Abstract
Ediacara fossils are among the oldest known macroscopic and complex life forms. Their bodyplan, ecology, and phylogenetic affinities have been controversial. On the basis of taphonomic observations, Seilacher [Seilacher, A. (1989) Lethaia 22, 229–239] proposed that the core elements of the Ediacara biota, the vendobionts, were constructed with serially or fractally arranged quilts or tube-like units. However, anatomy of quilt walls has been rarely reported, because most Ediacara fossils are preserved as casts and molds in siliciclastic rocks with inadequate morphological resolution. Here, we report an Ediacara form, uniquely preserved in situ and in three dimensions with its organic walls cast by early diagenetic calcite, from bituminous limestone of the 551- to 542-mega-annum Dengying Formation of South China. Despite diagenetic tampering, serial sections show that the Dengying form consists of biserially arranged, tube-like quilts, each with two vertical side walls, a floor, a roof, and an open distal end. Three-dimensional morphological complexity of the Dengying form excludes a microbial interpretation but is broadly consistent with vendobionts. Unlike classic frondose vendobionts sensu Seilacher, however, the Dengying form probably lacked a smooth margin and had distally open quilts. It probably lived procumbently at or near the water–sediment interface and shows evidence for substrate utilization. Despite its uncertain phylogeny, ontogeny, and functional biology, the Dengying form adds to Ediacaran biodiversity, places key constraints on the ecology and extinction of Ediacara organisms, and points to the need to explore an alternative taphonomic window for Ediacara biology.
Keywords: Neoproterozoic, Vendobionta, taphonomy, ecology
Ediacara fossils§ [≈575–542 mega-annum (Ma) (3, ¶)] were first described in the late 19th century (2), decades before the discovery of Burgess Shale fossils (4). In comparison to the Burgess Shale biota, however, the Ediacara biota is rather poorly understood. Various Ediacara fossils have been interpreted as xenophyophoran protists (5, 6), organisms with phototrophic symbionts (7), lichens (8), fungus-like multicellular eukaryotes (9), sponges (10), cnidarians or cnidarian-grade animals (11, 12), bilaterian animals (13–15), or vendobionts, macroscopic organisms with a quilted bodyplan and uncertain phylogenetic relationships with extant clades (16, 17). Although these divergent interpretations partly mirror the biodiversity in the late Ediacaran biosphere (18–20), they also illustrate the unyielding challenges in the interpretation of Ediacara fossils. A key challenge comes from the preservation of Ediacara fossils: almost all Ediacara fossils are preserved as casts and molds in siliciclastic rocks. Although some Ediacara casts and molds are preserved with incredible morphological resolution (21), the majority do not have sufficient morphological details, and few show direct evidence of organic walls or internal structures. Only two Ediacara assemblages are known to occur in nonsiliciclastic rocks: the terminal Neoproterozoic Dengying Formation in South China (22) and the Khatyspyt Formation in Siberia (23). Both assemblages occur in bituminous limestone, but neither has been studied in detail. To explore possible insight from the carbonate taphonomic window, we investigated the Dengying Formation and discovered a fossil form that may expand our knowledge about the bodyplan, taphonomy, and ecology of Ediacara organisms.
Geological and Stratigraphic Setting
About 20 specimens of the fossil form were collected from the middle Shibantan Member of the Dengying Formation at two localities (Muzhuxia, 30°45.03′N, 110°59.48′E; and Miaohe, 30°59.12′N, 111°13.27′E) in the Yangtze Gorges area, South China. The Dengying Formation in this area consists of three units: in stratigraphic order, the Hamajing, Shibantan, and Baimatuo members (Fig. 1). The Shibantan Member is characterized by dark gray, finely laminated, bituminous limestone (Fig. 2A) deposited in subtidal facies likely below fair weather wave base, whereas both the Hamajing and Baimatuo members consist of thick-bedded, light gray, peritidal dolostones (24). The Shibantan Member also contains, in addition to the recently discovered Ediacara form, abundant ribbon-like fossils (Vendotaenia antiqua), microbial structures (Fig. 2B), horizontal trace fossils (Fig. 2 B and C), and a rare Ediacara fossil described as Paracharnia dengyingensis (22, 24, 25). The biomineralized tubular fossil Sinotubulites baimatuoensis occurs in the uppermost Shibantan or lowermost Baimatuo members (24, 26), and Cloudina hartmannae occurs in correlative strata elsewhere in South China (27, 28). The Dengying Formation is overlain by chert, phosphorite, and dolostone of the Yanjiahe Formation, which contains basal Cambrian acritarchs and small shell fossils (29–31). The Dengying Formation in South China is radiometrically bracketed between 551.1 ± 0.7 Ma (32) and 538.2 ± 1.5 Ma (33), and it is probably older than 542 Ma (3).
