Abstract
Background
The appropriateness of ablation for liver cancer patients meeting the Milan criteria remains controversial.
Purpose
This study aims to evaluate the long-term outcomes of MR-guided thermal ablation for HCC patients meeting the Milan criteria and develop a nomogram for predicting survival rates.
Methods
A retrospective analysis was conducted from January 2009 to December 2021 at a single institution. Patients underwent MR-guided thermal ablation. Factors influencing progression-free survival (PFS) and overall survival (OS) were identified using univariate and multivariate Cox regression and stepwise regression. A nomogram was developed for survival prediction, followed by risk stratification and internal validation. Adverse events (AEs) were also analyzed.
Results
A total of 181 patients were included, with a mean follow-up of 73.8 ± 31.7 months. The cumulative local tumor progression rates at 1, 3, and 5 years were 0.80%, 1.27%, and 1.86%, respectively. The 1-, 3-, and 5-year PFS rates were 81.8%, 57.4%, and 38.1%, and OS rates were 98.3%, 87.8%, and 62.9%. Poorer outcomes were associated with age ≤ 60 years, tumor size > 2 cm, multiple tumors, cirrhosis, proximity to major vessels, and narrow ablation margins (P < 0.05). The nomogram accurately predicted 3- and 5-year survival, and internal validation confirmed the results. AEs occurred in 33.7% of patients, with pain being the most common.
Conclusion
MR-guided ablation is effective for HCC patients within the Milan criteria, especially for those with smaller tumors and better liver function. The nomogram and risk stratification model are valuable tools for predicting patient outcomes and guiding treatment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-025-13510-8.
Keywords: Hepatocellular carcinoma, Magnetic resonance imaging, Thermal ablation, Milan criteria
Background
HCC remains a significant contributor to cancer-related deaths globally [1]. Surgical intervention, ablation, and liver transplantation represent the optimal treatment strategies for achieving long-term survival in the early stages of liver cancer, with a 5- year survival rate ranging from 50% to 70% [2, 3]. In some countries, the preference for local treatments over surgical procedures has increased [4]. Ablation, in particular, offers several advantages such as a convenient procedure, shorter hospital stays, precise efficacy, and effective control over the ablation area. Currently, ablation procedures are predominantly guided by ultrasound (US) and computed tomography (CT). However, US imaging suffers from inadequate display and susceptibility to interference from ribs and lungs, limiting its effectiveness in monitoring treatment outcomes [5]. Although CT imaging is not affected by interference from gas and bone, it subjects patients to ionizing radiation, and metallic artifacts can obscure the visualization of tumor lesions [6]. Magnetic resonance imaging (MRI) is an ideal modality for guiding ablation procedures due to its multiparametric imaging capabilities, superior spatial resolution, excellent soft tissue contrast, and absence of ionizing radiation [7]. Most importantly, MRI enables the visualization of tissue function and temperature. Concurrently, the use of gadolinium contrast agents allows MRI to better detect small liver tumors that remain undetected or challenging to identify due to cirrhosis, fatty liver diseases, and other factors [8]. Consequently, MR-guided ablation procedures hold promise in improving outcomes and survival rates in patients with early-stage liver cancer.
Ablation has emerged as a potentially curative therapy for small HCC (< 3 cm), with most guidelines advocating local ablation as the first-line treatment for single tumors measuring less than 2 cm, boasting response rates of 70–90% [9–11]. However, the suitability of ablation for HCC patients within the Milan criteria remains a subject of debate [12–14]. A randomized trial revealed a lower recurrence rate with surgery compared to ablation for patients meeting these criteria [15]. It remains to be determined whether MRI can leverage its unique advantages to improve the prognosis for these patients.
Our study delves into the long-term clinical outcomes of MR-guided ablation for HCC patients who met the Milan criteria, drawing on a decade of experience at a single institution. The results highlight critical factors influencing PFS and OS, underscoring the significance of MRI's role in improving patient prognosis. Moreover, our research illustrates the development and validation of a predictive nomogram and risk stratification model, showcasing its potential to accurately forecast patient survival and recurrence rates, thereby optimizing individualized treatment strategies.
This comprehensive evaluation aims not only to underscore the efficacy of MR-guided ablation but also to provide a robust framework for future therapeutic approaches, ultimately striving to enhance survival outcomes and quality of life for HCC patients.
