. (2000) Nat. Neurosci. 3, 1256-1265. [DOI] [PubMed] [Google Scholar]
- 42.Simkus, C. R. & Stricker, C. (2002) J. Physiol. 545, 521-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verkhratsky, A. (2002) Cell Calcium 32, 393-404. [DOI] [PubMed] [Google Scholar]
- 44.Kleinfeld, D., Raccuia-Behling, F. & Chiel, H. J. (1990) Biophys. J. 57, 697-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loewenstein, Y., Mahon, S., Chadderton, P., Kitamura, K., Sompolinsky, H., Yarom, Y. & Hausser, M. (2005) Nat. Neurosci. 8, 202-211. [DOI] [PubMed] [Google Scholar]
- 46.Fuster, J. M. & Alexander, G. E. (1971) Science 173, 652-654. [DOI] [PubMed] [Google Scholar]
- 47.Kubota, K. & Niki, H. (1971) J. Neurophysiol. 34, 337-347. [DOI] [PubMed] [Google Scholar]
- 48.Anderson, J., Lampl, I., Reichova, I., Carandini, M. & Ferster, D. (2000) Nat. Neurosci. 3, 617-621. [DOI] [PubMed] [Google Scholar]
- 49.Hasselmo, M. E., Hay, J., Ilyn, M. & Gorchetchnikov, A. (2002) Neural Netw. 15, 689-707. [DOI] [PubMed] [Google Scholar]
- 50.Raghavachari, S., Kahana, M. J., Rizzuto, D. S., Caplan, J. B., Kirschen, M. P., Bourgeois, B., Madsen, J. R. & Lisman, J. E. (2001) J. Neurosci. 21, 3175-3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bringuier, V., Fregnac, Y., Baranyi, A., Debanne, D. & Shulz, D. E. (1997) J. Physiol. 500, 751-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.