Fig. 1.
Stratigraphic column showing fossil horizon (⋆). The thickness of the Hamajing Member varies from ≈20 m to > 100 m in the Yangtze Gorges area.
Fig. 2.
Finely laminated limestone (A), microbial structures (B), and horizontal trace fossils (arrows in B and C) from the Shibantan Member.
Morphologic Description and Interpretation
Specimens were found on the top bedding surface of finely laminated bituminous limestone and are typically overlain by a thin (≈150-μm) layer of fine silt (Fig. 3 C and D). Individual specimens can reach decimeters in horizontal dimension. They are usually preserved in clusters and typically, but not always, with a preferred orientation (Fig. 3 A and B). They are sometimes closely packed but never overlap or crosscut (Fig. 3 A–C). Viewed on bedding surface, each individual has a slightly zigzag central axis with alternate side branches on both sides (Fig. 3 A–F), thus forming a biserial branching structure. Side branches emerge from the central axis at variable angles (26.7–92.9°; mean, 51.7°; SD, 20.1°; n, 35); when the angle is acute, the alternate side branches appear to pseudomonopodially emerge from the central axis (see the specimens between arrows in Fig. 3B). Second-order side branches are rare but do occur (see the specimen bracketed by arrowheads in Fig. 3B). Side branches are distally terminated (black arrowheads in Fig. 3E), although some can further branch dichotomously (unlabeled white arrowheads in Fig. 3F). Side branches from different central axes can have close contact (arrow in Fig. 3E), but they never crosscut. Distal termini of side branches define the individuality of fossils; thus, individual fossils do not have a smoothly delineated margin.
Fig. 3.
Bedding surface views (A–F) and serial thin sections (G–L; perpendicular to bedding, upper bedding surface on top, nonpolarized transmitted light photomicrographs). (A) Clustered, preferentially oriented specimens with no evidence of overlapping or crosscutting. The specimen on the right, whose central axis (ca) is bracketed with arrowheads, is almost perpendicular to preferentially oriented specimens on left. (B) Detail showing two specimens (arrows) that stopped growing because of fast-growing neighbors. (C) Slab with densely packed specimens and thin layer of silts (si). The light color of the central axis and side branches is accentuated by residual silts. (D) Detail of rectangle area in C showing silt layer. (E) Polished thick section of D showing distally terminated side branches (black arrowheads). Under reflected light, light-colored carbonaceous/clay layer is flanked by dark-colored calcispar layers along central axis and side branches. Arrow points to close contact but no crosscut between side branches from two individuals; the contact can be confirmed by tracing at high magnification the carbonaceous/clay layer, which does not continue from one branch to another, and by the observation that the two side branches cannot be traced to the same central axis. (F) Specimen with short side branches (black arrows and arrowheads) and distally dichotomous side branches (white arrowheads). (G–K) Serial thin sections corresponding to labeled lines in F. Pendent vertical walls correspond to distal part of side branch (white arrows) or short side branch (black arrows). The arching vertical wall (arrowheads) is indicative of prior existence of floor. (K) Oblique section of central axis (ca). (L) Detail of I showing pendent vertical wall (arrow) and stylolite. (Scale bars: 1 mm, except where otherwise noted.)
In serial thin sections (Fig. 3 G–K; at ≈2-mm interval) perpendicular to bedding plane and to side branches, it can be seen that the central axis and side branches extend downwards to a depth of ≈1 mm (0.53–1.32 mm; mean, 1.00 mm; SD, 0.18 mm; n, 35), forming vertical side walls perpendicular to bedding plane. The vertical side walls are often truncated by stylolites (Fig. 3L; and 4 A–F). In a few cases where stylolitic dissolution is less severe, neighboring vertical side walls are connected at the lower and upper ends by, respectively, horizontal floor and roof walls (arrows in Fig. 4 A–D). When the floor or roof walls have been removed by stylolitic dissolution, their prior existence is indicated by the arching vertical walls (arrowheads in Figs. 3 G–J and 4D). However, some vertical side walls do not penetrate deep enough to reach the floor. These pendent vertical walls tend to be related to short side branches (black arrows in Fig. 3 F and H–J) and distal part of side branches (white arrows in Fig. 3 F–K).