Methods
Patient population
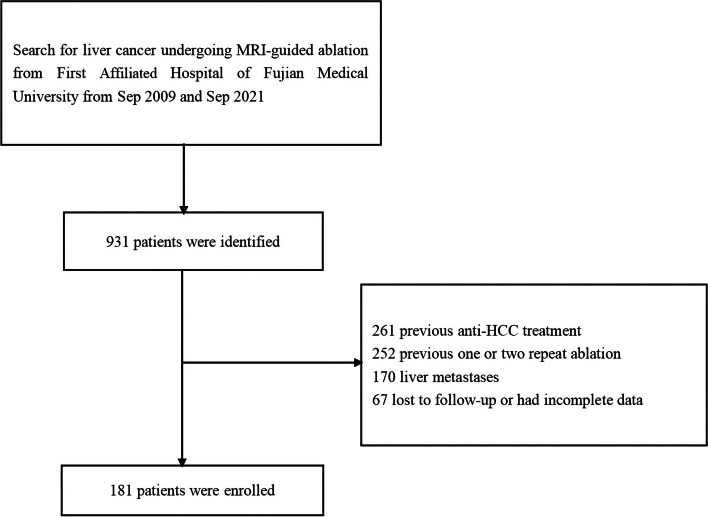
This retrospective study was approved by the ethics committee (MTCA, ECFAH of FMU [2015]084-2). It included all patients with primary liver cancer who underwent percutaneous MR-guided thermal ablation (including RFA or MWA) between January 2009 and December 2021. The inclusion criteria were as follows: a diagnosis of HCC based on the European Association for the Study of the Liver [16]; tumor diameter ≤ 5 cm; a maximum of three multiple tumors with the largest diameter ≤ 3 cm; absence of vascular invasion; Child–Pugh grade A or B; and an Eastern Cooperative Oncology Group (ECOG) score ≤ 2. Additionally, patients were included if they were unwilling to undergo surgery, faced high surgical risk due to severe cirrhosis, or had tumors that were unclear or difficult to treat with CT or US guidance, and if they preferred ablation under MR guidance. Exclusion criteria included previous surgery or radiofrequency treatment, benign liver tumors, concurrent antitumor therapies, platelet count ≤ 50 × 10^9/L, uncontrolled ascites, Child–Pugh grade C, loss to follow-up or absence of further imaging studies after ablation, severe concurrent diseases, acute or active infectious diseases, and pregnancy or breastfeeding. The enrollment process flow chart is presented in Fig. 1. According to the inclusion and exclusion criteria of this study, a total of 181 patients with HCC underwent MR-guided thermal ablation. As presented in Table 1, the median age of the patients was 57.7 ± 12.5 years. The majority of the patients were male (82.3%) and had contracted hepatitis B (79.6%). This retrospective study analyzes existing medical records, databases, and archived materials without involving direct interventions or invasive procedures on patients. This study strictly protects patient privacy and data security in accordance with ethics committee of the hospital. Since it poses no risk to patients’ rights and obtaining informed consent is practically difficult, this study has been granted exemption from informed consent.
Fig. 1.
Enrollment flow chart
Table 1.
Baseline characteristics of patients
| Variable | N=181 |
|---|---|
| Age, Mean ± SD(y) | 57.7 ± 12.51 |
| Sex(%) | |
| Male | 149 (82.3%) |
| Female | 32 (17.7%) |
| WBC, Mean ± SD | 3.77 ± 0.11 |
| HB, Mean ± SD | 135.0 ± 24.04 |
| PLT, Mean ± SD | 152.5 ± 48.79 |
| TB, Mean ± SD | 14.5 ± 4.74 |
| ALB, Mean ± SD | 39.2 ± 0.98 |
| PT, Mean ± SD | 12.6 ± 1.62 |
| Child-pugh classification | |
| A | 164 (90.6%) |
| B | 17 (9.4%) |
| AFP(ng/ml,%) | |
| ≤200 | 159 (87.9%) |
| >200 | 22 (12.1%) |
| BCLC stage | |
| 0 | 75 (41.4%) |
| A | 56 (31.0%) |
| B | 16 (8.8%) |
| ALBI score | |
| Grades 1 | 64 (35.4%) |
| Grades 2 | 115 (63.5%) |
| Grades 3 | 6 (3.3%) |
| Hepatitis(%) | |
| No | 30 (16.6%) |
| HBV | 144 (79.6%) |
| HCV | 4 (2.2%) |
| HBV+HCV | 3 (1.7%) |
| Liver cirrhosis(%) | |
| No | 33 (18.2%) |
| Yes | 148 (81.8%) |
| Tumor number(%) | |
| Single | 134 (74.0%) |
| Multiple | 47 (26.0%) |
| Tumor size(cm,%) | |
| ≤2 | 117 (64.6%) |
| >2 | 64 (35.4%) |
| Subcapsular(%) | |
| No | 87 (48.1%) |
| Yes | 94 (51.9%) |
| Adjacent peripheral organs(%) | |
| No | 164 (90.6%) |
| Yes | 17 (9.4%) |
| Adjacent major vessel(%) | |
| No | 105 (58.0%) |
| Hepatic vein | 25 (13.8%) |
| Potal vein | 51 (28.2%) |
| Ablation margin(mm,%) | |
| <5 | 142 (78.5%) |
| 5-10 | 39 (21.5%) |
| Ablation method | |
| RFA | 164 (90.6%) |
| MWA | 17 (9.4%) |
| Relapse pattern(%) | |
| No | 88 (48.6%) |
| LTP | 7 (3.9%) |
| Homohepatic segment | 14 (7.7%) |
| Different hepatic segment | 49 (27.1%) |
| Multiple hepatic segment | 23 (12.7%) |
| Extrahepatic metastasis | 3 (1.7%) |
| Gd-EOB-DTPA | |
| No | 143 (79.0%) |
| Yes | 38 (21.0%) |
| Ablation time, Mean ± SD(min) | 75.5 ± 27.57 |
Abbreviations: WBC White blood cell, HB Hemoglobin, PLT Platelets, TB Total bilirubin, ALB Albumin, PT Prothrombin time, AFP Alphafetoprotein, BCLC stage Barcelona Clinic Liver Cancer, ALBI Albumin-bilirubin, RFA Radiofrequency ablation, MWA Microwave ablation, LTP Local tumor progression, Gd-EOB-DTPA Gadoliniumethoxybenzyl-diethylenetriaminepentaacetic acid
MRI equipment and ablation system
Thermoablation therapy was strategically planned using pre-interventional MRI scans. Percutaneous thermoablation procedures were executed between 09/2009 and 10/2020 with a 1.5 T MR scanner (GE SignaInfinity Twinspeed, USA), followed by its transition to a 3.0 T MR scanner (Philips Ingenia 3.0 T, Netherlands) from 11/2020 to 2021. Both body array and loop array coils were utilized in these procedures. Depending on the specific interventions performed at 1.5 T and 3.0 T, different MR sequences were applied (Table 2). Between 9/2009 and 10/2020, the RITA system (MTC-3C, ANGIO, New York, USA) was employed and from 11/2020 to 9/2021, microwave ablation (MWA) system (MTC-3CA-II33, VISON Medical Equipment Co., Nanjing, China) was introduced.
