Abstract
Because evolutionary processes such as genetic drift and natural selection play a crucial role in determining the response that species will have to human-induced disturbances, there is increasing interest in the evolutionary aspects of conservation biology. Harvesting select individuals in natural plant populations can bring about unforeseen impacts that may negatively affect fitness. We analyzed how human harvesting affects two congeners known as snow lotus. Over a period of 100 years, there was a negative trend in plant height (r2 = 0.4361, P < 0.001) for the intensely collected and rare species, Saussurea laniceps, but not in the less intensely collected species, Saussurea medusa. Additionally, S. laniceps were significantly smaller in areas of high harvest than in areas with low harvest (Z = 4.91, P < 0.0001), but this was not so for S. medusa. Humans can unconsciously drive evolution and must be considered when managing threatened species.
Keywords: conservation, human selection, Tibetan medicine
The process of natural selection is well known to alter the traits of wild plants and animals (1, 2), as “artificial selection” alters the traits of crop plants and domesticated animals (3–5). Anthropogenic activities, such as harvesting of wild plants, also may lead to evolutionary change in natural populations, although this process is much less well understood. Harvesters who collect plants based on certain traits may unintentionally select against that trait in the remaining population. Here, we investigated whether human harvesting has selected for smaller plants in the rare Tibetan snow lotus, Saussurea laniceps.
Explorers and plant collectors have roamed many parts of the world to record and catalog the botanical diversity of our planet (6). Plant collections from these expeditions, typically housed in herbaria, provide scientists with records for taxonomic, systematic, and morphological studies. Because herbarium specimens provide us a glimpse of the past, we also can use these collections to examine possible changes in the distribution and phenotype of plants, particularly as a result of anthropogenic changes (7–17). However, with herbarium material alone, one cannot be certain whether a change in phenotype through time is due to a particular selective agent. To test for the role of human selection on harvested plants, we paired herbarium studies with field sampling and paired congeneric plant species that have experienced different levels of unconscious human selection.
Saussurea laniceps and Saussurea medusa (Asteraceae), known collectively as snow lotus (Fig. 1), are endemic to the eastern Himalayas. Both species have limited distributions on rocky habitats >4,000 m. Snow lotus is used in traditional Chinese and Tibetan medicine for the treatment of headaches and high blood pressure and to regulate menstrual cycles and treat menstrual problems (18). Although S. laniceps are primarily harvested as medicinal plants, they also have become popular souvenir items with tourists because they are strange-looking, rare, and grow in exotic locations (19). S. medusa are smaller and less frequently collected for medicine, sale, or souvenir. Larger individuals of S. laniceps are preferentially collected, because these are thought to be more potent and efficacious. Further, larger plants are easier to find. Regrettably, the whole plant of this monocarpic, long-lived species is harvested during the final flowering period, just before seed set, so that harvesting is a strong selective agent. Traditionally, snow lotus has been collected primarily on a small scale for local use. However, with better roads and the growing interest in alternative medicines, snow lotus has experienced increasingly intense harvest over the past 30 years.
Fig. 1.
Snow lotus used in traditional Tibetan and Chinese medicine. Shown are S. laniceps (a), the preferred species, and S. medusa (b), which is seldom collected or marketed.
Methods
Using historical herbarium samples and comparing them with those collected for the medicinal trade today, we documented the change in size (plant height) of S. laniceps and S. medusa over time. Both species reach their maximum size during flowering, therefore collections of flowering plants represent the plant at its largest size. We measured 218 individuals of S. laniceps and 309 individuals of S. medusa, the earliest dating back to 1872, collected by N. M. Przewalski (Zoological Institute of the Russian Academy of Science, St. Petersburg, Russia).
Plant height was measured on specimens of S. laniceps and S. medusa from eight different herbaria: the Missouri Botanical Garden (MO); the Gray Herbarium (GH) at Harvard University, Cambridge, MA; the Smithsonian Institution (US), Washington, DC; the Kunming Institute of Botany (KUN); the Beijing Institute of Botany (PE); the Royal Botanical Garden at Kew (K), London; the Natural History Museum in London (BM); and the Royal Botanical Garden at Edinburgh (E). These collections were the main repositories for specimens of the early explorers to northwest Yunnan, China. The collection dates of each specimen were recorded. The current sizes of plants were measured from recent collections being sold in the markets of northwest Yunnan.