Fig. 4.
Thin sections perpendicular to bedding plane (upper bedding surface on top; G is a scanning electron photomicrograph, and all others (A–F, H, and I) are transmitted light photomicrographs). (A and B) Serial thin sections corresponding to labeled lines in Fig. 3C. Tri-layered vertical side walls consist of a poorly defined carbonaceous/clay layer flanked by calcispar layers (light color). Stylolites partially truncate floor walls (arrows). (C and D) Partially preserved roof (arrows) and arching vertical side walls (arrowheads) indicative of prior existence of roof. (E and F) Tri-layered pendent vertical walls with poorly preserved crystal termini (serrate interface between carbonaceous/clay and calcispar layers). Stylolites (arrows) completely truncate roof walls. (G) Backscattered electron (BSE) image of a vertical wall consisting of carbonaceous/clay layer (CC) flanked by calcispar layers (CS). (H) Lensoidal pockets of calcispar (arrows) crosscut calcispar layer of vertical wall. (I) Lensoidal pockets of calcispar (arrow) in the same thin section, with surrounding microbial laminae. (Scale bar in C applies to C–F and H–I.)
Neither the roof/floor nor neighboring side branches are connected by a vertical distal wall. The absence of a distal wall is indicated by distally terminated side branches, clearly seen in a thick section parallel to bedding plane (Fig. 3E). Indeed, distally terminated side branches are clear on exposed bedding surface (Fig. 3B), which represents a natural section parallel to side branches because the frond-like morphology is only visible when the roof is removed by stylolitic dissolution or weathering. The absence of distal walls is probably biological, not preservational, because it is unlikely that taphonomic process (see Taphonomy) would selectively preserve side walls but not distal walls, or would preferentially destroy distal walls but not side walls.
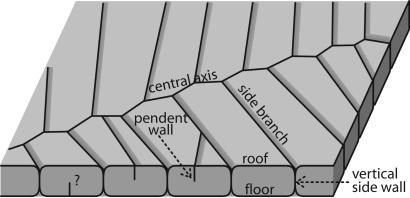
Combining observations on bedding plane and in perpendicular thin sections, we reconstruct the Dengying form as a biserially branching fossil consisting of tube-like units that are broadly similar but not identical to quilts described by Seilacher (16, 17). Each quilt is ≈2 mm in width (0.69–4.13 mm; mean, 1.96 mm; SD, 0.87 mm; n, 34) and >1 mm in depth; the exact depth cannot be accurately estimated because of stylolitic dissolution and compaction. Each quilt has two vertical side walls connected by roof and floor walls (Fig. 5), as well as an open distal end; thus, they could not have been filled with cytoplasm as proposed in the original Vendobionta hypothesis (16, 17). The Dengying form can also have secondary central axes and side branches, as well as pendent vertical walls to incompletely subdivide quilts. Pendent vertical walls tend to be associated with short side branches or the distal part of side branches, which are interpreted to be formed, through insertion or distal extension, relatively late in ontogeny.
Fig. 5.
Box diagram showing quilted bodyplan of the Dengying form.
Taphonomy
The Dengying form owes its preservation to a unique taphonomic pathway that, to the best of our knowledge, has not been documented in other Ediacara fossils. The vertical side walls that define the quilts are delineated by an ≈100-μm-thick carbonaceous/clay layer, flanked by two ≈200-μm-thick calcispar layers (Fig. 4 A–F; calcite verified by energy-dispersive X-ray spectroscopy analysis). The calcispars are turbid and rich in inclusions, although they become clearer and larger toward the carbonaceous/clay layer. The gradient of inclusion density and crystal size, along with poorly preserved crystal termini (Fig. 4 E and F) that point to the carbonaceous/clay layer, suggests that the calcispars are probably void-filling cements, growing centripetally toward the carbonaceous layer. Calcispars probably filled the void after decaying organic walls, thus casting quilt walls. The carbonaceous/clay layer is interpreted as residual insolubles that were formed through organic decay and sediment filtration and were trapped between centripetally growing calcispar layers. The roof and floor walls may have formed similarly; however, the tri-layer structure (i.e., the carbonaceous layer flanked by calcite layers) is only partially preserved because of extensive stylolitic dissolution (Fig. 4C). We hypothesize that microbial degradation of quilt walls beneath a thin layer of silts (Fig. 3C) or perhaps a microbial mat (Fig. 2B) may have created localized geochemical conditions where early diagenetic calcite precipitation was encouraged. This unique taphonomic pathway is responsible for the calcite casting of quilt walls and the three-dimensional preservation of the Dengying form.