Table 2.
Overview of the different applied MR sequences for thermoablation therapy performed at 1.5 T and 3.0 T
| 1.5T | 3.0T | |||||||||||
| Sequence | TE(ms) | TR(ms) | FA | GAP | FOV(mm) | NEX | TE(ms) | TR(ms) | FA | GAP | FOV(mm) | NEX |
| Fs FRFSE T2WI | 2600 | 85 | 90/180 | 5mm/1mm | 270×360 | 1 | 67.3 | 2600 | 90 | 5mm/1mm | 380×380 | 1 |
| 3D Dyn T1WI | 5 | 1.2 | 45deg | 3mm | 270×360 | 1 | 0 | 4.2 | 10 | 3mm | 380×380 | 1 |
Abbreviations: T1WI T1-weighted image, T2WI T2-weighted image, TR Repetition time, TE Echo time, FA Fractional anisotropy, FOV Field of view, NEX Number of excitation
MR‑guided thermal procedure
All patients underwent a comprehensive set of pre-treatment examinations, including complete blood count, liver and kidney function tests, electrolyte levels, coagulation profile, and cardiopulmonary function tests within three days prior to thermal ablation. Within a week before the procedure, enhanced CT or MR scans were performed to ensure accurate tumor visualization. Patients fasted for 4-6 h and practiced breathholding exercises before thermal ablation to facilitate MR scanning and enhance puncture efficiency. During the procedure, patients' vital signs were monitored using respiratory and ECG-gated devices. Two radiologists, each with over five years of experience in hepatobiliary interventions, conducted the ablation. After positioning with vitamin E pills, the dermal needle entry point was identified, and the angle and depth of the needle were measured. Guided by multiplanar MR scans, ablation electrodes were inserted into the target lesion, and thermal ablation was performed according to the manufacturer’s guidelines. When necessary, gadoliniumethoxybenzyl-diethylenetriaminepentaacetic acid (Gd-EOB-DTPA) agents were injected 30 min before ablation (Fig. 2). Real-time MRI signal and temperature monitoring within the ablation zone were selectively employed to ensure precision and safety (Figure.S1 and Video S1-2). This proactive surveillance provided dynamic feedback, enabling the medical team to promptly assess the ablation area's status and make rapid, targeted adjustments, enhancing both efficiency and accuracy. Immediately following ablation, efficacy was evaluated. Complete ablation was indicated by low signal intensity at the tumor location and high signal intensity at the ablation margin in fsT1WI (Figs. 3 and 4). For tumors located subcapsularly, adjacent to major blood vessels, or vital organs where the ablation margin failed to achieve the required 5-10 mm thermal margin, complete ablation was characterized by the entire low signal intensity tumor being covered by a high signal intensity ablation margin. In cases of incomplete ablation, supplementary ablation was performed. After completing the ablation, the needle was removed, and a subsequent fsT2WI and fsT1WI sequence scan of the entire liver was conducted to assess immediate complications. The specific steps for this process are detailed in a previous article [17].
Fig. 2.
MR-guided microwave ablation in a 63-year-old male patient with hepatocellular carcinoma. a Axial enhanced arterial phase MRI shows significant enhancement in the tumor nodule. b Axial non-contrast CT and (c) axial T1-weighted imaging show unclear nodule delineation. d Localization with gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid demonstrates the tumor clearly in hepatobiliary phase imaging. Post-ablation, (e) axial T1-weighted imaging reveals the nodule encompassed by a hyperintense rim, and (f) the ablation zone appears hypointense on axial T2-weighted imaging
Fig. 3.
MR-guided RFA in a 59-year-old male patient with hepatocellular carcinoma. Pre-RFA, (a) axial T2-weighted imaging reveals high signal intensity within the tumor. b Axial T1-weighted imaging demonstrates low signal intensity. c Contrast-enhanced MRI shows high signal intensity in the area. Following the placement of the radiofrequency electrode at the nodule's edge, (d) axial T1-weighted and coronal 3D T1-weighted imaging (e) confirms complete nodule overlap. Immediately after RFA, axial T2-weighted imaging showed the nodule fully replaced by low signal intensity (f). Complete tumor ablation was confirmed at the 2-month follow-up using enhanced MRI (g, h, i)
Fig. 4.
MR-guided MWA in a 45-year-old female patient with hepatocellular carcinoma. Pre-MWA, (a) axial T2-weighted imaging reveals high signal intensity within the tumor. b Axial T1-weighted imaging demonstrates low signal intensity. c Contrast-enhanced MRI shows high signal intensity in the area. The microwave electrode was precisely inserted into the tumor nodule, as seen on (d) axial T1-weighted imaging. Immediately after MWA, (e) axial T1-weighted imaging shows the nodule encompassed by a hyperintense rim, and (f) the ablation zone appears hypointense on axial T2-weighted imaging. Complete tumor ablation is confirmed at the 2-month follow-up using enhanced MRI (g, h, i)
Follow-up
Technical success encompassed achieving complete ablation without any evidence of enhancement on axial MRI/CT scans 4 ~ 8 weeks post-procedure. Patients were subsequently followed for 2 ~ 3 months in the first year and then every 6 months thereafter. OS was defined as the interval between the ablation and the death due to any cause, with the endpoint censored at the date of the last follow-up visit when the patients were still alive. PFS represented the time elapsed between the ablation and the first documented HCC recurrence or death. LTP was defined as local recurrence occurring at or near the edge of the ablation zone more than two months after the initial ablation procedure. In managing recurrences, considerations were made based on the patient’s overall performance, liver function, degree of cirrhosis, tumor size, number of nodules and the location of the recurrent tumor. The efficacy and safety of the procedure were evaluated using the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) classification score system [18].