We also measured the heights of S. laniceps and S. medusa plants belonging to present-day populations in heavily harvested and seldom-harvested areas. To determine heavily harvested and protected sites and thus to categorize collection pressures, we interviewed doctors and collectors. The protected sites were sacred Tibetan lands on the side of Khawa Karpo, one of the most sacred Tibetan mountains (20); the heavily harvested sites were common property alpine areas. We then measured flowering plants, from the top of the flower to ground level, that were found in these areas.
Statistically, we analyzed plant size over time, which was not normally distributed, by using Spearman's rho, a nonparametric correlation, and analyzed the relationship of plant height by year of collection. We tested for a difference in plant size between harvested and protected sites, which had unequal sample sizes that were not normally distributed, by using a Wilcoxon signed-rank test. All statistical tests were carried out by using jmp software (version 5.1, SAS Institute, Cary, NC).
Results
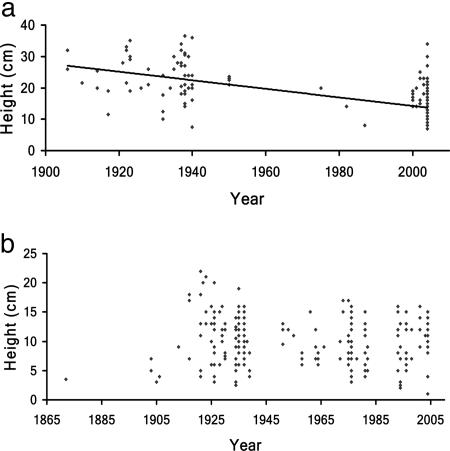
Saussurea laniceps showed a significant decline in size over time (Fig. 2a, r2 = 0.4361, P < 0.001), whereas S. medusa showed no significant change in size (Fig. 2b). We repeated this analysis by including only herbarium specimens to ensure that the results were not an artifact of the most recent plants measured in the medicinal shops rather than on herbarium sheets; the results of this test were not qualitatively changed and were still significant (r2 = 0.130, P < 0.001).
Fig. 2.
Heights of snow lotus plants at flowering. (a) Decline in height of S. laniceps from herbarium specimens and field collections over the past 100 years (r2 = 0.4361, P < 0.0001). (b) Height of S. medusa collections from the past 120 years, with no significant decline.
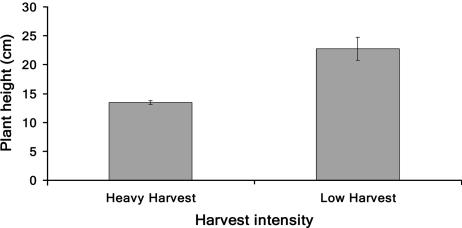
Furthermore, we compared the size of flowering plants that grew in heavily harvested areas with those that were protected in Tibetan sacred areas where very little harvesting took place (Fig. 3). We found that S. laniceps in the heavily harvested areas were on average 9 cm smaller than plants found in low-harvested areas (Z = 4.91, P < 0.0001). There was no significant difference in the height of S. medusa.
Fig. 3.
Height of S. laniceps plants found at heavily harvested and protected sites. Plants found in heavily harvested sites were on average 9 cm smaller than plants found in protected areas (average plant height ± SE; low harvest, 22.7 cm ± 2.0; high harvest, 13.4 cm ± 0.4).
Discussion
Selection caused by humans is a powerful force whether conscious or unconscious, “artificial” or natural (21, 22). Paradoxically, with unconscious human selection, when a species posses a certain trait that is valued by people (e.g., large size), individuals with that trait will be preferentially harvested, and this selection will leave individuals that possess less desirable traits (e.g., small plants). Evidence for this process has been reported from fisheries that harvest larger fish, resulting in smaller fish and lower yields (23). In our case, we observed that human harvesting of larger individuals of a rare plant for medicinal purposes resulted in the rapid evolution of smaller individuals over only 100 years. If plants that are smaller have decreased fitness because of lower seed yield (24–28), then the conservation status of this rare plant may be further threatened by this short-term evolutionary change. The immediate demographic effects of harvesting as well as the dwarfing of plants in response to unconscious anthropogenic selection may put threatened plants at greater risk of extinction.
Methodologically, our study demonstrates that herbarium specimens allow us to examine phenotypic changes due to selection in extant populations, which can facilitate conservation assessment and monitoring. This research is yet another example of how herbarium and museum collections are essential to conservation biology (29–31).
Acknowledgments
We thank T. Knight, L. Harmon, and J. Chase for careful reading of the manuscript and all herbaria that provided us access to their collections and facilities. This work was supported by National Science Foundation Grants 0413496 and 408123, the Mellon Foundation, and The Nature Conservancy.