Crosscutting relationships and petrographic analysis indicate that the calcispar layer was formed after burial but before diagenetic compaction. First, the calcitic side walls are sometimes cut by lensoidal pockets of spars (arrows in Fig. 4H). The exact origin of these pockets of spars is unclear, but they clearly were formed before compaction because microbial laminae go around them (Fig. 4I). Second, the calcispars are typically cut by stylolites (Fig. 4 A–F), indicating that calcispar precipitation predated diagenetic compaction. Third, the turbid calcispars are very different from typically clear meteoric cements, and the abundance of inclusions indicates that they were formed before the consolidation of micritic matrix. Finally, all quilts appear to be filled with micrites that are identical, in texture and composition, to those outside the quilts; no geopetal structures occur in any quilts examined by us. Thus, the calcispars are early diagenetic in origin.
Further observations suggest that the Dengying fossils were preserved in situ. It is unlikely that such sheet-like organisms had been transported from elsewhere or sunken from the water column, because they show no evidence of folding, deformation, or overlapping. In addition, an allochthonous origin is inconsistent with the finely grained microlaminate and quiet subtidal depositional environment of the Shibantan Member. More likely, the Dengying organisms were preserved where they lived. In this regard, it is interesting to note that the Dengying fossils are covered by a thin silt layer (Fig. 3 C and D), which may have buried the Dengying organism in situ.
Ecology
Because the Dengying form was preserved in situ, it follows that it probably lived procumbently at or near the water–sediment interface. The uniformity of sediments inside and outside the quilts, the lack of any holdfasts or stalks, the lack of any folded specimens, and the nonoverlapping relationship in densely populated clusters are all consistent with a procumbent lifestyle. The preferred orientation in some (but not all) populations may be taken as evidence for current alignment of erect benthos. However, closely located specimens are not consistently oriented. For example, one specimen near an oriented cluster of specimens is oriented almost perpendicular to the clustered specimens (Fig. 3A). In addition, an erect life position is inconsistent with the lack of holdfasts, and current alignment is inconsistent with the lack of folding and overlapping. Furthermore, there appears to be evidence for substrate utilization: the arrowed specimens in Fig. 3B appear to have stopped growing because of fast-growing neighbors. This interdigitate spatial relationship would be rather fortuitous if the Dengying form was erect organisms felled by currents. Instead, we interpret the interdigitate relationship as evidence for spatial utilization by substrate exclusion. Interestingly, there is no evidence for active spatial competition by overgrowth, which is common among modern bryozoans and coralline algae. The absence of overgrowth indicates that growth of the Dengying form depended on direct contact with sediment substrate.
Thus, our preferred interpretation is that the Dengying form lived procumbently, although we are uncertain whether it reclined on the sediment surface or grew within sediments; the former interpretation appears to be inconsistent with the lack of geopetal structures in the quilts, and the latter raises questions about functional biology of the Dengying form.