Statistical analysis
Continuous data, summarized as the mean (standard deviation), were compared using Student’s t-test or the Mann–Whitney U-test. Categorical data were expressed as numbers (percentages) and compared using the chi-square test or Fisher’s exact test. PFS and OS curves were presented using the Kaplan–Meier method, and differences in PFS and OS between groups were tested using the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed to identify independent risk factors for PFS and OS. Variables with P < 0.1 in univariate analysis were further evaluated by multivariate analysis. To detect collinearity, the variance inflation factor (VIF) for each variable was calculated, with VIF values exceeding 10 indicating potential collinearity issues. Stepwise regression was used to identify the optimal subset of features for constructing the forest plot. The survminer package was used to draw the forest plot, and the rms package was used to construct the nomogram. A random seed was set to ensure reproducibility of the selection process, enhancing the robustness of our findings, and 80% of the data were randomly selected for internal validation of the model. All statistical analyses were two-sided, and P < 0.05 was considered statistically significant. Statistical analysis was performed using R version 4.3.2 (R Project, Vienna, Austria).
Results
Univariate and multivariate analyses of PFS and OS for patients
The PFS and OS outcomes of the study are summarized in Table 3, presenting the results of both univariate and multivariate Cox regression analyses. The data reveal that age ≤ 60 years and tumor size > 2 cm are significantly associated with increased tumor recurrence rates and shorter OS (P < 0.05). Additionally, patients with multiple tumors, cirrhosis, tumors adjacent to majorvessels, and narrow ablation margins exhibit a similar trend, indicating poorer PFS (P < 0.05).
Table 3.
Univariate and multivariable analysis of PFS and OS in all patient
| Variable | PFS | OS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age (≤ 60y vs > 60y) | 2.066 | 1.365–3.127 | 0.001* | 1.827 | 1.189–2.806 | 0.006* | 2.12 | 1.277–3.519 | 0.004* | 2.037 | 1.226–3.386 | 0.006* |
| Sex (male vs female) | 0.534 | 0.284–1.002 | 0.051 | 1.224 | 0.651–2.300 | 0.530 | ||||||
| AFP level (< 200 vs ≥ 200 ng/ml) | 1.323 | 0.870–2.011 | 0.190 | 1.237 | 0.741–2.067 | 0.416 | ||||||
| Hepatitis (yes vs no) | 1.312 | 0.902–1.908 | 0.155 | 0.802 | 0.482–1.336 | 0.397 | ||||||
| Cirrhosis (yes vs no) | 1.856 | 0.945–3.501 | 0.077 | 1.327 | 0.712–2.314 | < 0.001* | 0.412 | 0.261–0.646 | < 0.001* | |||
| Child–pugh (A vs B) | 0.787 | 0.381–1.626 | 0.518 | 0.62 | 0.225–1.709 | 0.355 | ||||||
| Tumor size (> 2 vs ≤ 2 cm) | 2.007 | 1.337–3.012 | 0.001* | 1.749 | 1.146–2.669 | 0.010* | 2.018 | 1.234–3.299 | 0.005* | 1.93 | 1.179–3.16 | 0.009* |
| Tumor number (multiple vs single) | 1.814 | 1.183–2.782 | 0.006* | 1.657 | 1.051–2.61 | 0.030* | 0.881 | 0.499–1.554 | 0.662 | |||
| Subcapsular (yes vs no) | 1.507 | 0.998–2.276 | 0.051 | 1.246 | 0.760–2.043 | 0.383 | ||||||
| Adjacent peripheralorgans (yes vs no) | 1.15 | 0.578–2.287 | 0.691 | 1.285 | 0.585–2.822 | 0.532 | ||||||
| Adjacent major vessel (yes vs no) | 1.151 | 0.764–1.735 | 0.501 | 1.501 | 0.997–2.221 | < 0.001* | 0.957 | 0.577–1.589 | 0.866 | |||
| Ablation edge (0 ~ 5 vs 5 ~ 10 mm) | 0.551 | 0.307–0.990 | 0.046* | 1.154 | 0.667–1.812 | < 0.001* | 0.718 | 0.365–1.413 | 0.338 | |||
| Gd-EOB-DTPA (yes vs no) | 0.531 | 0.363–0.964 | 0.036* | 1.323 | 0.851–2.001 | 0.234 | ||||||
| Ablationmethod (MWA vs RFA) | 0.113 | 0.015–0.781 | 0.027* | 17.098 | 8.823–32.183 | < 0.001* |
Abbreviations: AFP α‐fetoprotein, RFA Radiofrequency ablation, MWA Microwave ablation, Gd-EOB-DTPA Gadolinium-ethoxybenzyl-diethylenetriaminepentaacetic acid
*P < 0.05
PFS and OS outcomes
The mean follow-up duration was 73.4 ± 31.7 month. The cumulative LTP rates at 1-, 3-, and 5-years were 0.80%, 1.27% and 1.86%, respectively (Fig. 5c). The 1-, 3-, and 5-year PFS rates for all patients were 81.8%, 57.4%, and 38.1%. The 1-, 3-, and 5-year OS rates for all patients were 98.3%, 87.8%, and 62.9%, respectively (Fig. 5a-b). Analysis of OS data revealed no significant differences in survival rates between patients with solitary recurrences in homohepatic segments and those with recurrences in different hepatic segments. Both groups exhibited higher survival rates compared to patients with multiple hepatic segment recurrences (median survival times of 91.8, 84.9, and 40.4 months, respectively; P < 0.001) (Fig. 5d).
Fig. 5.