Author contributions: W.L. and J.S. designed research; W.L. performed research; W.L. analyzed data; and W.L. and J.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Endler, J. A. (1986) Natural Selection in the Wild (Princeton Univ. Press, Princeton).
- 2.Kingsolver, J. G., Hoekstra, H. E., Hoekstra, J. M., Berrigan, D., Vignieri, S. N., Hill, C. E., Hoang, A., Gibert, P. & Beerli, P. (2001) Am. Nat. 157, 245-261. [DOI] [PubMed] [Google Scholar]
- 3.Darwin, C. (1859) On the Origin of Species (John Murray, London).
- 4.Vavilov, N. (1922) J. Genet. 12, 47-89. [Google Scholar]
- 5.Harlan, J. R. (1992) Crops and Man (Am. Soc. Agron./Crop Sci. Soc. Am., Madison, WI).
- 6.Lyte, C. (1983) The Plant Hunters (Orbis, London).
- 7.Jones, P., Beebe, S., Tohme, J. & Galwey, N. (1997) Biodiversity Conserv. 6, 947-958. [Google Scholar]
- 8.Prance, G. T. (2001) Taxon 50, 345-359. [Google Scholar]
- 9.Ter Stege, H., Jansen-Jacobs, M. J. & Datadin, V. K. (2000) Biodiversity Conserv. 9, 215-240. [Google Scholar]
- 10.Funk, V. A., Zermoglio, M. F. & Nasir, N. (1999) Biodiversity Conserv. 8, 727-751. [Google Scholar]
- 11.Prasad, S. N., Vijayan, L., Balachandran, S., Ramachandran, V. S. & Verghese, C. P. A. (1998) Curr. Sci. 75, 211-219. [Google Scholar]
- 12.Primack, D., Imbres, C., Primack, R. B., Miller-Rushing, A. J. & Tredici, P. D. (2004) Am. J. Bot. 91, 1260-1264. [DOI] [PubMed] [Google Scholar]
- 13.Saltonstall, K. (2002) Proc. Natl. Acad. Sci. USA 99, 2445-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skov, F. & Borchesenius, F. (1997) Ecography 20, 347-355. [Google Scholar]
- 15.Skov, F. (2000) Taxon 49, 503-515. [Google Scholar]
- 16.Delisle, F., Lavoie, C., Jean, M. & Lachance, D. (2003) J. Biogeogr. 30, 1033-1042. [Google Scholar]
- 17.McGraw, J. B. (2001) Biol. Conserv. 98, 25-32. [Google Scholar]
- 18.Yang, J. S., & Chuchengjiangcuo (1989) Diqing Zang yao (Yunnan min zu chu ban she, Kunming Shi).
- 19.Yang, Q. S., Chen, S. T. & Zhou, Z. K. (2003) Acta Bot. Yunn. 25, 297-302. [Google Scholar]
- 20.Anderson, D., Salick J., Moseley, R. K. & Ou, X. K. (2005) Biodiversity Conserv., in press.
- 21.Salick, J. (1992) Evol. Biol. 26, 247-285. [Google Scholar]
- 22.Salick, J. & Merrick, L. (1990) in Agroecology, eds. Carroll, C. R., Vandermeer, J. H. & Rosset, P. M. (McGraw–Hill, New York), pp. 517-548.
- 23.Conover, D. O. & Munch, S. B. (2002) Science 297, 94-96. [DOI] [PubMed] [Google Scholar]
- 24.Weiner, J. & Thomas, S. C. (1986) Oikos 47, 211-222. [Google Scholar]
- 25.Herrera, C. M. (1993) Ecol. Monogr. 63, 251-275. [Google Scholar]
- 26.Ollerton, J. & Lack, A. (1998) Plant Ecol. 139, 35-47. [Google Scholar]
- 27.Charron, D. & Gagnon, D. (1991) J. Ecol. 79, 1137-1146. [Google Scholar]
- 28.Nantel, P., Gagnon, D. & Nault, A. (1996) Conserv. Biol. 10, 608-621. [Google Scholar]
- 29.Wilis, F., Moat, J. & Paton, A. (2003) Biodiversity Conserv. 12, 1537-1552. [Google Scholar]
- 30.Lienert, J., Fischer, M. & Diemer, M. (2002) Biol. Conserv. 103, 65-76. [Google Scholar]
- 31.MacDougall, A. S., Loo, J. A., Claden, S. R., Goltz, J. G. & Hinds, H. R. (1998) Biol. Conserv. 86, 325-338. [Google Scholar]