Discussion
The Dengying form defies close morphological comparison to any described Ediacara fossils, most of which have well defined outer boundary and distinct individuality. Therefore, alternative interpretations must be considered and excluded. First, we can rule out the possibility that the Dengying form is a weathering structure, because it is crosscut by stylolites. Second, it does not appear to be an abiogenic fractal structure because of its tubular constructional elements, poor fractality, and consistent stratigraphic occurrences at multiple localities. Third, we know of no sedimentary structures that morphologically resemble the Dengying form. The Ediacara form Arumberia banksi was once interpreted as a cup-shaped organism (34, 35) but later as the sole cast of flute-like sedimentary structures (36). Whereas the interpretation of A. banksi is still uncertain (37), the biserially branching system and three-dimensional nature of the Dengying form are different from the fine ridges and grooves of A. banksi and, for that matter, impressions or casts of other known sedimentary structures. Fourth, early diagenetic structures such as molar-tooth (38) and syneresis structures lack the tri-layered walls and branching system characteristic of the Dengying form; instead, molar-tooth and syneresis structures are filled with homogenous calcimicrospars or sediments derived from overlying beds. Neither these nor any other early diagenetic structures (Fred Read, personal communication) have the three-dimensional organization of the Dengying form. Fifth, microbially induced sedimentary structures, such as wrinkles, petees, and dendrolites, are not close analogs of the Dengying form either. Microbial wrinkles are characterized by buckled microbial laminae (39), petees by polygonal pattern on bedding plane (37), and dendrolites by vertically oriented branching system (40). To the best of our knowledge, no published microbial structures have morphological complexity comparable to the Dengying form described here. Finally, some undermat tunnels made by living staphylinid beetles (41) may have a branching system and tubular structures similar to the Dengying form. However, staphylinid tunnels disturb mat laminae to make doming roofs that are laterally continuous with mat laminae, and they should have blind distal ends if lined with diagenetic calcispars. In contrast, the Dengying form has tri-layered calcisparic quilt walls that do not disturb microbial laminae, and its tubular quilts have open distal ends.
Therefore, the Vendobionta model (16, 17) is the closest, albeit imperfect, guide for the morphological interpretation of the Dengying form. As mentioned above, two related features, distally open quilts and lack of a smooth outer boundary, distinguish the Dengying form from vendobionts sensu Seilacher (16). However, an undescribed form from Ediacaran siliciclastic rocks in Australia (Jim Gehling, personal communication) is broadly similar to the Dengying form in the lack of definitive outer boundary. Thus, structures similar to the Dengying form may have a wide geographic and environmental distribution, not restricted to the Yangtze Gorges area and to carbonate facies.
Much remains to be understood about the Dengying form. It remains uncertain whether the quilts had cellular walls, whether they grew indeterminately, whether they represent individuals in a colony, whether and how the Dengying form grew vertically, how it reproduced, how it acquired nutrients from the environment, and how it is phylogenetically related to other Ediacara fossils and to extant macroscopic life. Answers to these questions require careful characterization of the much better preserved internal anatomy of the Dengying form.
Conclusions and Implications
Despite uncertainties in its phylogenetic and ontogenetic interpretation, the Dengying form makes several contributions to our understanding of the taphonomy of Ediacara fossils. First, the silt layer that overlies the Dengying fossil-bearing bed represents a taphonomic analog to Ediacara preservation under microbial mats, volcanic ashes, or sand deposits (37, 42, 43). In essence, silt-masking in the Dengying Formation is similar to volcanic ash-masking in the Mistaken Point Formation of Newfoundland; in both cases, the masking deposits are distinct from background sedimentation and are relatively thick compared with the limited relief of Ediacara fossils, allowing an easy split along the top bedding surface where fossils are visible. Second, three-dimensional preservation in a carbonate sedimentary environment, through calcite casting of decaying organic walls, represents a distinct taphonomic pathway. In this regard, careful investigation of the Khatyspyt Formation in Siberia (23) would provide additional insight into Ediacara taphonomic diversity.
The Dengying fossils also have broad implications for the paleoecology and extinction of Ediacara fossils. The procumbent lifestyle of the Dengying fossils at or near the water–sediment interface suggests that it contributed insignificantly to the tiering structure of Ediacaran epibenthic communities (44). The lifestyle also indicates that the Dengying form is ecologically similar, and perhaps convergently so given its quilted body plan, to crustose fungi, lichens, algae, and large protists, some of which do interact by substrate exclusion. In addition to the Dengying form described here, several vendobionts, including Dickinsonia (17) and Newfoundland spindles (44), have been interpreted as procumbent recliners (41). Other vendobionts, such as Ernietta (45, 46), Pteridinium (42, 47), Namalia (42), Ventogyrus (47), and perhaps Charnia (48) and Rangea (42), may have lived within sediments. These ecological interpretations, if reliable, are incompatible with a photosynthetic trophic strategy but more consistent with osmoheterotrophy or saprotrophy, ecologically analogous to modern fungi (9). Furthermore, it is possible that the procumbent and underground lifestyle diminished after the initial evolution of animal bioturbation, an animal innovation that had significant ecological and evolutionary consequences on nonmobile benthic organisms (49).