Kaplan-Meier survival analysis for all patients. a Overall survival probability. b Progression-free survival. c Cumulative incidence plot of local tumor progression. d Survival curves stratified by different relapse patterns
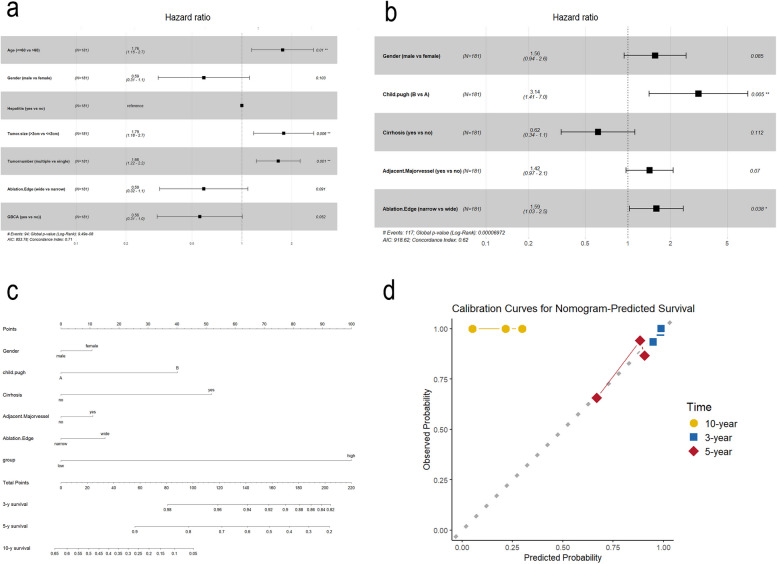
Model development and forest plot analysis
Using stepwise regression, we developed the optimal model for both OS and PFS. Forest plots were constructed to visually represent the significant factors impacting OS and PFS. These plots highlight the variables that remained significant in the final multivariate Cox proportional hazards regression analysis. Age ≤ 60 years, tumor size > 2 cm, and multiple tumors significantly impacted PFS, while Child-Pugh grade B liver function affected OS. The identified risk factors, along with their hazard ratios and confidence intervals, are detailed in the forest plots (Fig. 6a-b).
Fig. 6.
Prognostic factors influencing overall and progression-free survival. a Forest plot of hazard ratios for PFS. b Forest plot showing hazard ratios for additional prognostic factors impacting OS. c Nomogram predicting 3-, 5-, and 10-year survival probabilities based on key variables. d Calibration curves for nomogram-predicted survival at 3, 5, and 10 years
Nomogram construction and risk stratification
Based on the OS model, we constructed a nomogram to predict 3-, 5-, and 10-year survival rates (Fig. 6c). The calibration curves demonstrated that the nomogram accurately predicted 3-and 5-year survival outcomes (Fig. 6d). Based on the risk scores, the cohort was stratified into high- and low-risk groups. Results showed that patients in the low-risk group had significantly higher survival rates and lower recurrence rates compared to those in the high-risk group (Fig. 7a). To further validate the accuracy of this risk assessment system, 80% of the patients were randomly selected for internal validation. The findings consistently showed that patients in the low-risk group had significantly higher survival rates and lower recurrence rates compared to the high-risk group (Fig. 7c).
Fig. 7.
Kaplan-Meier analysis of OS and PFS in all patients and the internal validation cohort based on risk scores. a Overall survival curves by risk groups. b Progression-free survival curves by risk groups. c Overall survival curves for the internal validation cohort. d Progression-free survival curves for the internal validation cohort
Adverse events
Regarding the safety of thermal ablation, a total of 61 (33.7%) patients experienced AEs of any grade during the treatment period, with pain being the most common complication. Grade 3 or 4 AEs were recorded in 15 (8.3%) patients, and no fatal AEs were reported (Table 4). The most common grade 3 or 4 AEs were biliary- cardiac reflex (10, 5.5%), infection (3, 1.6%),liver abscess (1, 0.6%),and hemorrhage (1, 0.6%).
Table 4.
Treatment-related adverse events
| Adverse Events | All Grades | Grade 1/2 | Grade 3/4 |
|---|---|---|---|
| Fever or infection | 21 (11.6%) | 18 (9.9%) | 3 (1.6%) |
| Nausea | 15 (8.3%) | 15 (8.3%) | 0 (0%) |
| Pain | 48 (26.5%) | 46 (25.4%) | 0 (0%) |
| Biliary-cardiac reflex | 12 (6.6%) | 2 (1.1%) | 10 (5.5%) |
| Liver abscess | 1 (0.6%) | 0 (0%) | 1 (0.6%) |
| Pleural effusion | 9 (5%) | 9 (5%) | 0 (0%) |
| Hemorrhage | 13 (7.2%) | 12 (6.7%) | 1 (0.6%) |
| Pneumothorax | 6 (3.3%) | 6 (3.3%) | 0 (0%) |
Discussion
This study provides a comprehensive evaluation of MR-guided thermal ablation for HCC patients meeting the Milan criteria, leveraging a decade of experience at a single institution. The median OS among the 181 patients in the study cohort was 73.8 months, with 1-, 3- and 5-year OS rates of 98.3%, 87.8% and 62.9%, respectively. Although these outcomes cannot rival liver transplantation or resection [19], they surpass the results of liver cancer ablation under CT or ultrasound guidance (97%, 67% and 41%) [20]. The results underscore the significant impact of patient age, tumor size, number of tumors, liver function, and tumor proximity to major vessels on PFS and OS. These findings align with previous research suggesting that smaller tumor size and better liver function are critical determinants of favorable outcomes [14, 21, 22]. For instance, a study by Han et al. [23] indicated that smaller HCCs (< 3 cm) treated with thermal ablation exhibited significantly lower recurrence rates compared to larger tumors, which supports our observations. Additionally, the presence of multiple tumors and poor liver function has been correlated with increased recurrence and decreased survival rates in studies [24, 25], further validating our findings.
The use of MRI for guiding ablation procedures offers distinct advantages, including superior imaging capabilities, excellent soft tissue contrast, and the absence of ionizing radiation. These benefits are particularly important for patients with tumors that are difficult to visualize using traditional ultrasound or CT imaging. MRI's ability to provide real-time monitoring of tissue function and temperature significantly enhances the precision and safety of the ablation procedure. A study by Lin et al. [26] highlighted MRI's superiority in detecting small HCCs and accurately guiding ablation needles, which translates into improved treatment outcomes and lower recurrence rates. Moreover, the use of Gd-EOB-DTPA agents in MRI has been shown to improve the detection of small liver tumors, particularly inpatients with underlying cirrhosis or fatty liver disease [27].
Thermal ablation for early-stage HCC commonly faces the drawback of a higher recurrence rate compared to surgical resection [28], particularly for local recurrences that are independent of the ablation method. A meta-analysis [29] revealed no significant differences in LTP between MWA, RFA and cryoablation, potentially due to the inability of the ablation margin to meet surgical resection criteria. Although researchers have explored enhancing local control with tyrosine kinase inhibitors, transarterial chemoembolization (TACE), no-touch RFA and liposomal doxorubicin [30–33], local recurrence remains a challenge. In this long-term follow-up study, the cumulative LTP rates at 1-, 3- and 5-years were 0.80%, 1.27% and 1.86%, respectively, appearing significantly lower than previously reported. Several factors may contribute to the reduced local recurrence: first, MRI vividly delineates tumor boundaries; second, MRI accurately assesses ablation margins from multiple angles; and third, for tumors adjacent to major vessels or dangerous organs, our team determines whether ablation is complete by monitoring MRI signals or temperature changes around the ablation needle in realtime, effectively minimizing residual tumor.
Previous studies [34, 35] have shown that older patients are often diagnosed with liver cancer during routine screening and typically present poorer prognostic features compared to younger patients, which may explain the differences in clinicopathological features and prognosis observed in our study. As reported in prior literature [36], cirrhosis is a known risk factor for liver cancer survival, closely linked to recurrence and prognosis. However, in our study, cirrhosis was found to be a protective factor for OS in univariate analysis. This could be due to confounding factors not controlled for in this analysis. Similarly, contrast enhancement and ablation modality did not significantly affect recurrence or survival outcomes in multivariate analysis and were therefore excluded. In the univariate analysis of survival, multiple tumors appeared as a protective factor, which may be explained by our aggressive management strategy for recurrent HCC. This suggests that the apparent protective role of cirrhosis in OS could be influenced by confounding factors, which were accounted for in multivariate analysis. Additionally, larger tumor size correlates with poorer differentiation, increasing the likelihood of hematogenous micrometastasis and resulting in shorter survival [37].
Our study also highlights the development of a predictive nomogram and risk stratification model, which accurately forecasted 3- and 5-year survival rates. This model enables clinicians to identify high-risk patients who may benefit from more intensive follow-up and tailored therapeutic strategies. The internal validation of this model confirms its robustness and utility in clinical practice. Nomograms have become increasingly valuable in oncology for their ability to provide individualized risk assessments and treatment predictions. For instance, a study by Tian et al. [38] demonstrated the effectiveness of a nomogram in predicting survival in HCC patients after resection, suggesting that such tools can significantly enhance personalized treatment planning. Our nomogram's accurate predictions of survival and recurrence rates underscore its potential as a critical tool in the management of HCC patients undergoing MR-guided ablation.
The observed adverse events were consistent with those reported in other studies, with pain being the most common complication. The incidence of grade 3 or 4 adverse events was relatively low, and no fatal events were recorded, indicating that MR-guided ablation is a safe procedure for this patient population. A meta-analysis by Chen et al. [39] comparing the safety profiles of RFA and MWA also reported similar findings, with both modalities showing low rates of severe complications. Our study's adverse event profile is inline with these results, reinforcing the safety of MR-guided thermal ablation as a treatment modality for HCC.
Despite the promising findings, this study has several limitations, including its retrospective design and the potential for selection bias. MR-guided thermal ablation is highly selective and involves higher costs and equipment requirements compared to traditional US- or CT-guided procedures, making it challenging to obtain external validation data and increasing the risk of selection bias. Future prospective studies with larger sample sizes are needed to validate these results and further refine the predictive models. Additionally, exploring the integration of MR-guided ablation with other therapeutic modalities, such as TACE or systemic therapies, may offer valuable insights into comprehensive treatment strategies for HCC.
Conclusions
MR-guided ablation is a promising treatment for HCC patients within the Milan criteria, particularly for those with smaller tumors and better liver function. The developed nomogram and risk stratification model provide valuable tools for predicting patient outcomes and guiding treatment decisions.
Supplementary Information
Supplementary Material 1: Figure.S1 T1WI images of the needle in place were transmitted to the interventional MR platform [Interventional MRI Suite (iSuite), Philips Research, Hamburg, Germany] System. Two temperature measurement points were placed at the center and the edge of the lesion, respectivel. Video S1 The ablation power was set to 75W and the effective ablation time was 3min. The temperature mapping (Tmap) sequence was used to collect the thermometry data and to display the pseudo-color overlay in real time on iSuite platform. Video S2 Magnetic resonance T2WI is applied throughout the ablation process, allowing for real-time monitoring of tumor and surrounding area signal changes during ablation.
Acknowledgements
We would like to thank Editage (www.mjeditor.com) for English language editing.
The ethics committee
Ethics Committee of First Affiliated Hospital of Fujian Medical University.