Regardless of how the Dengying form is related to other members of the Ediacara biota, it is clear that it significantly expands our view about vendobiont biodiversity, taphonomy, and ecology. Thus, Ediacaran limestones may represent an under-explored taphonomic window onto the Ediacara biota, which is just as fascinating as the Burgess Shale biota (4).
Acknowledgments
We thank J. Gehling, A. Knoll, M. Kowalewski, J. Lipps, G. Narbonne, F. Read, and three anonymous reviewers for discussion and comments. This study was supported by National Science Foundation Grant EAR-0354807 (to S.X.), Virginia Tech ASPIRES Program Grant 232830 (to S.X.), National Natural Science Foundation of China Grants 40372006 and 40472003 (to C.Z. and X.Y.), Chinese Academy of Science Grant KZCX-SW-141 (to X.Y.), and Chinese Ministry of Science and Technology Grants G20000777000 and 2003CB716805 (to X.Y. and C.Z.).
Author contributions: S.X. designed research; S.X., B.S., C.Z., G.X., and X.Y. performed research; S.X., B.S., and C.Z. analyzed data; and S.X. and B.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: Ma, mega-annum.
Footnotes
In this paper, “Ediacaran fossils” refer to fossils of the Ediacaran Period (1), whereas “Ediacara fossils” refer to macroscopic soft-bodied fossils similar to those preserved in the Ediacara Member of South Australia (2).
Bowring, S., Myrow, P., Landing, E., Ramezani, J. & Grotzinger, J. (2003) Geophys. Res. Abstr. 5, 13219.
References
- 1.Knoll, A. H., Walter, M. R., Narbonne, G. M. & Christie-Blick, N. (2004) Science 305, 621-622. [DOI] [PubMed] [Google Scholar]
- 2.Narbonne, G. M. (2005) Annu. Rev. Earth Planet. Sci. 33, 421-442. [Google Scholar]
- 3.Amthor, J. E., Grotzinger, J. P., Schröder, S., Bowring, S. A., Ramezani, J., Martin, M. W. & Matter, A. (2003) Geology 31, 431-434. [Google Scholar]
- 4.Briggs, D. E. G., Erwin, D. H. & Collier, F. J. (1994) The Fossils of the Burgess Shale (Smithsonian Inst. Press, Washington, DC).
- 5.Zhuravlev, A. Y. (1993) Neues Jahrb. Geol. Paläontol. Abh. 190, 299-314. [Google Scholar]
- 6.Seilacher, A., Grazhdankin, D. & Legouta, A. (2003) Paleontol. Res. 7, 43-54. [Google Scholar]
- 7.McMenamin, M. A. S. (1986) Palaios 1, 178-182. [Google Scholar]
- 8.Retallack, G. J. (1994) Paleobiology 20, 523-544. [Google Scholar]
- 9.Peterson, K. J., Waggoner, B. & Hagadorn, J. W. (2003) Integr. Comp. Biol. 43, 127-136. [DOI] [PubMed] [Google Scholar]
- 10.Gehling, J. G. & Rigby, J. K. (1996) J. Paleontol. 70, 185-195. [Google Scholar]
- 11.Jenkins, R. J. F. (1992) in Origin and Early Evolution of Metazoa, eds. Lipps, J. H. & Signor, P. W. (Plenum, New York), pp. 131-176.
- 12.Conway Morris, S. (1993) Nature 361, 219-225. [Google Scholar]
- 13.Glaessner, M. F. (1984) The Dawn of Animal Life: A Biohistorical Study (Cambridge Univ. Press, Cambridge, U.K.).