Abbreviations
- HCC
Hepatocellular carcinoma
- PFS
Progression-free survival
- OS
Overall survival
- LTP
Local tumor progression
- CT
Computed tomography
- MRI
Magnetic resonance imaging
- ECOG
Eastern Cooperative Oncology Group
- CIRSE
Cardiovascular and Interventional Radiological Society of Europe
- RFA
Radiofrequency ablation
- MWA
Microwave ablation
- Gd-EOB-DTPA
Gadolinium-ethoxybenzyldiethylenetriaminepentaacetic acid
- fsT1WI
Fat-suppressed T1-weighted imaging
- fsT2WI
Fat-suppressed T2-weighted imaging
- TACE
Transarterial chemoembolization
- Tmap
Temperature mapping
Authors’ contributions
Z.Y.L contributed to the study concept and design. F.Q.W, P.S.H, B.Z, R.G, Y.Y and J.C contributed to the acquisition of clinical data. F.Q.W wrote the first draft of the manuscript. P.S.H, B.Z, R.G, Y.Y and J.C supervised and oversaw the study. P.S.H contributed to the statistical analysis. All authors contributed to the article and approved the submitted version. F.Q.W is the guarantor of the whole study.
Funding
Funded by the Fujian Province Double High Project.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fu-Qun Wei and Pei-Shu Huang are joint first authors.
References
- 1.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345–62. [DOI] [PubMed] [Google Scholar]
- 4.Koga H, Iwamoto H, Suzuki H, Shimose S, Nakano M, Kawaguchi T. Clinical practice guidelines and real-life practice in hepatocellular carcinoma: a Japanese perspective. Clin Mol Hepatol. 2023;29(2):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song KD, Lee MW, Rhim H, Kang TW, Cha DI, Sinn DH, Lim HK. Percutaneous US/MRI fusion-guided radiofrequency ablation for recurrent subcentimeter hepatocellular carcinoma: technical feasibility and therapeutic outcomes. Radiology. 2018;288(3):878–86. [DOI] [PubMed] [Google Scholar]
- 6.Benson R, Pathy S, Kumar L, Mathur S, Dadhwal V, Mohanti BK. Locally advanced cervical cancer - neoadjuvant chemotherapy followed by concurrent chemoradiation and targeted therapy as maintenance: a phase II study. J Cancer Res Ther. 2019;15(6):1359–64. [DOI] [PubMed] [Google Scholar]
- 7.Xu B, Huo Z, Huang H, Ji W, Bian Z, Jiao J, Sun J, Shao J. The expression and prognostic value of the epidermal growth factor receptor family in glioma. BMC Cancer. 2021;21(1):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricke J, Steffen IG, Bargellini I, Berg T, Bilbao Jaureguizar JI, Gebauer B, Iezzi R, Loewe C, Karçaaltincaba M, Pech M, et al. Gadoxetic acid-based hepatobiliary MRI in hepatocellular carcinoma. JHEP Rep. 2020;2(6):100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, MD). 2018;67(1):358–80. [DOI] [PubMed] [Google Scholar]
- 10.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vogel A, Cervantes A, Chau I, Daniele B, Llovet JM, Meyer T, Nault JC, Neumann U, Ricke J, Sangro B, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv238–55. [DOI] [PubMed] [Google Scholar]
- 12.Thamtorawat S, Hicks RM, Yu J, Siripongsakun S, Lin WC, Raman SS, McWilliams JP, Douek M, Bahrami S, Lu DS. Preliminary outcome of microwave ablation of hepatocellular carcinoma: breaking the 3-cm barrier? J Vasc Interv Radiol. 2016;27(5):623–30. [DOI] [PubMed] [Google Scholar]
- 13.Groeschl RT, Gamblin TC, Turaga KK. Ablation for hepatocellular carcinoma: validating the 3-cm breakpoint. Ann Surg Oncol. 2013;20(11):3591–5. [DOI] [PubMed] [Google Scholar]
- 14.Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, Zeng Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–12. [DOI] [PubMed] [Google Scholar]
- 16.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–32. [DOI] [PubMed] [Google Scholar]
- 17.Lin XC, Yan Y, Lin L, Lin QF, Chen J, Lin ZY, Chen J. Magnetic resonance-guided thermal ablation for small liver malignant tumor located on segment II or IVa abutting the heart: a retrospective cohort study. Int J Hyperthermia. 2021;38(1):1359–65. [DOI] [PubMed] [Google Scholar]
- 18.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol. 2017;40(8):1141–6. [DOI] [PubMed] [Google Scholar]
- 19.Koh JH, Tan DJH, Ong Y, Lim WH, Ng CH, Tay PWL, Yong JN, Muthiah MD, Tan EX, Pang NQ, et al. Liver resection versus liver transplantation for hepatocellular carcinoma within Milan criteria: a meta-analysis of 18,421 patients. Hepatobiliary Surg Nutr. 2022;11(1):78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conticchio M, Inchingolo R, Delvecchio A, Laera L, Ratti F, Gelli M, Anelli F, Laurent A, Vitali G, Magistri P, et al. Radiofrequency ablation vs surgical resection in elderly patients with hepatocellular carcinoma in Milan criteria. World J Gastroenterol. 2021;27(18):2205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Duijnhoven FH, Jansen MC, Junggeburt JM, van Hillegersberg R, Rijken AM, van Coevorden F, van der Sijp JR, van Gulik TM, Slooter GD, Klaase JM, et al. Factors influencing the local failure rate of radiofrequency ablation of colorectal liver metastases. Ann Surg Oncol. 2006;13(5):651–8. [DOI] [PubMed] [Google Scholar]
- 22.Demirtas CO, D’Alessio A, Rimassa L, Sharma R, Pinato DJ. ALBI grade: Evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP Rep. 2021;3(5):100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Ni JY, Li SL, Deng HX, Liang HM, Xu YY, Huang ZM, Zhang TQ, Huang JH. Radiofrequency versus microwave ablation for hepatocellular carcinoma within the Milan criteria in challenging locations: a retrospective controlled study. Abdom Radiol (NY). 2021;46(8):3758–71. [DOI] [PubMed] [Google Scholar]
- 24.Lee MW, Han S, Gu K, Rhim H. Local ablation therapy for hepatocellular carcinoma: clinical significance of tumor size, location, and biology. Invest Radiol. 2025;60(1):53–9. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Chen Y, Ye F, Cao X, Xin Y, Wang Y, Lei Y, Li X, Feng D, Zhou X, et al. Late recurrence of hepatocellular carcinoma after radiofrequency ablation: a multicenter study of risk factors, patterns, and survival. Eur Radiol. 2021;31(5):3053–64. [DOI] [PubMed] [Google Scholar]
- 26.Lin ZY, Fang Y, Chen J, Lin QF, Yan Y, Chen J, Li YL. Feasibility and efficacy study of microwave ablation of recurrent small HCC guided by enhanced liver-specific magnetic resonance imaging contrast agent. Int J Hyperthermia. 2020;37(1):1330–5. [DOI] [PubMed] [Google Scholar]
- 27.Yinzhong W, Xiaoxue T, Jinhui T, Pengcheng Y, Xiaoying L, Junqiang L. Is gadoxetic acid disodium (Gd-EOB-DTPA)-enhanced magnetic resonance imaging an accurate diagnostic method for hepatocellular carcinoma? A systematic review with meta-analysis. Curr Med Imaging. 2022;18(6):633–47. [DOI] [PubMed] [Google Scholar]
- 28.Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver resection versus local ablation therapies for hepatocellular carcinoma within the Milan criteria: a systematic review and meta-analysis. Ann Surg. 2021;273(4):656–66. [DOI] [PubMed] [Google Scholar]
- 29.Gupta P, Maralakunte M, Kumar MP, Chandel K, Chaluvashetty SB, Bhujade H, Kalra N, Sandhu MS. Overall survival and local recurrence following RFA, MWA, and cryoablation of very early and early HCC: a systematic review and Bayesian network meta-analysis. Eur Radiol. 2021;31(7):5400–8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Wang X, Li R, Wang C, Wang J, Xie X, Li Y, Li S, Mao X, Liang P. Sorafenib as adjuvant therapy following radiofrequency ablation for recurrent hepatocellular carcinoma within Milan criteria: a multicenter analysis. J Gastroenterol. 2022;57(9):684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao S, Zou Y, Lyu T, Fan Z, Guan H, Song L, Tong X, Wang J. Long-term outcomes of combined transarterial chemoembolization and radiofrequency ablation versus RFA monotherapy for single hepatocellular carcinoma ≤3 cm: emphasis on local tumor progression. Int J Hyperthermia. 2022;39(1):1–7. [DOI] [PubMed] [Google Scholar]
- 32.Han S, Lee MW, Lee YJ, Hong HP, Lee DH, Lee JM. No-touch radiofrequency ablation for early hepatocellular carcinoma: 2023 Korean society of image-guided tumor ablation guidelines. Korean J Radiol. 2023;24(8):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tak WY, Lin SM, Wang Y, Zheng J, Vecchione A, Park SY, Chen MH, Wong S, Xu R, Peng CY, et al. Phase III HEAT study adding lyso-thermosensitive liposomal doxorubicin to radiofrequency ablation in patients with unresectable hepatocellular carcinoma lesions. Clin Cancer Res. 2018;24(1):73–83. [DOI] [PubMed] [Google Scholar]
- 34.Lam CM, Chan AO, Ho P, Ng IO, Lo CM, Liu CL, Poon RT, Fan ST. Different presentation of hepatitis B-related hepatocellular carcinoma in a cohort of 1863 young and old patients - implications for screening. Aliment Pharmacol Ther. 2004;19(7):771–7. [DOI] [PubMed] [Google Scholar]
- 35.Yan H, Wang X, Liu X, Wang P, Yu L, Zhou D, Yang Z. The survival strength of younger patients in BCLC stage 0-B of hepatocellular carcinoma: basing on competing risk model. BMC Cancer. 2022;22(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang SL, Liu LP, Sun YF, Yang XR, Fan J, Ren JW, Chen GG, Lai PB. Distinguished prognosis after hepatectomy of HBV-related hepatocellular carcinoma with or without cirrhosis: a long-term follow-up analysis. J Gastroenterol. 2016;51(7):722–32. [DOI] [PubMed] [Google Scholar]
- 37.Shinkawa H, Tanaka S, Kabata D, Takemura S, Amano R, Kimura K, Kinoshita M, Kubo S. The prognostic impact of tumor differentiation on recurrence and survival after resection of hepatocellular carcinoma is dependent on tumor size. Liver Cancer. 2021;10(5):461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian Y, Wang Y, Wen N, Lin Y, Liu G, Li B. Development and validation of nomogram to predict overall survival and disease-free survival after surgical resection in elderly patients with hepatocellular carcinoma. Front Oncol. 2024;14:1395740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zufry H, Hariyanto TI. Comparative efficacy and safety of radiofrequency ablation and microwave ablation in the treatment of benign thyroid nodules: a systematic review and meta-analysis. Korean J Radiol. 2024;25(3):301–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Figure.S1 T1WI images of the needle in place were transmitted to the interventional MR platform [Interventional MRI Suite (iSuite), Philips Research, Hamburg, Germany] System. Two temperature measurement points were placed at the center and the edge of the lesion, respectivel. Video S1 The ablation power was set to 75W and the effective ablation time was 3min. The temperature mapping (Tmap) sequence was used to collect the thermometry data and to display the pseudo-color overlay in real time on iSuite platform. Video S2 Magnetic resonance T2WI is applied throughout the ablation process, allowing for real-time monitoring of tumor and surrounding area signal changes during ablation.
Data Availability Statement
No datasets were generated or analysed during the current study.