- 14.Gehling, J. G. (1991) Geol. Soc. India Mem. 20, 181-224. [Google Scholar]
- 15.Fedonkin, M. A. & Waggoner, B. M. (1997) Nature 388, 868-871. [Google Scholar]
- 16.Seilacher, A. (1989) Lethaia 22, 229-239. [Google Scholar]
- 17.Seilacher, A. (1992) J. Geol. Soc. (London) 149, 607-613. [Google Scholar]
- 18.Runnegar, B. (1995) Neues Jahrb. Geol. Paläontol. Abh. 195, 303-318. [DOI] [PubMed] [Google Scholar]
- 19.Narbonne, G. M. (1998) GSA Today 8 (2), 1-6. [Google Scholar]
- 20.Waggoner, B. (2003) Integr. Comp. Biol. 43, 104-113. [DOI] [PubMed] [Google Scholar]
- 21.Narbonne, G. M. (2004) Science 305, 1141-1144. [DOI] [PubMed] [Google Scholar]
- 22.Sun, W. (1986) Precambrian Res. 31, 361-375. [Google Scholar]
- 23.Fedonkin, M. A. (1990) in The Vendian System: Paleontology, eds. Sokolov, B. S. & Iwanowski, A. B. (Springer, Heidelberg), Vol. 1, pp. 71-120. [Google Scholar]
- 24.Zhao, Z., Xing, Y., Ding, Q., Liu, G., Zhao, Y., Zhang, S., Meng, X., Yin, C., Ning, B. & Han, P. (1988) The Sinian System of Hubei (China Univ. of Geosciences Press, Wuhan, China).
- 25.Dzik, J. (2002) J. Morphol. 252, 315-334. [DOI] [PubMed] [Google Scholar]
- 26.Chen, M., Chen, Y. & Qian, Y. (1981) Bull. Tianjin Inst. Geol. Min. Res., Chinese Acad. Geol. Sci. 3, 117-124. [Google Scholar]
- 27.Bengtson, S. & Yue, Z. (1992) Science 257, 367-369. [DOI] [PubMed] [Google Scholar]
- 28.Hua, H., Chen, Z., Yuan, X., Zhang, L. & Xiao, S. (2005) Geology 33, 277-280. [Google Scholar]
- 29.Chen, P. (1984) Prof. Pap. Stratigra. Palaeontol. 13, 49-66. [Google Scholar]
- 30.Ding, L., Li, Y. & Chen, H. (1992) Acta Micropalaeontol. Sinica 9, 303-309. [Google Scholar]
- 31.Yin, C., Gao, L. & Xing, Y. (2003) Acta Palaeontol. Sinica 42, 76-88. [Google Scholar]
- 32.Condon, D., Zhu, M., Bowring, S., Wang, W., Yang, A. & Jin, Y. (2005) Science 308, 95-98. [DOI] [PubMed] [Google Scholar]
- 33.Jenkins, R. J. F., Cooper, J. A. & Compston, W. (2002) J. Geol. Soc. (London) 159, 645-658. [Google Scholar]
- 34.Glaessner, M. F. & Walter, M. R. (1975) Alcheringa 1, 59-69. [Google Scholar]
- 35.Bland, B. H. (1984) Geol. Mag. 121, 624-633. [Google Scholar]
- 36.McIlroy, D. & Walter, M. R. (1997) Alcheringa 21, 79-80. [Google Scholar]
- 37.Gehling, J. G. (1999) Palaios 14, 40-57. [Google Scholar]
- 38.James, N. P., Narbonne, G. M. & Sherman, A. G. (1998) J. Sediment. Res. 68, 716-722. [Google Scholar]
- 39.Hagadorn, J. W. & Bottjer, D. J. (1999) Palaios 14, 73-85. [Google Scholar]
- 40.Riding, R. (2000) Sedimentology 47, Suppl. 1, 179-214. [Google Scholar]
- 41.Seilacher, A. (1999) Palaios 14, 86-93. [Google Scholar]
- 42.Grazhdankin, D. & Seilacher, A. (2002) Palaeontology 45, 57-78. [Google Scholar]
- 43.Narbonne, G. M. & Gehling, J. G. (2003) Geology 31, 27-30. [Google Scholar]
- 44.Clapham, M. E. & Narbonne, G. M. (2002) Geology 30, 627-630. [Google Scholar]
- 45.Jenkins, R. J. F., Plummer, P. S. & Moriarty, K. C. (1981) Trans. R. Soc. South Australia 105, 67-83. [Google Scholar]
- 46.Dzik, J. (1999) Geology 27, 519-522. [Google Scholar]
- 47.Ivantsov, A. Y. & Grazhdankin, D. (1997) Paleontol. J. 31, 1-16. [Google Scholar]
- 48.Grazhdankin, D. (2004) Paleobiology 30, 203-221. [Google Scholar]
- 49.Bottjer, D. J., Hagadorn, J. W. & Dornbos, S. Q. (2000) GSA Today 10 (9), 1-7. [Google Scholar]